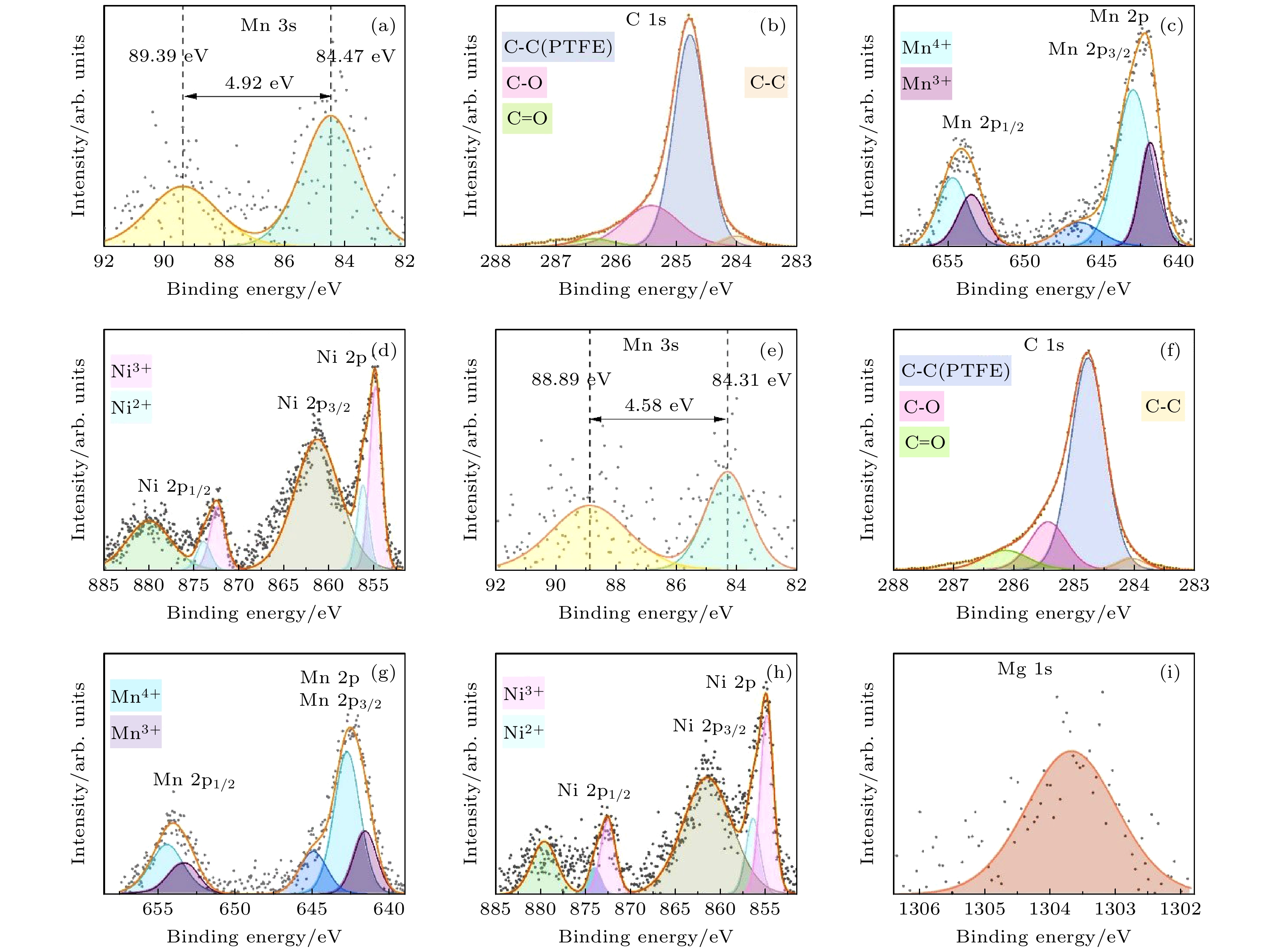
-
钠离子电池层状氧化物正极材料具有高容量、易合成等优势, 表现出巨大的应用潜力. 为了开发出高容量、长循环的正极材料, 本文提出对NaNi0.4Cu0.1Mn0.4Ti0.1O2 (NCMT)用Mg2+部分取代Ni2+的改性策略, 设计并合成了高容量、长循环的NaNi0.35Mg0.05Cu0.1Mn0.4Ti0.1O2 (NCMT-Mg)正极材料. 该材料在2.4—4.3 V电压范围内, 显示165 mAh·g–1的高可逆比容量. 在0.1 C的倍率下循环350周后, 仍有111 mAh·g–1的可逆比容量, 容量保持率为67.3%, 相较于未掺杂的原始样品提升了约13%. 本文对其进行了系统表征并揭示了其高电压循环稳定的机理, 为开发出高性能钠离子正极材料提供了重要参考.Driven by global demand for new energy, Li-ion batteries (LIBs) have developed rapidly due to their competitive performance. Although LIBs show the advantages of high capacity and good cycling stability, their disadvantages such as uneven distribution of lithium resources are gradually exposed. Therefore, with abundant reserves, Na-ion batteries (NIB) have become one of the most promising solutions to make up for the deficiency of Li-ion battery. The NIBs layered oxide cathodes have the most potential applications of cathode material due to their high specific capacity (167 mAh·g–1 in 2.4–4.3 V) and simple synthesis method. However, improving the cycling stability of layered cathode materials is one of the keys to their large-scale industrialization. To develop high capacity and cycling stability cathode materials, the Mg2+ is substituted for Ni2+ in NaNi0.4Cu0.1Mn0.4Ti0.1O2 (NCMT), thereby obtaining a NaNi0.35Mg0.05Cu0.1Mn0.4Ti0.1O2 (NCMT-Mg) cathode material. The NCMT-Mg has a high reversible specific capacity of 165 mAh·g–1 in a voltage window of 2.4–4.3 V. The reversible specific capacity of about 110 mAh·g–1 at 0.1 C after 350 cycles with a capacity retention of 67.3% is about 13% higher than the counterpart of NCMT. The irreversible reaction is suppressed from P'3 phase to X phase for NCMT. The ex-XRD spectrometers further prove that the NCMT-Mg shows a P3 and X mixed phase after being initially charged to 4.3 V, but the NCMT shows an X phase. The irreversible phase transition is suppressed to increase the cycling stability. The inactive Mg2+ replaces Ni2+, reducing the charge compensation and stabilizing the structure, the inactive Mg2+ can activate the charge compensation of Ni2+/Cu2+. The electrochemical activity increases from 77% to 86%. The high capacity and excellent cycling stability prove that the NCMT-Mg structure remains intact after various current rates have been tested. The long cycling stability mechanism is further systematically studied by using various technologies. The present work will provide an important reference for developing high-performance Na-ion cathode materials.
-
Keywords:
- Na-ion batteries /
- layered oxide cathodes /
- cathode material /
- cycling stability
[1] 陆雅翔, 赵成龙, 容晓晖, 陈立泉, 胡勇胜 2018 67 120601
 Google Scholar
Google Scholar
Lu Y X, Zhao C L, Rong X H, Chen L Q, Hu Y S 2018 Acta Phys. Sin. 67 120601
 Google Scholar
Google Scholar
[2] Li Y, Lu Y, Zhao C, Hu Y S, Titirici M M, Li H, Yong-Sheng H, Chen L 2017 Energy Storage Mater 7 130
 Google Scholar
Google Scholar
[3] Ding F, Li J, Deng F, Xu G, Liu Y, Yang K, Kang F 2017 ACS Appl. Mater. Interfaces 9 27936
 Google Scholar
Google Scholar
[4] Braconnier J, Delmas C, Fouassier C, Hagenmuller P 1980 Mater. Res. Bull. 15 1797
 Google Scholar
Google Scholar
[5] Nagelberg A, Worrell W 1979 J. Solid State Chem. 29 345
 Google Scholar
Google Scholar
[6] Whittingham M 1978 Prog Solid State Chem. 12 41
 Google Scholar
Google Scholar
[7] Whittingham M S 1976 Science 192 1126
 Google Scholar
Google Scholar
[8] Sun Y, Guo S, Zhou H 2019 Energy Environ. Sci. 12 825
 Google Scholar
Google Scholar
[9] Kubota K, Kumakura S, Yoda Y, Kuroki K, Komaba S 2018 Adv. Energy Mater. 8 1703415
 Google Scholar
Google Scholar
[10] Liu Q, Hu Z, Chen M, Zou C, Jin H, Wang S, Chou S L, Dou S X 2019 Small 15 1805381
 Google Scholar
Google Scholar
[11] Bauer A, Song J, Vail S, Pan W, Barker J, Lu Y 2018 Adv. Energy Mater. 8 1702869
 Google Scholar
Google Scholar
[12] Wang L, Lu Y, Liu J, Xu M, Cheng J, Zhang D, Goodenough J B 2013 Angew. Chem. Int. Ed. 52 1964
 Google Scholar
Google Scholar
[13] Wang S, Wang L, Zhu Z, Hu Z, Zhao Q, Chen J. 2014 Angew. Chem. Int. Ed. 53 5892
 Google Scholar
Google Scholar
[14] Wang Q, Zhao C, Lu Y, Li Y, Zheng Y, Qi Y, Rong X, Jiang L, Qi X, Shao Y, Pan D, Li B, Hu Y S, Chen L 2017 Small 13 1701835
 Google Scholar
Google Scholar
[15] Wang Y S, Xiao R J, Hu Y S, Avdeev M, Chen L Q 2015 Nat. Commun. 6 6954
 Google Scholar
Google Scholar
[16] Wu F, Zhao C, Chen S, Lu Y, Hou Y, Hu Y S, Maier J, Yu Y 2018 Mater. Today 21 960
 Google Scholar
Google Scholar
[17] Xu S Y, Wu X Y, Li Y M, Hu Y S, Chen L Q 2014 Chin. Phys. B 23 118202
 Google Scholar
Google Scholar
[18] Li Y, Yang Z, Xu S, Mu L, Gu L, Hu Y S, Li H, Chen L 2015 Adv. Sci. 2 1500031
 Google Scholar
Google Scholar
[19] Delmas C, Fouassier C, Hagenmuller P 1980 Physica B & C 99 81
[20] 穆林沁, 戚兴国, 胡勇胜, 李泓, 陈立泉, 黄学杰. 2016 储能科学与技术 5 324
Mu L Q, Qi X G, Hu Y S, Li H, Chen L Q, Huang X J 2016 Energy Storage Sci. Technol. 5 324
[21] Su D, Wang C, Ahn H J, Wang G 2013 Chem. Eur. J. 19 10884
 Google Scholar
Google Scholar
[22] Caballero A, Hernan L, Morales J, Sanchez L, Pena J S, Aranda M A G 2002 J. Mater. Chem. 12 1142
 Google Scholar
Google Scholar
[23] Rai A K, Anh L T, Gim J, Mathew V, Kim J 2014 Ceram. Int. 40 2411
 Google Scholar
Google Scholar
[24] Xia X, Dahn J R 2012 J. Electrochem. Soc. 159 A1048
 Google Scholar
Google Scholar
[25] Yang L, Sun S, Du K, Zhao H, Yan D, Yang H Y, Bai Y 2021 Ceram. Int. 47 28521
 Google Scholar
Google Scholar
[26] Huang J Y, Yu T Y, Sun Y K 2018 J. Mater. Chem. A 6 16854
 Google Scholar
Google Scholar
[27] Hong N, Wu K, Peng Z, Zhu Z, Jia G, Wang M 2020 J. Phys. Chem. C 124 22925
 Google Scholar
Google Scholar
[28] Pang W L, Zhang X H, Guo J Z, Li J Y, Yan X, Hou B H, Wu X L 2017 J. Power Sources 356 80
 Google Scholar
Google Scholar
[29] Lee E, Lu J, Ren Y, Luo X, Zhang X, Wen J, Johnson C S 2014 Adv. Energy Mater. 4 1400458
 Google Scholar
Google Scholar
[30] Zheng S, Zhong G, McDonald M J, Gong Z, Liu R, Wen W, Yang Y 2016 J. Mater. Chem. A 4 9054
 Google Scholar
Google Scholar
[31] Natasha A C, Ma M, Xiao J, et al. 2006 MRS Online Proceedings Library 972 1
[32] Yuan D D, Wang Y X, Cao Y L, Ai X P, Yang H X 2015 ACS Appl. Mater. Interfaces 7 8585
 Google Scholar
Google Scholar
[33] Yabuuchi N, Yano M, Yoshida H, Kuze S, Komaba S 2013 J. Electrochem. Soc. 160 A3131
 Google Scholar
Google Scholar
[34] Zhao H, Li J, Liu W, Xu H, Gao X, Shi J, Ding X 2021 Electrochim. Acta 388 138561
 Google Scholar
Google Scholar
[35] Kubota K, Fujitani N, Yoda Y, Kuroki K, Tokita Y, Komaba S 2021 J. Mater. Chem. A 9 12830
 Google Scholar
Google Scholar
[36] Zhang X, Zhou Y N, Yu L, Zhang S Y, Xing X X, Wang W, Xu S 2021 Mater. Chem. Front. 5 5344
 Google Scholar
Google Scholar
[37] Yao H R, Wang P F, Gong Y, Zhang J, Yu X, Gu L, Wan L J 2017 J. Am. Chem. Soc. 139 8440
 Google Scholar
Google Scholar
[38] Wang Q, Mariyappan S, Vergnet J, Abakumov A M, Rousse G, Rabuel F 2019 Adv. Energy Mater. 9 1901785
 Google Scholar
Google Scholar
[39] Zhao C, Wang Q, Yao Z, Wang J, Sánchez-Lengeling B, Ding F, Hu Y S 2020 Science 370 708
[40] Dang R, Li N, Yang Y, Wu K, Li Q, Lee Y L, Liu X F, Hu Z B, Xiao X 2020 J. Power Sources 464 228190
 Google Scholar
Google Scholar
[41] 彭佳悦, 祖晨曦, 李泓 2013 储能科学与技术 2 55
 Google Scholar
Google Scholar
Peng J Y, Zu C X, Li H 2013 Energy Storage Sci. Technol. 2 55
 Google Scholar
Google Scholar
-
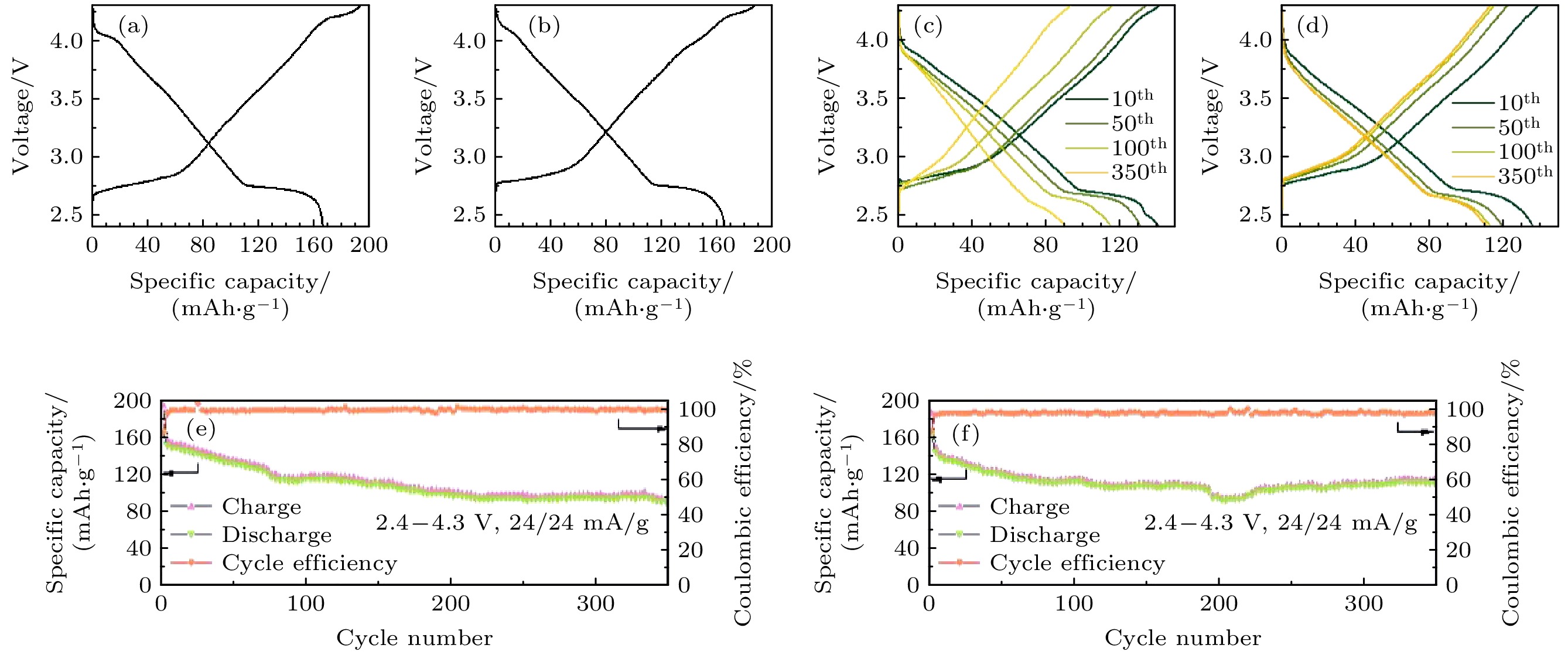
图 3 NCMT和NCMT-Mg两种正极材料的电化学性能. 使用不同正极组装的电池NCMT (a)和NCMT-Mg (b) 的第1周, 和第10周、第50周、第100周和第350周充放电曲线((c), (d)); 在0.1 C电流倍率下两材料的循环性能((e), (f))
Fig. 3. Electrochemical performance of NCMT and NCMT-Mg based batteries. The batteries are assembled using different samples NCMT (a) and NCMT-Mg (b) initial charge and discharge curves. The batteries are assembled using different samples (c) NCMT and (d) NCMT-Mg charge and discharge curves for the, 10 th, 50 th, 100 th, and 350 th cycles. Cycling performance of the (e) NCMT and (f) NCMT-Mg at 0.1 C.
图 4 使用NCMT (a), NCMT-Mg (b) 组装的电池在2.4—4.3 V范围内前两周循环dQ/dV曲线; 使用NCMT (c), NCMT-Mg (d) 组装的电池充电至4.3 V的非原位XRD衍射图谱
Fig. 4. dQ/dV curves of the first two cycles between 2.4 and 4.3 V of batteries assembled using different samples NCMT (a) and NCMT-Mg (b). Ex-XRD curves of the first to fifth cycles charge to 4.3 V of NCMT (c) and NCMT-Mg (d).
-
[1] 陆雅翔, 赵成龙, 容晓晖, 陈立泉, 胡勇胜 2018 67 120601
 Google Scholar
Google Scholar
Lu Y X, Zhao C L, Rong X H, Chen L Q, Hu Y S 2018 Acta Phys. Sin. 67 120601
 Google Scholar
Google Scholar
[2] Li Y, Lu Y, Zhao C, Hu Y S, Titirici M M, Li H, Yong-Sheng H, Chen L 2017 Energy Storage Mater 7 130
 Google Scholar
Google Scholar
[3] Ding F, Li J, Deng F, Xu G, Liu Y, Yang K, Kang F 2017 ACS Appl. Mater. Interfaces 9 27936
 Google Scholar
Google Scholar
[4] Braconnier J, Delmas C, Fouassier C, Hagenmuller P 1980 Mater. Res. Bull. 15 1797
 Google Scholar
Google Scholar
[5] Nagelberg A, Worrell W 1979 J. Solid State Chem. 29 345
 Google Scholar
Google Scholar
[6] Whittingham M 1978 Prog Solid State Chem. 12 41
 Google Scholar
Google Scholar
[7] Whittingham M S 1976 Science 192 1126
 Google Scholar
Google Scholar
[8] Sun Y, Guo S, Zhou H 2019 Energy Environ. Sci. 12 825
 Google Scholar
Google Scholar
[9] Kubota K, Kumakura S, Yoda Y, Kuroki K, Komaba S 2018 Adv. Energy Mater. 8 1703415
 Google Scholar
Google Scholar
[10] Liu Q, Hu Z, Chen M, Zou C, Jin H, Wang S, Chou S L, Dou S X 2019 Small 15 1805381
 Google Scholar
Google Scholar
[11] Bauer A, Song J, Vail S, Pan W, Barker J, Lu Y 2018 Adv. Energy Mater. 8 1702869
 Google Scholar
Google Scholar
[12] Wang L, Lu Y, Liu J, Xu M, Cheng J, Zhang D, Goodenough J B 2013 Angew. Chem. Int. Ed. 52 1964
 Google Scholar
Google Scholar
[13] Wang S, Wang L, Zhu Z, Hu Z, Zhao Q, Chen J. 2014 Angew. Chem. Int. Ed. 53 5892
 Google Scholar
Google Scholar
[14] Wang Q, Zhao C, Lu Y, Li Y, Zheng Y, Qi Y, Rong X, Jiang L, Qi X, Shao Y, Pan D, Li B, Hu Y S, Chen L 2017 Small 13 1701835
 Google Scholar
Google Scholar
[15] Wang Y S, Xiao R J, Hu Y S, Avdeev M, Chen L Q 2015 Nat. Commun. 6 6954
 Google Scholar
Google Scholar
[16] Wu F, Zhao C, Chen S, Lu Y, Hou Y, Hu Y S, Maier J, Yu Y 2018 Mater. Today 21 960
 Google Scholar
Google Scholar
[17] Xu S Y, Wu X Y, Li Y M, Hu Y S, Chen L Q 2014 Chin. Phys. B 23 118202
 Google Scholar
Google Scholar
[18] Li Y, Yang Z, Xu S, Mu L, Gu L, Hu Y S, Li H, Chen L 2015 Adv. Sci. 2 1500031
 Google Scholar
Google Scholar
[19] Delmas C, Fouassier C, Hagenmuller P 1980 Physica B & C 99 81
[20] 穆林沁, 戚兴国, 胡勇胜, 李泓, 陈立泉, 黄学杰. 2016 储能科学与技术 5 324
Mu L Q, Qi X G, Hu Y S, Li H, Chen L Q, Huang X J 2016 Energy Storage Sci. Technol. 5 324
[21] Su D, Wang C, Ahn H J, Wang G 2013 Chem. Eur. J. 19 10884
 Google Scholar
Google Scholar
[22] Caballero A, Hernan L, Morales J, Sanchez L, Pena J S, Aranda M A G 2002 J. Mater. Chem. 12 1142
 Google Scholar
Google Scholar
[23] Rai A K, Anh L T, Gim J, Mathew V, Kim J 2014 Ceram. Int. 40 2411
 Google Scholar
Google Scholar
[24] Xia X, Dahn J R 2012 J. Electrochem. Soc. 159 A1048
 Google Scholar
Google Scholar
[25] Yang L, Sun S, Du K, Zhao H, Yan D, Yang H Y, Bai Y 2021 Ceram. Int. 47 28521
 Google Scholar
Google Scholar
[26] Huang J Y, Yu T Y, Sun Y K 2018 J. Mater. Chem. A 6 16854
 Google Scholar
Google Scholar
[27] Hong N, Wu K, Peng Z, Zhu Z, Jia G, Wang M 2020 J. Phys. Chem. C 124 22925
 Google Scholar
Google Scholar
[28] Pang W L, Zhang X H, Guo J Z, Li J Y, Yan X, Hou B H, Wu X L 2017 J. Power Sources 356 80
 Google Scholar
Google Scholar
[29] Lee E, Lu J, Ren Y, Luo X, Zhang X, Wen J, Johnson C S 2014 Adv. Energy Mater. 4 1400458
 Google Scholar
Google Scholar
[30] Zheng S, Zhong G, McDonald M J, Gong Z, Liu R, Wen W, Yang Y 2016 J. Mater. Chem. A 4 9054
 Google Scholar
Google Scholar
[31] Natasha A C, Ma M, Xiao J, et al. 2006 MRS Online Proceedings Library 972 1
[32] Yuan D D, Wang Y X, Cao Y L, Ai X P, Yang H X 2015 ACS Appl. Mater. Interfaces 7 8585
 Google Scholar
Google Scholar
[33] Yabuuchi N, Yano M, Yoshida H, Kuze S, Komaba S 2013 J. Electrochem. Soc. 160 A3131
 Google Scholar
Google Scholar
[34] Zhao H, Li J, Liu W, Xu H, Gao X, Shi J, Ding X 2021 Electrochim. Acta 388 138561
 Google Scholar
Google Scholar
[35] Kubota K, Fujitani N, Yoda Y, Kuroki K, Tokita Y, Komaba S 2021 J. Mater. Chem. A 9 12830
 Google Scholar
Google Scholar
[36] Zhang X, Zhou Y N, Yu L, Zhang S Y, Xing X X, Wang W, Xu S 2021 Mater. Chem. Front. 5 5344
 Google Scholar
Google Scholar
[37] Yao H R, Wang P F, Gong Y, Zhang J, Yu X, Gu L, Wan L J 2017 J. Am. Chem. Soc. 139 8440
 Google Scholar
Google Scholar
[38] Wang Q, Mariyappan S, Vergnet J, Abakumov A M, Rousse G, Rabuel F 2019 Adv. Energy Mater. 9 1901785
 Google Scholar
Google Scholar
[39] Zhao C, Wang Q, Yao Z, Wang J, Sánchez-Lengeling B, Ding F, Hu Y S 2020 Science 370 708
[40] Dang R, Li N, Yang Y, Wu K, Li Q, Lee Y L, Liu X F, Hu Z B, Xiao X 2020 J. Power Sources 464 228190
 Google Scholar
Google Scholar
[41] 彭佳悦, 祖晨曦, 李泓 2013 储能科学与技术 2 55
 Google Scholar
Google Scholar
Peng J Y, Zu C X, Li H 2013 Energy Storage Sci. Technol. 2 55
 Google Scholar
Google Scholar
计量
- 文章访问数: 11157
- PDF下载量: 326
- 被引次数: 0














 下载:
下载: