-
三维石墨烯为开发高能量密度的电极提供了有效的途径. 与二维石墨烯相比, 三维石墨烯具有三维导电网络, 极大地改善锂离子和电子传输的能力, 同时能够承受电极循环期间的结构和体积变化. 本文发展了低压封闭化学气相沉积法(CVD), 以泡沫镍为模板, 采用聚甲基丙烯酸甲酯为固态碳源来制备缺陷可控的三维石墨烯泡沫. 分别研究了碳源添加量、反应时间及氢气含量对三维石墨烯泡沫形貌及结构的影响, 发展了一种新型的三维石墨烯泡沫制备工艺, 所制备的三维石墨烯泡沫具有缺陷密度可控, 质量轻及化学性能稳定的特点. 以三维石墨烯泡沫为导电框架和活性物载体来制备ZnO/石墨烯泡沫(ZnO/GF)复合电极并作为锂离子电池负极, 循环200圈后仍能保持851.5 mA·h·g-1的高比容量, ZnO/三维石墨烯电极表现出较高的可逆容量以及优异的循环性能.Three-dimensional graphene provides a promising approach to developing high-energy-density electrodes. Compared with two-dimensional (2D) graphene, three-dimensional (3D) graphene has a three-dimensional conductive network, which greatly improves the ability of lithium ions and electron to transport and can tolerate the changes of structural and volume in the cycling process. In this paper, 3D graphene with controllable defects is prepared by using an innovative low-pressure closed chemical vapor deposition method, through using nickel foam as the template and polymethyl methacrylate as a solid carbon source. The effects of the amount of carbon source addition, reaction time and hydrogen content on the morphology and structure of graphene foam are analyzed. The experimental results indicate that the amount of carbon source added, the reaction time, and the hydrogen content have significant effects on the morphology and structure of graphene. The defect density and the number of layers of as-prepared graphene are directly proportional to the amount of carbon source added. There is a threshold for the reaction time. After reaching a certain reaction time, graphene with good structure and morphology can be formed. The optimal reaction time is about 20 min. The hydrogen content promotes the high-temperature pyrolysis of solid carbon source. The sample has a highest defect density at 0.5 kPa hydrogen content. In summary, the low-pressure closed CVD method has strong safety and can synthesize 3D graphene with excellent controllable structure and defects. The 3D graphene foam with a complete structure of 2–5 layers can be prepared under the conditions of 1000 ℃, 500 μL carbon source addition, 20 min reaction time and 0.5 kPa hydrogen content, displaying the best physical chemistry performance. The graphene foam prepared in this experiment has the characteristics of convenient and controllable defect density, light weight and stable chemical properties. When ZnO/GF electrode prepared with 3D GF as a conductive frame and active carrier is used as an anode, the lithium ion battery has a high specific capacity of 851.5 mA·h·g–1 after 200 cycles, which exhibites high reversible capacity and good cycling performance. Although ZnO/GF electrode displays excellent lithium storage performance, the GF prepared based on the 3D Ni foam has a low spatial structure density and the surface loading of the ZnO/GF composite electrode is still low, resulting in a low energy density. Therefore, the following researchers should focus on the structural design of 3D graphene host/current collector to obtain a 3D graphene frame with high conductivity and high loading capacity.
-
Keywords:
- chemical vapor deposition /
- controllable preparation /
- three-dimensional grapheme foam /
- cycling performance
[1] Fang H, Zou W, Yan J, Xing Y L, Zhang S C 2018 ChemElectroChem 5 2458
 Google Scholar
Google Scholar
[2] Peng H J, Huang J Q, Cheng X B, Zhang Q 2017 Adv. Energy Mater. 24 54
[3] Xu Y X, Lin Z Y, Huang X Q, Wang Y, Huang Y, Duan X F 2013 Adv. Mater. 25 5779
 Google Scholar
Google Scholar
[4] Wei S Y, Ma L, Hendrickson K E, Tu Z Y, Archer L A 2015 J. Am. Chem. Soc. 37 12143
[5] Fang H, Meng F T, Chen G Y, Wang L X, Zhang S C, Yan J, Zhang L S, Zhang Y X 2019 Int. J. Electrochem. Sci. 14 7937
[6] Li G R, Lei W, Luo D, Deng Y P 2018 Adv. Energy Mater. 8 1702381
 Google Scholar
Google Scholar
[7] Geim A K, Novoselov K S 2007 Nat. Mater. 3 183
[8] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[9] Guo H, Wang X Y, Bao D L, Lu L L, Zhang Y Y, Li Geng, Wang Y L, Du S X, Gao H J 2019 Chin. Phys. B 28 078103
 Google Scholar
Google Scholar
[10] Novoselov K S, Geim A K, Morozov S V, Jiang D, Katsnelson M L, Grigorieva I V, Dubonos S V, Firsov A A 2005 Nature 7065 197
[11] Li X S, Magnuson C W, Venugopal A, An J H, Suk J W, Han B Y, Borysiak M, Cai W W, Velamakanni A, Zhu Y W, Fu L F, Vogel E M, VoelkL E, Colombo L G, Ruoff R S 2010 Nano Lett. 11 4328
[12] Li X S, Cai W W, Colombo L G, Ruoff R S 2009 Nano Lett. 12 4268
[13] Ito Y, Christodoulou C, Nardi M V, Koch N, Sachdec H, Mullen K 2014 ACS Nano 8 3337
 Google Scholar
Google Scholar
[14] 任文才, 高力波, 马来鹏 2011 新型炭材料 1 71
Ren W C, Gao L B, Ma L P 2011 New Carbon Mater. 1 71
[15] Ci L J, Xu Z P, Wang L L, Gao W, Ding F, Kelly K F, Yakobson B I, Ajayan P M 2008 Nano Res. 1 116
 Google Scholar
Google Scholar
[16] Rodrı′guez-Manzo J A, Pham-Huu C, Banhart F 2011 ACS Nano 2 1529
[17] Ji H X, Hao Y F, Ren Y J, Charlton M, Lee W H, Wu Q Z, Li H F, Zhu Y W, Wu Y P, Piner R, Ruoff R S 2011 ACS Nano 9 7656
[18] Raccichini R, Varzi A, Passerini S, Scrosati B 2015 Nat. Mater. 14 271
 Google Scholar
Google Scholar
[19] Bi H, Chen I W, Lin T 2015 Adv. Mater. 39 5943
[20] Ito Y, Tanabe Y, Qiu H J, Sugawara K, Heguri S, Tu N H, Huynh K K, Fujita T, TakahashivT, Tanigaki K, Chen M 2014 Angew Chem. Int. Ed. 53 4822
 Google Scholar
Google Scholar
[21] Dong X C, Wang X W, Wang L H, Song H, Zhang H, Huang W, Chen P 2012 ACS Appl. Mater. Interfaces 6 3129
[22] Dong X C, Ma Y W, Zhu G Y, Huang Y X, Wang J, Chan-Park M B, Wang L H, Huang W, Chen P 2012 J. Mater. Chem. 22 17044
 Google Scholar
Google Scholar
[23] Li Z C, Wu P, Wang C X, Fan X D, Zhang W H, Zhai X F, Zeng C G, Li Z Y, Yang J L, Hou J G 2011 ACS Nano 5 3385
 Google Scholar
Google Scholar
[24] Xiao X Y, Beechem T E, Brumbach M T, Lambert T N, Davis D J, Michael J R, Washburn C M, Wang J, Brozik S M, Wheeler D R, Burcker D B, Polsky R 2012 ACS Nano 6 3573
 Google Scholar
Google Scholar
[25] Fernandez Merino M J, Guardia L, Paredes J I, Villar-Rodil S, Solis-Fernandez P, Martinez-Alonso A, Tascon J M D 2010 J. Phys. Chem. C 114 6426
[26] Zhang L B, Chen G Y, Hedhili M N, Zhang H N, Wang P 2012 Nanoscale 4 7038
 Google Scholar
Google Scholar
[27] Fang H, Meng F T, Yan J, Chen G Y, Zhang L S, Wu S D, Zhang S C, Wan L Z, Zhang Y X 2019 RSC Adv. 9 20107
 Google Scholar
Google Scholar
[28] Fang H, Chen G Y, Wang L X, Yan J, Zhang L S, Gao K Z, Zhang Y X, Wang L Z 2018 RSC Adv. 8 38550
 Google Scholar
Google Scholar
[29] Wang W X, Yang P H, Jian Z X, Li H L, Xing Y L, Zhang S C 2018 J. Mater. Chem. A 6 13797
 Google Scholar
Google Scholar
[30] Wang W X, Zhang S C, Xing Y L, Wang S B, Ren Y B 2016 RSC Adv. 6 75414
 Google Scholar
Google Scholar
[31] Chen Z P, Ren W C, Gao L B, Liu B L, Pei S F, Cheng H M 2011 Nat. Mater. 10 424
 Google Scholar
Google Scholar
[32] Compton O C, Nguyen S T 2010 Small 6 711
 Google Scholar
Google Scholar
[33] Wu J, Xu H, Zhang J 2014 Acta Chim. Sin. 72 301
 Google Scholar
Google Scholar
[34] Malard L M, Pimenta M A, Dresselhaus G, Dresselhaus M S 2009 Phys. Rep. 473 51
 Google Scholar
Google Scholar
[35] Ferrari A C, Basko D M 2013 Nat. Nanotechnol. 8 235
 Google Scholar
Google Scholar
[36] Chae S J, Güneş F, Kim K K, Kim E S, Han G H, Kim S M, Shin H J, Yoon S M, Chio J Y, Park M H, Yang C W, Pribat D, Lee Y H 2009 Adv. Mater. 21 2328
 Google Scholar
Google Scholar
[37] Zhang Y, Gomez L, Ishikawa F N, Madaria A, Ryu K, Wang C, Badmaev A, Zhou C W 2010 J. Phys. Chem. Lett. 1 3101
 Google Scholar
Google Scholar
[38] Ashkenov N, Mbenkum B N, Bundesmann C, Riede V, Lorenz M, Spemann D, Kaidashev E M, Kasic A, Schubert M, Grundmann M, Wagner G, Neumann H, Darakchieva V, Arwin H, Monemar B 2003 J. Appl. Phys. 93 126
 Google Scholar
Google Scholar
[39] Bundesmann C, Ashkenov N, Schubert M, Spemann D, Butz T, Kaidashev E M, Lorenz M, Grundmann M 2003 App. Phys. Lett. 83 1974
 Google Scholar
Google Scholar
[40] Fang H, Zhang L S, Xing Y L, Zhang S C, Wu S D 2018 Int. J. Electrochem. Sci. 13 8736
[41] Liu J, Zhang Y H, Bai Z M, Huang Z A, Gao Y K 2019 Chin. Phys. B 28 048101
 Google Scholar
Google Scholar
-
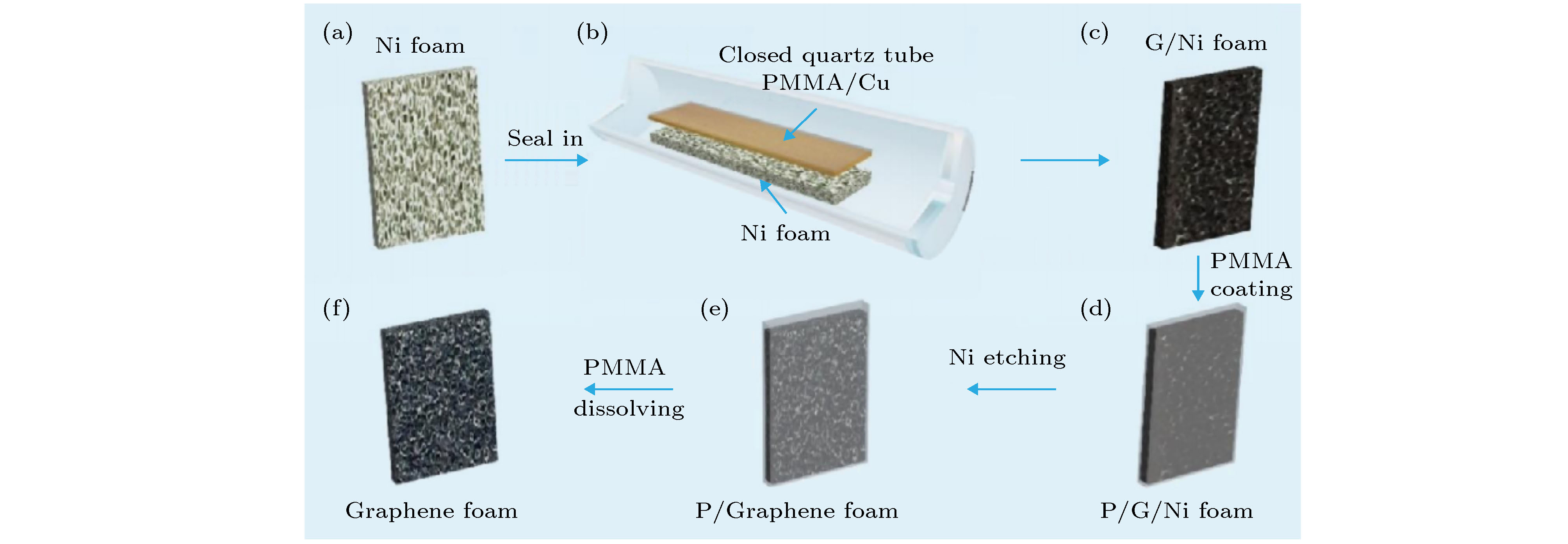
图 1 自支撑3D GF的合成过程示意图 (a)−(c)密封的石英管中泡沫镍上生长石墨烯; (d) 具有薄层PMMA膜的G/Ni泡沫涂层; (e) 去除泡沫镍后, PMMA保护的G/Ni泡沫; (f) 自支撑3D GF
Fig. 1. Schematic of the synthesis process of a self-supporting 3D GF: (a)−(c) Low pressure closed CVD method uses a sealed quartz tube to grow graphene on nickel foam; (d) G/Ni foam coating with thin PMMA film; (e) etching to remove nickel foam After that, PMMA protected G/Ni foam; (f) self-supporting 3D GF after dissolving the thin PMMA layer with hot acetone.
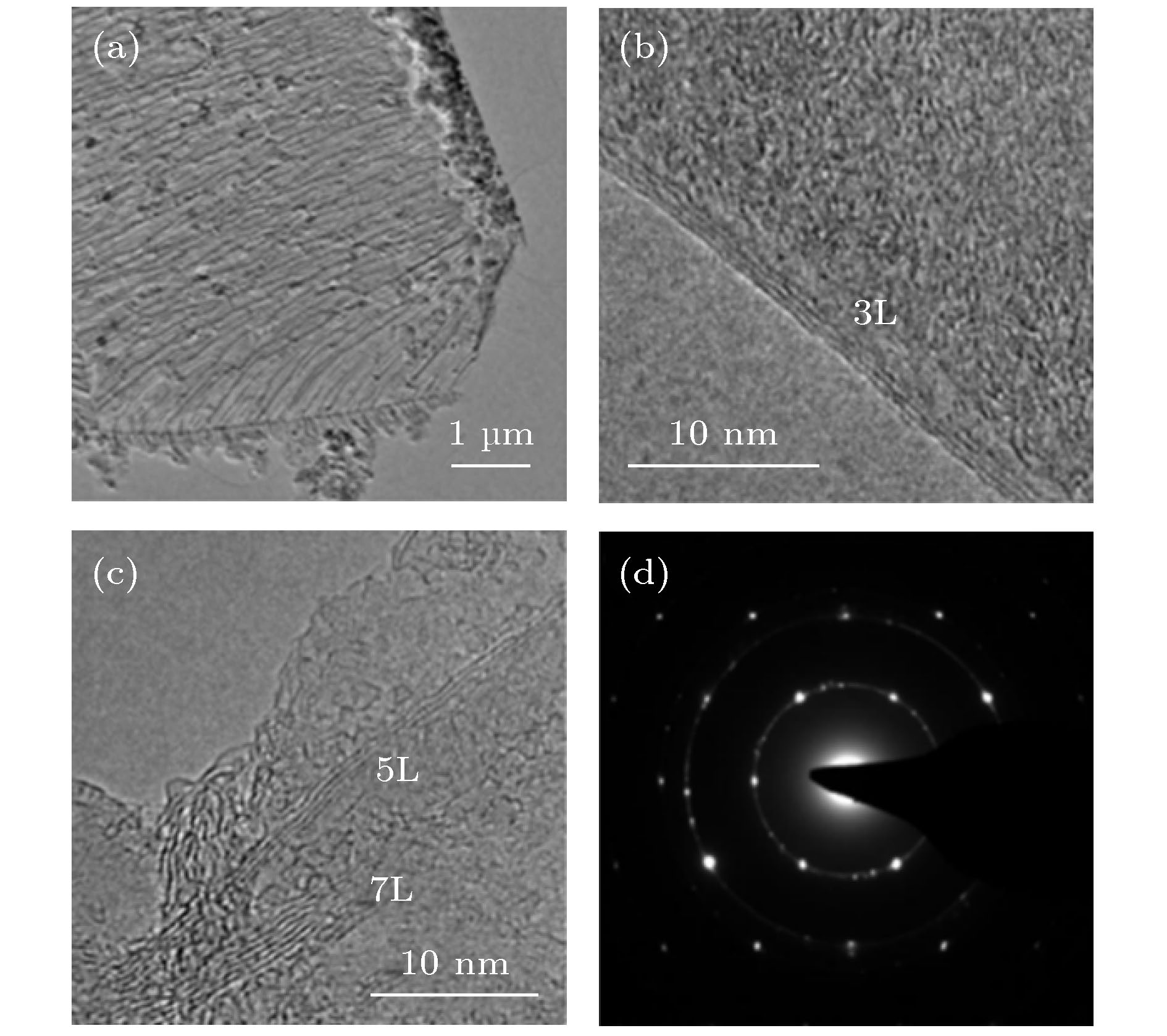
图 3 低压封闭CVD制备的GF400石墨烯样品的TEM图像 (a) 石墨烯薄膜的低倍TEM图像, 显示出典型的褶皱形貌; (b), (c) 石墨烯薄膜边缘的HRTEM图像; (d) 石墨烯薄膜的电子衍射图案
Fig. 3. TEM image of GF400 graphene sample prepared by low pressure closed CVD: (a) Low magnification TEM image of graphene film, showing typical wrinkle morphology; (b), (c) HRTEM image of graphene film edge; (d) electron diffraction pattern of graphene film.
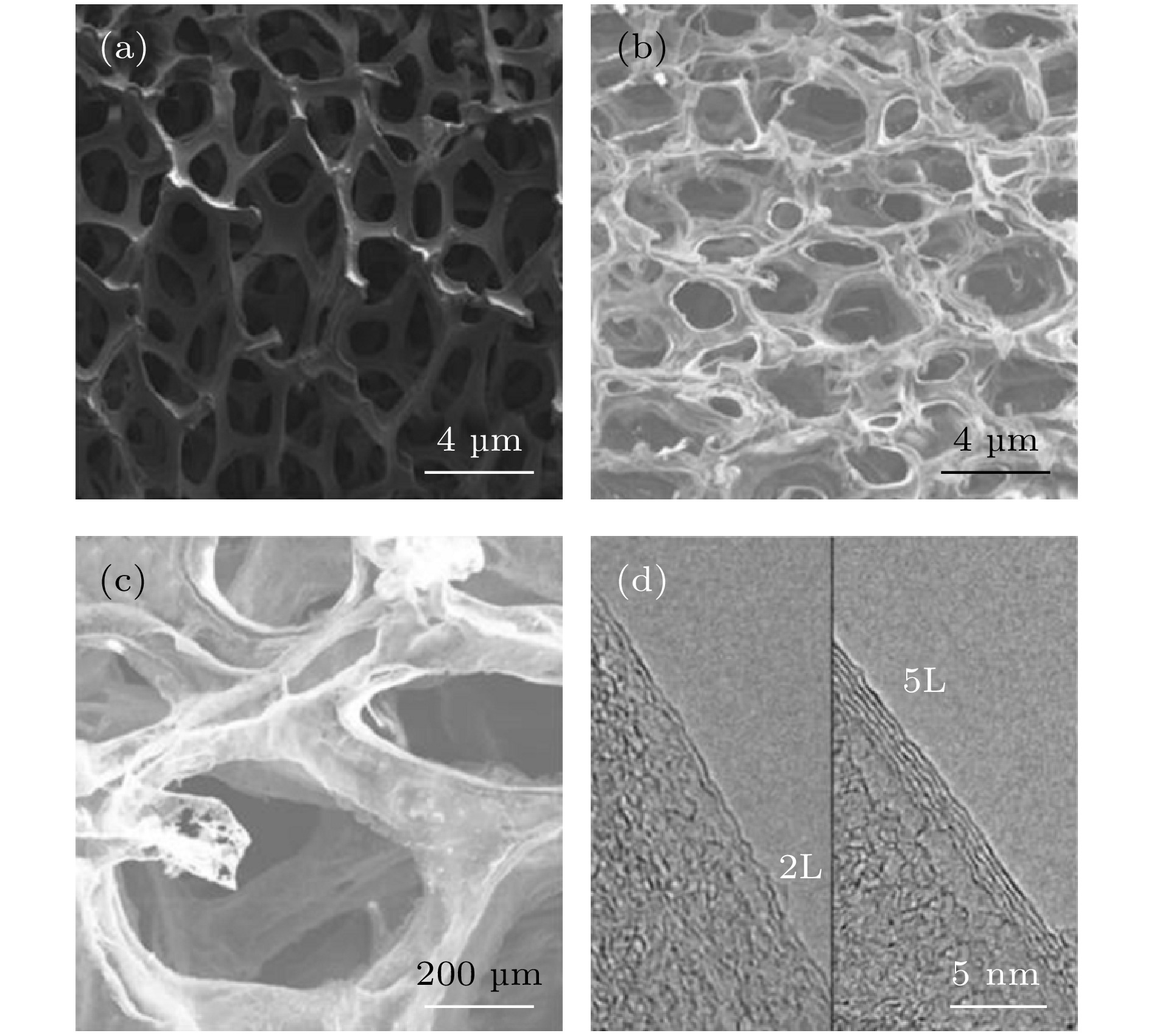
图 7 样品SEM图像 (a)低压封闭CVD方法制备的G/Ni泡沫; (b)低放大倍数的自支撑3D GF; (c)高放大倍数的自支撑3D GF; (d)石墨烯片边缘的高分辨TEM图像, 双层(2 L)和五层(5 L)石墨烯的层间距约为0.33 nm, 样品由500 μL PMMA添加量制备
Fig. 7. SEM images of 3 D GF: (a) G/Ni foam prepared by low-pressure closed CVD method; (b) self-supporting 3D GF with low magnification; (c) self-supporting 3D GF with high magnification; (d) high-resolution TEM image of graphene sheet edge. The interlayer spacing of double-layer (2 L) and five-layer (5 L) graphene is about 0.33 nm, and the samples were prepared with 500 μL of PMMA addition.
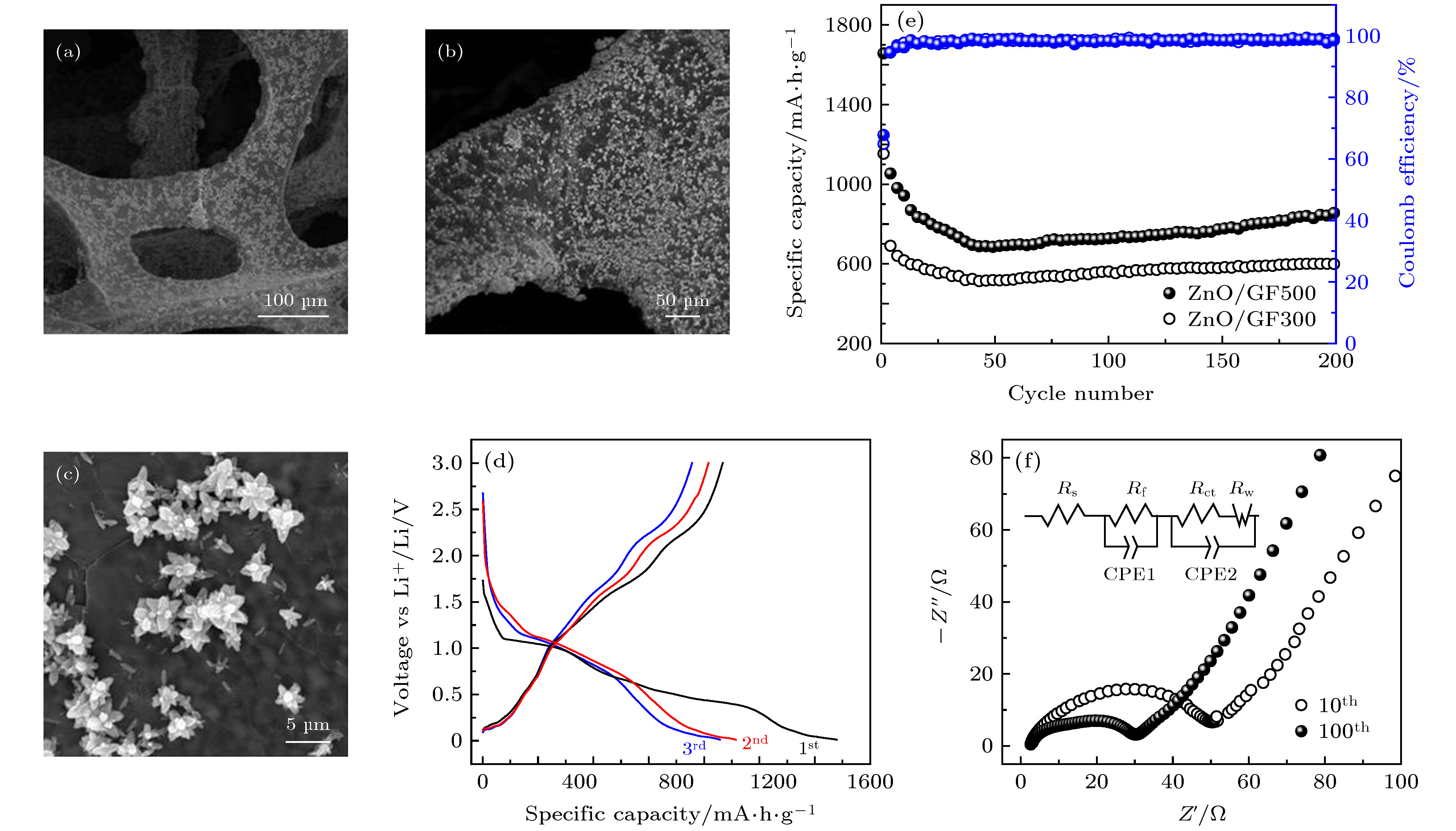
图 8 (a)−(c) 具有不同放大率的ZnO/GF电极SEM图像; (d) ZnO/GF500在电流密度为0.2 A·g–1时的充放电曲线; (e) ZnO/GF300和ZnO/GF500电极在0.2 A·g–1的电流密度下的循环曲线; (f) 在电流密度为0.2 A·g–1下的ZnO/GF500电极第10次和第100次循环后的Nyqusit谱图, 插图为阻抗谱图模拟出的等效电路图
Fig. 8. (a)−(c) FE-SEM images of ZnO/GF electrodes with different magnifications; (d) charge-discharge curves of ZnO/GF500 composite; (e) cycle curves of ZnO/GF300 and ZnO/GF500 at current densities of 0.2 A·g–1; (f) the nyqusit spectrum of ZnO/GF500 electrode after 10th and 100th cycles at a current density of 0.2 A·g–1 (inset is the equivalent circuits of ZnO/GF electrode).
表 1 ZnO/GF500电极基于等效电路图的各参数数值
Table 1. Values for all the parameters of ZnO/GF500 electrode based on the equivalent circuits.
圈数 Rs/Ω Rf/Ω Rct/Ω CPE1/F CPE2/F 10 5.6 15.2 42.3 6.4 × 10–5 6.5 × 10–4 100 4.2 12.4 21.7 1.9 × 10–4 7.8 × 10–4 -
[1] Fang H, Zou W, Yan J, Xing Y L, Zhang S C 2018 ChemElectroChem 5 2458
 Google Scholar
Google Scholar
[2] Peng H J, Huang J Q, Cheng X B, Zhang Q 2017 Adv. Energy Mater. 24 54
[3] Xu Y X, Lin Z Y, Huang X Q, Wang Y, Huang Y, Duan X F 2013 Adv. Mater. 25 5779
 Google Scholar
Google Scholar
[4] Wei S Y, Ma L, Hendrickson K E, Tu Z Y, Archer L A 2015 J. Am. Chem. Soc. 37 12143
[5] Fang H, Meng F T, Chen G Y, Wang L X, Zhang S C, Yan J, Zhang L S, Zhang Y X 2019 Int. J. Electrochem. Sci. 14 7937
[6] Li G R, Lei W, Luo D, Deng Y P 2018 Adv. Energy Mater. 8 1702381
 Google Scholar
Google Scholar
[7] Geim A K, Novoselov K S 2007 Nat. Mater. 3 183
[8] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[9] Guo H, Wang X Y, Bao D L, Lu L L, Zhang Y Y, Li Geng, Wang Y L, Du S X, Gao H J 2019 Chin. Phys. B 28 078103
 Google Scholar
Google Scholar
[10] Novoselov K S, Geim A K, Morozov S V, Jiang D, Katsnelson M L, Grigorieva I V, Dubonos S V, Firsov A A 2005 Nature 7065 197
[11] Li X S, Magnuson C W, Venugopal A, An J H, Suk J W, Han B Y, Borysiak M, Cai W W, Velamakanni A, Zhu Y W, Fu L F, Vogel E M, VoelkL E, Colombo L G, Ruoff R S 2010 Nano Lett. 11 4328
[12] Li X S, Cai W W, Colombo L G, Ruoff R S 2009 Nano Lett. 12 4268
[13] Ito Y, Christodoulou C, Nardi M V, Koch N, Sachdec H, Mullen K 2014 ACS Nano 8 3337
 Google Scholar
Google Scholar
[14] 任文才, 高力波, 马来鹏 2011 新型炭材料 1 71
Ren W C, Gao L B, Ma L P 2011 New Carbon Mater. 1 71
[15] Ci L J, Xu Z P, Wang L L, Gao W, Ding F, Kelly K F, Yakobson B I, Ajayan P M 2008 Nano Res. 1 116
 Google Scholar
Google Scholar
[16] Rodrı′guez-Manzo J A, Pham-Huu C, Banhart F 2011 ACS Nano 2 1529
[17] Ji H X, Hao Y F, Ren Y J, Charlton M, Lee W H, Wu Q Z, Li H F, Zhu Y W, Wu Y P, Piner R, Ruoff R S 2011 ACS Nano 9 7656
[18] Raccichini R, Varzi A, Passerini S, Scrosati B 2015 Nat. Mater. 14 271
 Google Scholar
Google Scholar
[19] Bi H, Chen I W, Lin T 2015 Adv. Mater. 39 5943
[20] Ito Y, Tanabe Y, Qiu H J, Sugawara K, Heguri S, Tu N H, Huynh K K, Fujita T, TakahashivT, Tanigaki K, Chen M 2014 Angew Chem. Int. Ed. 53 4822
 Google Scholar
Google Scholar
[21] Dong X C, Wang X W, Wang L H, Song H, Zhang H, Huang W, Chen P 2012 ACS Appl. Mater. Interfaces 6 3129
[22] Dong X C, Ma Y W, Zhu G Y, Huang Y X, Wang J, Chan-Park M B, Wang L H, Huang W, Chen P 2012 J. Mater. Chem. 22 17044
 Google Scholar
Google Scholar
[23] Li Z C, Wu P, Wang C X, Fan X D, Zhang W H, Zhai X F, Zeng C G, Li Z Y, Yang J L, Hou J G 2011 ACS Nano 5 3385
 Google Scholar
Google Scholar
[24] Xiao X Y, Beechem T E, Brumbach M T, Lambert T N, Davis D J, Michael J R, Washburn C M, Wang J, Brozik S M, Wheeler D R, Burcker D B, Polsky R 2012 ACS Nano 6 3573
 Google Scholar
Google Scholar
[25] Fernandez Merino M J, Guardia L, Paredes J I, Villar-Rodil S, Solis-Fernandez P, Martinez-Alonso A, Tascon J M D 2010 J. Phys. Chem. C 114 6426
[26] Zhang L B, Chen G Y, Hedhili M N, Zhang H N, Wang P 2012 Nanoscale 4 7038
 Google Scholar
Google Scholar
[27] Fang H, Meng F T, Yan J, Chen G Y, Zhang L S, Wu S D, Zhang S C, Wan L Z, Zhang Y X 2019 RSC Adv. 9 20107
 Google Scholar
Google Scholar
[28] Fang H, Chen G Y, Wang L X, Yan J, Zhang L S, Gao K Z, Zhang Y X, Wang L Z 2018 RSC Adv. 8 38550
 Google Scholar
Google Scholar
[29] Wang W X, Yang P H, Jian Z X, Li H L, Xing Y L, Zhang S C 2018 J. Mater. Chem. A 6 13797
 Google Scholar
Google Scholar
[30] Wang W X, Zhang S C, Xing Y L, Wang S B, Ren Y B 2016 RSC Adv. 6 75414
 Google Scholar
Google Scholar
[31] Chen Z P, Ren W C, Gao L B, Liu B L, Pei S F, Cheng H M 2011 Nat. Mater. 10 424
 Google Scholar
Google Scholar
[32] Compton O C, Nguyen S T 2010 Small 6 711
 Google Scholar
Google Scholar
[33] Wu J, Xu H, Zhang J 2014 Acta Chim. Sin. 72 301
 Google Scholar
Google Scholar
[34] Malard L M, Pimenta M A, Dresselhaus G, Dresselhaus M S 2009 Phys. Rep. 473 51
 Google Scholar
Google Scholar
[35] Ferrari A C, Basko D M 2013 Nat. Nanotechnol. 8 235
 Google Scholar
Google Scholar
[36] Chae S J, Güneş F, Kim K K, Kim E S, Han G H, Kim S M, Shin H J, Yoon S M, Chio J Y, Park M H, Yang C W, Pribat D, Lee Y H 2009 Adv. Mater. 21 2328
 Google Scholar
Google Scholar
[37] Zhang Y, Gomez L, Ishikawa F N, Madaria A, Ryu K, Wang C, Badmaev A, Zhou C W 2010 J. Phys. Chem. Lett. 1 3101
 Google Scholar
Google Scholar
[38] Ashkenov N, Mbenkum B N, Bundesmann C, Riede V, Lorenz M, Spemann D, Kaidashev E M, Kasic A, Schubert M, Grundmann M, Wagner G, Neumann H, Darakchieva V, Arwin H, Monemar B 2003 J. Appl. Phys. 93 126
 Google Scholar
Google Scholar
[39] Bundesmann C, Ashkenov N, Schubert M, Spemann D, Butz T, Kaidashev E M, Lorenz M, Grundmann M 2003 App. Phys. Lett. 83 1974
 Google Scholar
Google Scholar
[40] Fang H, Zhang L S, Xing Y L, Zhang S C, Wu S D 2018 Int. J. Electrochem. Sci. 13 8736
[41] Liu J, Zhang Y H, Bai Z M, Huang Z A, Gao Y K 2019 Chin. Phys. B 28 048101
 Google Scholar
Google Scholar
计量
- 文章访问数: 11291
- PDF下载量: 138
- 被引次数: 0













 下载:
下载: