-
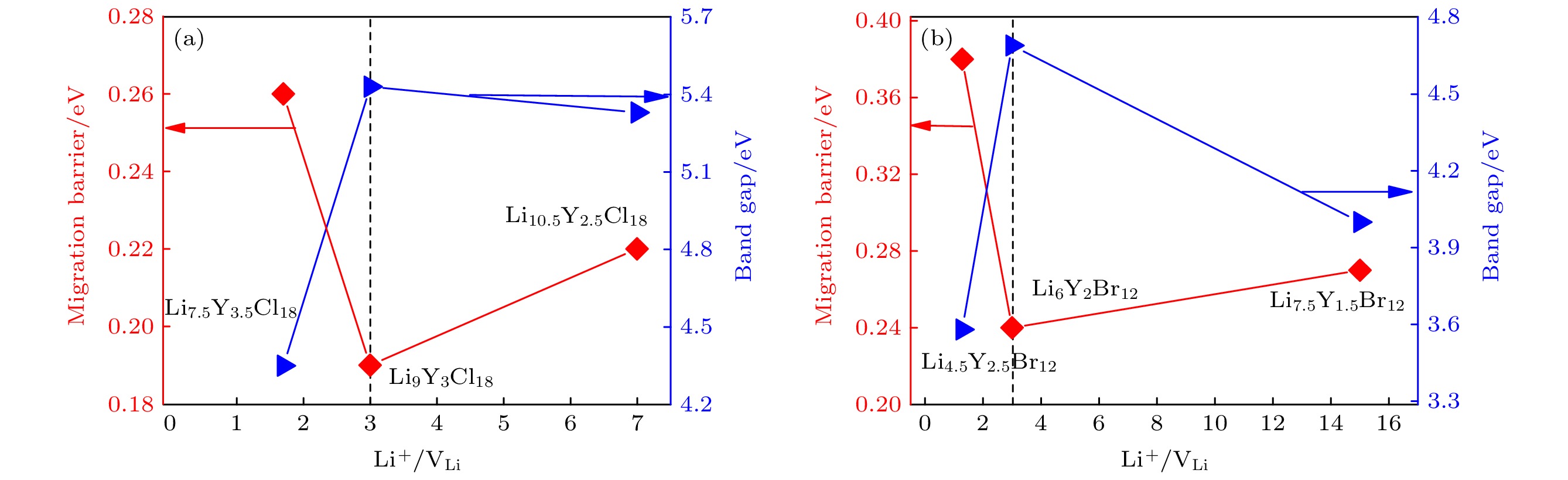
三元锂金属卤化物作为极具潜力的固体电解质材料受到人们的广泛关注. 本文利用基于密度泛函理论的第一性原理方法研究了一系列具有不同Li离子浓度的LixYCl3+x (x = 2.14, 3.00, 4.20)和LixYBr3+x (x = 1.8, 3.0, 5.0)材料的结构、电子性质和迁移特性. 研究结果表明, Li离子和Li空位浓度的变化对材料的性能有显著影响, 而且随着x值的增加, Li离子的含量增加, 相应的Li空位浓度降低, 结构的稳定性增强、带隙增大、离子迁移能垒降低, 从而可以调控该类材料的性能. 此外, 计算结果进一步表明, 在所有考虑的结构中, 具有最佳Li离子与Li空位平衡的Li3YCl6和Li3YBr6组分展现出最高的结构稳定性、最大的带隙和最低的迁移能垒. 本文为设计性能更好的卤化物固态电解质提供了一种新策略和新思路.Ternary lithium metal halides have attracted much attention as potential solid electrolytes. In this work, we study the structural, electronic and ionic diffusion properties of a series of LixYCl3+x (x = 2.14, 3.00, 4.20) and LixYBr3+x (x = 1.8, 3.0, 5.0) by using first-principles calculation based on density functional theory. The calculation results show that the Li-ion concentration has a significant effect on the properties of the materials, and with the increase of x value, Li-ion number becomes higher, structure turns more stable, band gap gets larger, and migration barrier lowers, thus the performance of the material can be tuned. In addition, the calculation results further show that Li3YCl6 and Li3YBr6 with the best balance between Li-ion carrier concentration and vacancy concentration exhibit the highest structural stability, the largest band gaps, and the lowest migration barriers in all similar structures. Our study provides a new strategy and idea for designing better-performance halide solid electrolytes.
-
Keywords:
- all-solid-state lithium-ion batteries /
- ternary halides /
- concentration regulation /
- first-principles calculations
[1] Famprikis T, Canepa P, Dawson J A, Islam M S, Masquelier C 2019 Nat. Mater. 18 1278
 Google Scholar
Google Scholar
[2] Manthiram A, Yu X, Wang S 2017 Nat. Rev. Mater. 2 16103
 Google Scholar
Google Scholar
[3] Kamaya N, Homma K, Yamakawa Y, Hirayama M, Kanno R, Yonemura M, Kamiyama T, Kato Y, Hama S, Kawamoto K, Mitsui A 2011 Nat. Mater. 10 682
 Google Scholar
Google Scholar
[4] Kraft M A, Ohno S, Zinkevich T, Koerver R, Culver S P, Fuchs T, Senyshyn A, Indris S, Morgan B J, Zeier W. G 2018 J. Am. Chem. Soc. 140 16330
 Google Scholar
Google Scholar
[5] Zhou L, Assoud A, Zhang Q, Wu X, Nazar L F 2019 J. Am. Chem. Soc. 141 19002
 Google Scholar
Google Scholar
[6] Zhu Y Z, He X F, Mo Y F, 2015 ACS Appl. Mater. Interfaces 7 23685
 Google Scholar
Google Scholar
[7] Nolan A M, Zhu Y Z, He X F, Bai Q, Mo Y F 2018 Joule 2 2016
 Google Scholar
Google Scholar
[8] Li X, Liang J W, Yang X F, Adair K R, Wang C H, Zhao F P, Sun X L 2020 Energy Environ. Sci. 13 1429
 Google Scholar
Google Scholar
[9] Shannon R D, 1976 Acta Crystallogr. Sect. A:Found. Adv. 32 751
 Google Scholar
Google Scholar
[10] Pauling L 1960 The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry (New York: Cornell University Press) pp505–562
[11] Lutz H D, Schmidt W, Haeuseler H 1981 J. Phys. Chem. Solids 42 287
 Google Scholar
Google Scholar
[12] Lutz H D, Kuske P, Wussow K 1988 Solid State Ionics 28 1282
 Google Scholar
Google Scholar
[13] Steiner H J, Lutz H D 1992 Z. Anorg. Allg. Chem. 613 26
 Google Scholar
Google Scholar
[14] Asano T, Sakai A, Ouchi S, Sakaida M, Miyazaki A, Hasegawa S 2018 Adv. Mater. 30 1803075
 Google Scholar
Google Scholar
[15] Li X N, Liang J W, Luo J, et al. 2019 Energy Environ. Sci. 12 2665
 Google Scholar
Google Scholar
[16] Li X N, Liang J W, Chen N, et al. 2019 Angew. Chem. 131 16579
 Google Scholar
Google Scholar
[17] Wang S, Bai Q, Nolan A M, Liu Y S, Gong S, Sun Q, Mo Y F 2019 Angew. Chem. Int. Ed. 58 8039
 Google Scholar
Google Scholar
[18] Wang Y, Richards W D, Ong S P, Miara L J, Kim J C, Mo Y F, Ceder G 2015 Nat. Mater. 14 1026
 Google Scholar
Google Scholar
[19] Schlem R, Bernges T, Li C, Kraft M A, Minafra N, Zeier W G 2020 ACS Appl. Energy Mater. 3 3684
 Google Scholar
Google Scholar
[20] Wang K, Ren Q Y, Gu Z Q, Duan C M, Wang J Z, Zhu F, Fu Y Y, Hao J P, Zhu J F, He L H, Wang C W, Lu Y Y, Ma J, Ma C 2021 Nat. Commun. 12 4410
 Google Scholar
Google Scholar
[21] Liang J W, Li X N, Wang S, et al. 2020 J. Am. Chem. Soc. 142 7012
 Google Scholar
Google Scholar
[22] Schlem R, Muy S, Prinz N, Banik A, Shao-Horn Y, Zobel M, Zeier W G 2020 Adv. Energy Mater. 10 1903719
 Google Scholar
Google Scholar
[23] Liu Z T, Ma S, Liu J, Xiong S, Ma Y F, Chen H L 2021 ACS Energy Lett. 6 298
 Google Scholar
Google Scholar
[24] Li X N, Liang J W, Adair K R, et al. 2020 Nano Lett. 20 4384
 Google Scholar
Google Scholar
[25] He B, Chi S T, Ye A J, Mi P H, Zhang L W, Pu B W, Zou Z Y, Ran Y B, Zhao Q, Wang D, Zhang W Q, Zhao J T, Adams S, Avdeev M, Shi S Q 2020 Sci. Data 7 151
 Google Scholar
Google Scholar
[26] 钟淑琳, 仇家豪, 罗文葳, 吴木生 2021 70 158203
 Google Scholar
Google Scholar
Zhong S L, Qiu J H, Luo W W, Wu M S 2021 Acta Phys. Sin. 70 158203
 Google Scholar
Google Scholar
[27] Shi S Q, Gao J, Liu Y, Zhao Y, Wu Q, Ju W W, Ouyang C Y, Xiao R J 2016 Chin. Phys. B 25 018212
 Google Scholar
Google Scholar
[28] Kresse G, Furthmüeller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[29] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[30] Perdew J P, Ernzerhof M, Burke K 1996 J. Chem. Phys. 105 9982
 Google Scholar
Google Scholar
[31] Dudarev S L, Botton G A, Savrasov S Y, Humphreys C J, Sutton A P 1998 Phys. Rev. B 57 1505
 Google Scholar
Google Scholar
[32] Stevanović V, Lany S, Zhang X W, Zunger A 2012 Phys. Rev. B 85 115104
 Google Scholar
Google Scholar
[33] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[34] Henkelman G, Uberuaga B P, Jónsson H 2000 J. Chem. Phys. 113 9901
 Google Scholar
Google Scholar
[35] 郑路敏, 钟淑英, 徐波, 欧阳楚英 2019 68 138201
 Google Scholar
Google Scholar
Zheng L M, Zhong S Y, Xu B, Ouyang C Y 2019 Acta Phys. Sin. 68 138201
 Google Scholar
Google Scholar
[36] Ouyang C Y, Shi S Q, Wang Z X, Huang X J, Chen L Q 2004 Phys. Rev. B 69 104303
 Google Scholar
Google Scholar
[37] Wu M S, Xu B, Lei X L, Huang K L, Ouyang C Y 2018 J. Mater. Chem. A 6 1150
 Google Scholar
Google Scholar
[38] Wang Y C, Lü J, Zhu L, Ma Y M 2010 Phys. Rev. B 82 094116
 Google Scholar
Google Scholar
[39] Wang Y C, Lü J, Zhu L, Ma Y M 2012 Comput. Phys. Commun. 183 2063
 Google Scholar
Google Scholar
[40] Hongzhiwei Technology 2021 Device Studio (Version 2021A) China
[41] Sun W H, Dacek S T, Ong S P, Hautier G, Jain A, Richards W D, Gamst A C, Persson K A, Ceder G 2016 Sci. Adv. 2 e1600225
 Google Scholar
Google Scholar
[42] Bartel C J, Millican S L, Deml A M, Rumptz J R, Tumas W, Weimer A W, Lany S, Stevanović V, Musgrave C B, Holder A M 2018 Nat. Commun. 9 4168
 Google Scholar
Google Scholar
[43] Aykol M, Dwaraknath S S, Sun W H, Persson K A 2018 Sci. Adv. 4 eaaq0148
 Google Scholar
Google Scholar
[44] Zhao Q, Pan L, Li Y J, Chen L Q, Shi S Q 2018 Rare Met. 37 497
 Google Scholar
Google Scholar
[45] 任元, 邹喆乂, 赵倩, 王达, 喻嘉, 施思齐 2020 69 226601
 Google Scholar
Google Scholar
Ren Y, Zou Z Y, Zhao Q, Wang D, Yu J, Shi S Q 2020 Acta Phys. Sin. 69 226601
 Google Scholar
Google Scholar
[46] Qiu J H, Wu M S, Luo W W, Xu B, Liu G, Ouyang C Y, 2021 J. Phys. Chem. C 125 23510
 Google Scholar
Google Scholar
[47] Pan L, Zhang L W, Ye A J, Chi S T, Zou Z Y, He B, Chen L L, Zhao Q, Wang D, Shi S Q 2019 J. Materiomics 5 688
 Google Scholar
Google Scholar
[48] He B, Mi P H, Ye A J, Chi S T, Jiao Y, Zhang L W, Pu B W, Zou Z Y, Zhang W Q, Avdeev M, Adams S, Zhao J T, Shi S Q 2021 Acta Mater. 203 116490
 Google Scholar
Google Scholar
[49] Momma K, Izumi F 2011 J. Appl. Crystallogr. 44 1272
 Google Scholar
Google Scholar
[50] Chen H, Adams S 2017 IUCrJ 4 614
 Google Scholar
Google Scholar
-
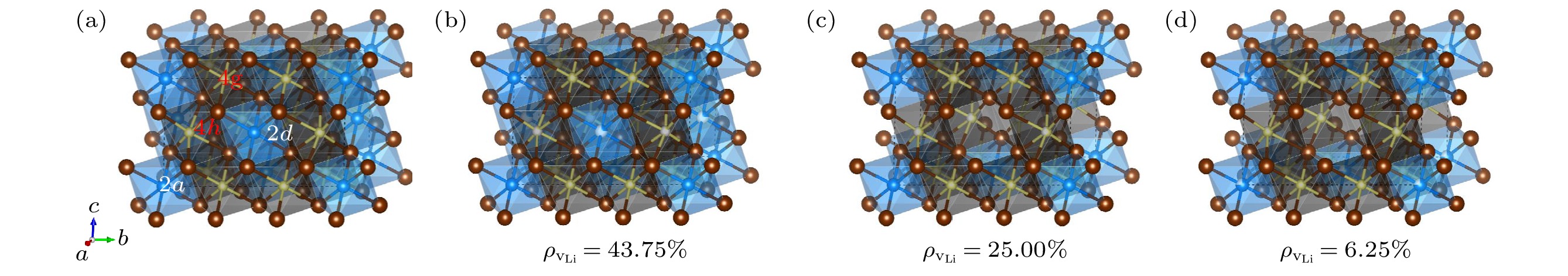
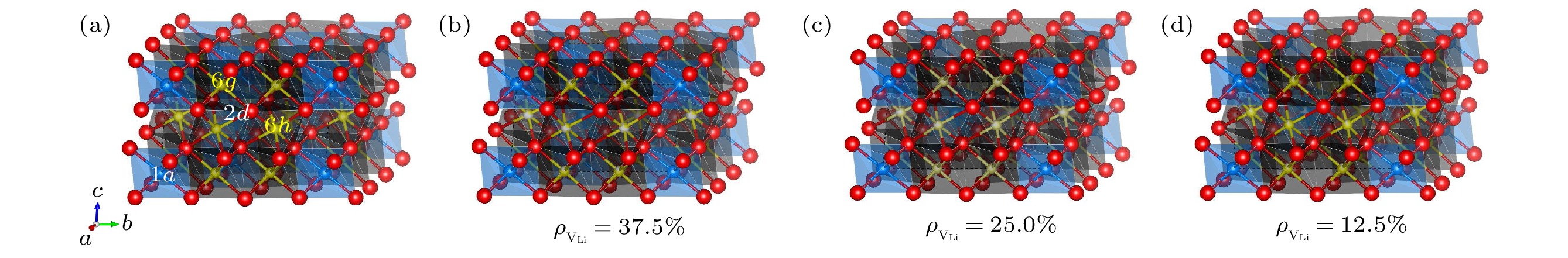
图 1 原子结构示意图 (a) Li12Y5Cl18; (b) Li7.5Y3.5Cl18; (c) Li9Y3Cl18; (d) Li10.5Y2.5Cl18 (黄色、蓝色和红色圆球分别代表Li, Y和Cl原子, 有部分白色的小球代表对应原子在该位置的部分占据)
Fig. 1. Schematic diagram of atomic structures: (a) Li12Y5Cl18; (b) Li7.5Y3.5Cl18; (c) Li9Y3Cl18; (d) Li10.5Y2.5Cl18 (The yellow, blue and red spheres represent Li, Y and Cl atoms, the spheres with a partially white indicate the partial occupancy of the corresponding atoms).
图 2 原子结构示意图 (a) Li8Y4Br12; (b) Li4.5Y2.5Br12; (c) Li6Y2Br12; (d) Li7.5Y1.5Br12 (黄色、蓝色和咖啡色圆球分别代表Li, Y和Br原子, 有部分白色的小球代表对应原子在该位置的部分占据)
Fig. 2. Schematic diagram of atomic structures: (a) Li8Y4Br12; (b) Li4.5Y2.5Br12; (c) Li6Y2Br12; (d) Li7.5Y1.5Br12 (The yellow, blue and brown spheres represent Li, Y and Br atoms, the spheres with a partially white indicate the partial occupancy of the corresponding atoms).
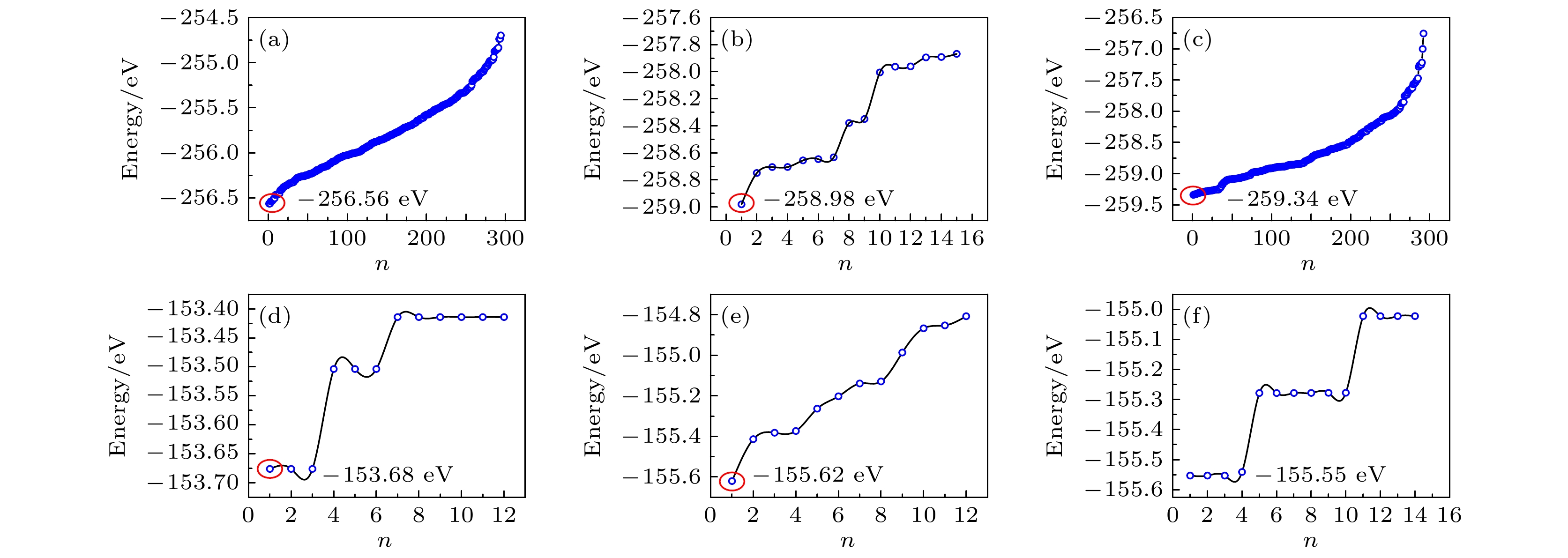
图 3 超胞中所有可能构型n的总能. 1×1×2超胞 (a) Li7.5Y3.5Cl18; (b) Li9Y3Cl18; (c) Li10.5Y2.5Cl18. 2×1×1超胞 (d) Li4.5Y2.5Br12; (e) Li6Y2Br12; (f) Li7.5Y1.5Br12
Fig. 3. The total energies of all possible configurations n. 1×1×2 supercell: (a) Li7.5Y3.5Cl18; (b) Li9Y3Cl18; (c) Li10.5Y2.5Cl18. 2×1×1 supercell: (d) Li4.5Y2.5Br12; (e) Li6Y2Br12; (f) Li7.5Y1.5Br12.
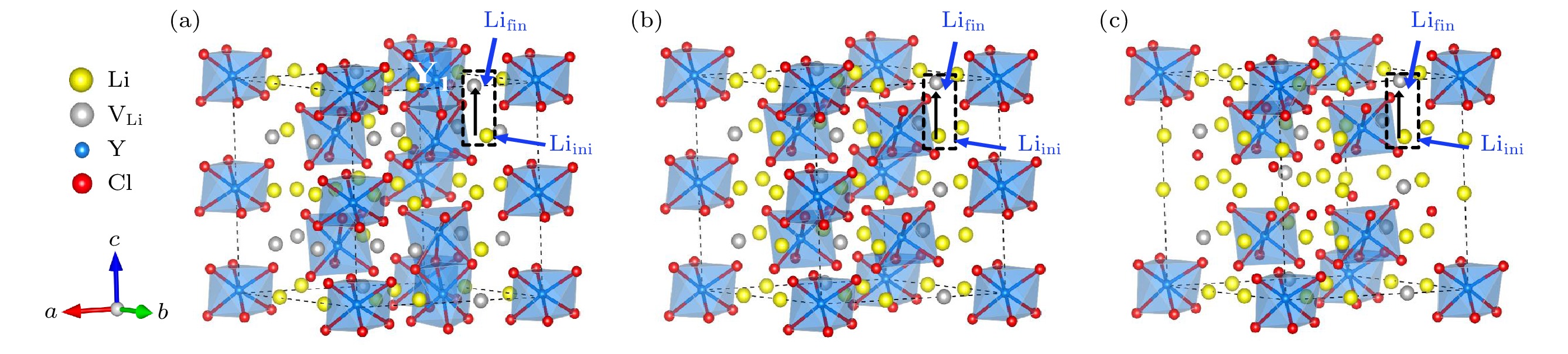
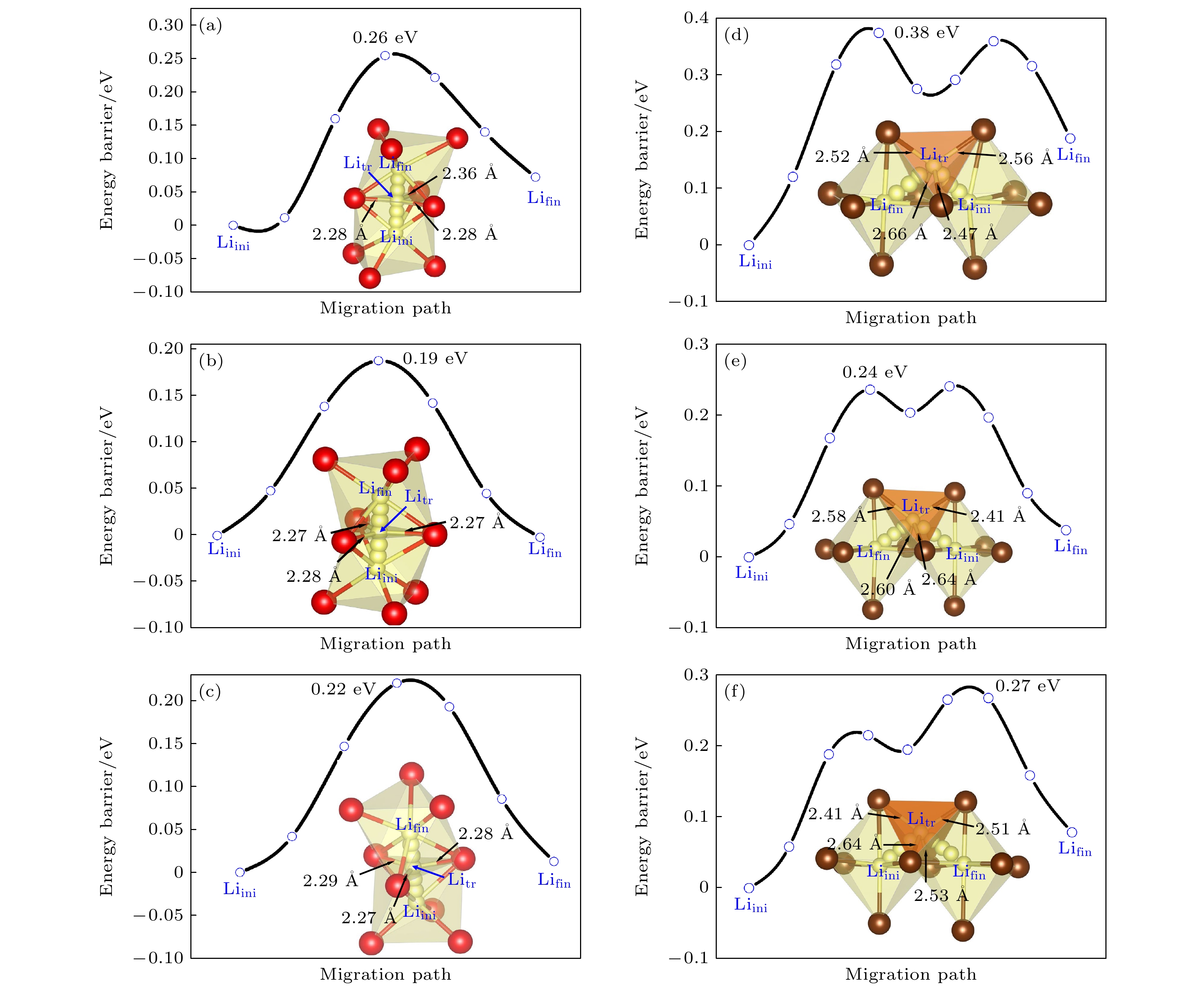
图 4 DFT计算的不同构型的基态结构 (灰色小球代表Li空位, 黑色箭头代表虚线框中的Li离子向Li空位迁移的迁移路径) (a) Li15Y7Cl36; (b) Li18Y6Cl36; (c) Li21Y5Cl36
Fig. 4. Ground-state structures of different configurations calculated by DFT (The grey spheres represent Li vacancies, black arrows represent the migration paths of Li ions in the dashed box to Li vacancies): (a) Li15Y7Cl36; (b) Li18Y6Cl36; (c) Li21Y5Cl36.
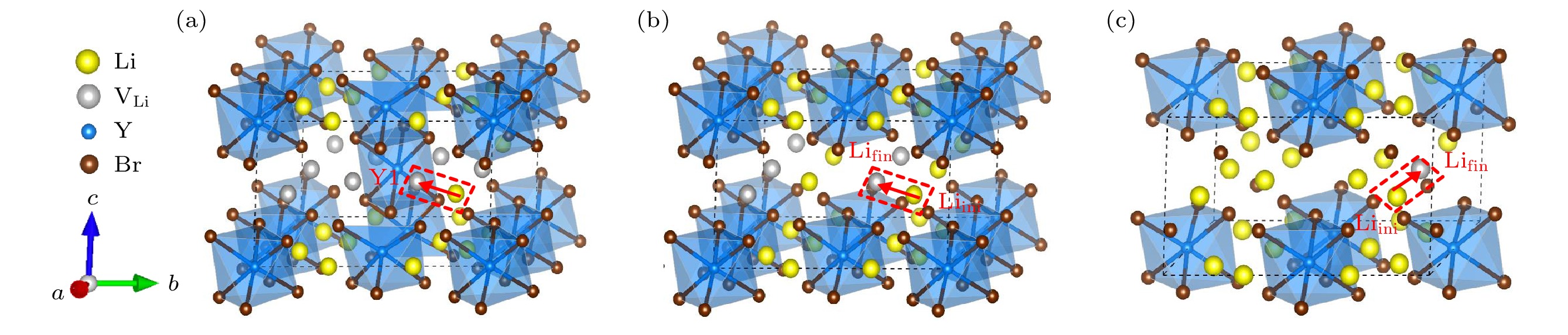
图 5 DFT计算的不同构型的基态结构 (红色箭头代表虚线框中的Li离子向Li空位迁移的迁移路径) (a) Li9Y5Br24; (b) Li12Y4Br24; (c) Li15Y3Br24
Fig. 5. Ground-state structures of different configurations calculated by DFT (Red arrows represent the migration paths of Li ions in the dashed box to Li vacancies): (a) Li9Y5Br24; (b) Li12Y4Br24; (c) Li15Y3Br24.
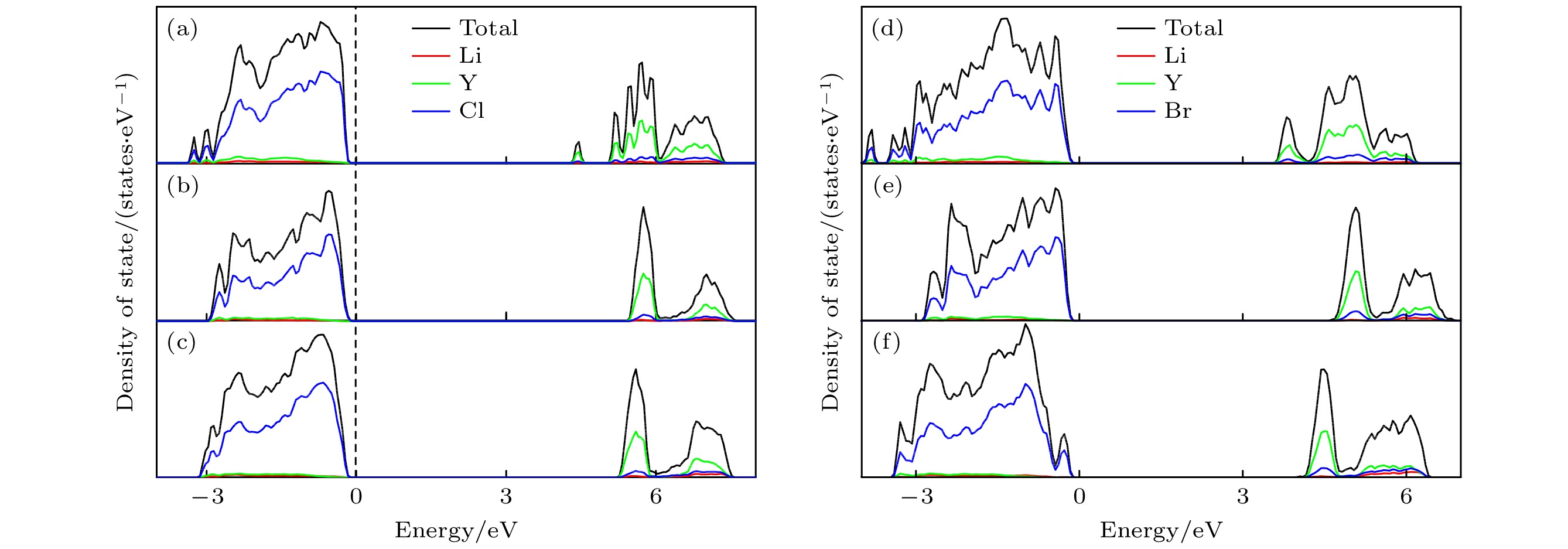
图 6 所有组分的DOS图及带隙 (a) Li15Y7Cl36 (4.35 eV); (b) Li18Y6Cl36 (5.43 eV); (c) Li21Y5Cl36 (5.33 eV); (d) Li9Y5Br24 (3.58 eV); (e) Li12Y4Br24 (4.69 eV); (f) Li15Y3Br24 (4.00 eV)
Fig. 6. DOS plots and band gap results for all components: (a) Li15Y7Cl36 (4.35 eV); (b) Li18Y6Cl36 (5.43 eV); (c) Li21Y5Cl36 (5.33 eV); (d) Li9Y5Br24 (3.58 eV); (e) Li12Y4Br24 (4.69 eV); (f) Li15Y3Br24 (4.00 eV).
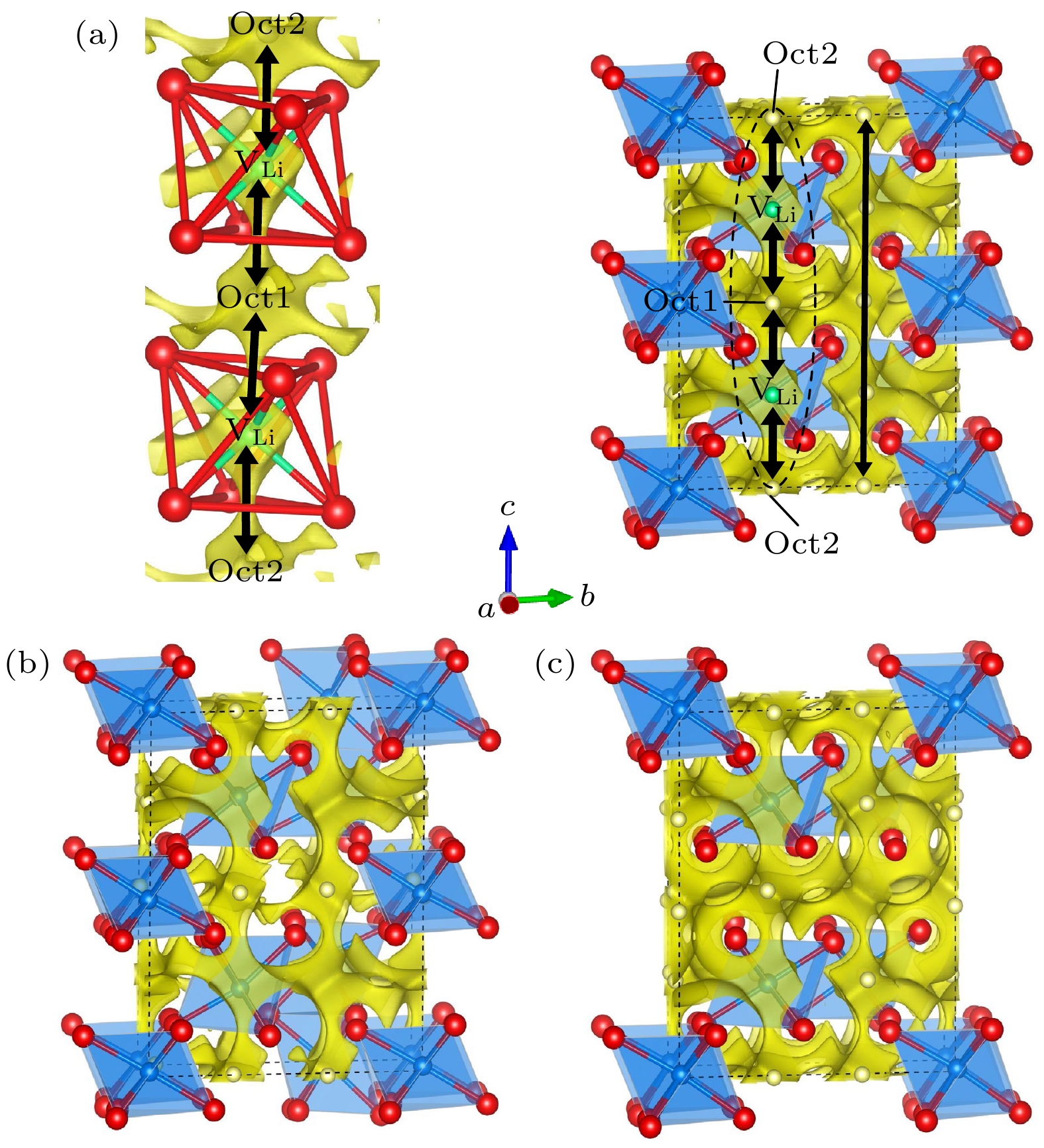
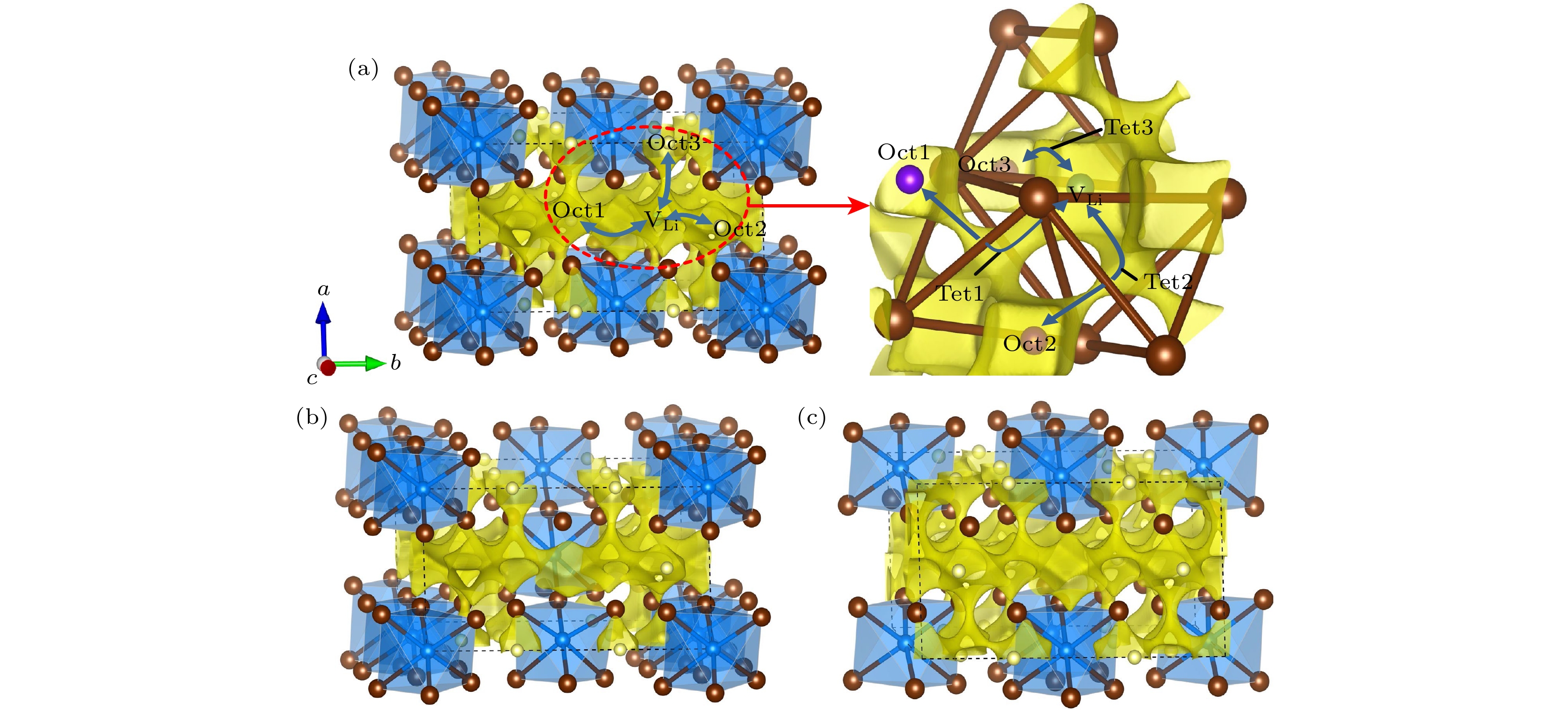
图 7 (a) Li18Y6Cl36, (b) Li15Y7Cl36, (c) Li21Y5Cl36的晶体结构与BVSE计算的Li离子等位面势图的叠加图(黄色区域是Li离子的分布密度, 代表Li离子的传导通道)
Fig. 7. Crystal structures of (a) Li18Y6Cl36, (b) Li15Y7Cl36, (c) Li21Y5Cl36 superimposed with the Li-ion potential map calculated using BVSE (The yellow isosurfaces correspond to Li-ions probability density, indicating Li-ion conduction path).
图 8 (a) Li12Y4Br24, (b) Li9Y5Br24, (c) Li15Y3Br24的晶体结构与BVSE计算的Li离子等位面势图的叠加图(黄色区域是Li离子的分布密度, 代表Li离子的传导通道)
Fig. 8. Crystal structures of (a) Li12Y4Br24, (b) Li9Y5Br24, (c) Li15Y3Br24 superimposed with the Li-ion potential map calculated using BVSE (The yellow isosurfaces correspond to Li-ions probability density, indicating Li-ion conduction path).
表 1 LixYCl3+x和LixYBr3+x卤化物的形成能
Table 1. Formation energies of LixYCl3+x and LixYBr3+x halides.
化学式 空间群 晶格常数/Å 反应方程式 Ef/(meV·atom–1) Li15Y7Cl36 $p\bar{3}m1$ a = 11.22
c = 12.3415LiCl+7YCl3→Li15Y7Cl36 32 Li18Y6Cl36 18LiCl+6YCl3→Li18Y6Cl36 13 Li21Y5Cl36 21LiCl+5YCl3→Li21Y5Cl36 19 Li9Y5Br24 C2/m a = 13.93
b = 12.02
c = 6.959LiBr+5YBr3→Li9Y5Br24 17 Li12Y4Br24 12LiBr+4YBr3→Li12Y4Br24 2 Li15Y3Br24 15LiBr+3YBr3→Li15Y3Br24 30 -
[1] Famprikis T, Canepa P, Dawson J A, Islam M S, Masquelier C 2019 Nat. Mater. 18 1278
 Google Scholar
Google Scholar
[2] Manthiram A, Yu X, Wang S 2017 Nat. Rev. Mater. 2 16103
 Google Scholar
Google Scholar
[3] Kamaya N, Homma K, Yamakawa Y, Hirayama M, Kanno R, Yonemura M, Kamiyama T, Kato Y, Hama S, Kawamoto K, Mitsui A 2011 Nat. Mater. 10 682
 Google Scholar
Google Scholar
[4] Kraft M A, Ohno S, Zinkevich T, Koerver R, Culver S P, Fuchs T, Senyshyn A, Indris S, Morgan B J, Zeier W. G 2018 J. Am. Chem. Soc. 140 16330
 Google Scholar
Google Scholar
[5] Zhou L, Assoud A, Zhang Q, Wu X, Nazar L F 2019 J. Am. Chem. Soc. 141 19002
 Google Scholar
Google Scholar
[6] Zhu Y Z, He X F, Mo Y F, 2015 ACS Appl. Mater. Interfaces 7 23685
 Google Scholar
Google Scholar
[7] Nolan A M, Zhu Y Z, He X F, Bai Q, Mo Y F 2018 Joule 2 2016
 Google Scholar
Google Scholar
[8] Li X, Liang J W, Yang X F, Adair K R, Wang C H, Zhao F P, Sun X L 2020 Energy Environ. Sci. 13 1429
 Google Scholar
Google Scholar
[9] Shannon R D, 1976 Acta Crystallogr. Sect. A:Found. Adv. 32 751
 Google Scholar
Google Scholar
[10] Pauling L 1960 The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry (New York: Cornell University Press) pp505–562
[11] Lutz H D, Schmidt W, Haeuseler H 1981 J. Phys. Chem. Solids 42 287
 Google Scholar
Google Scholar
[12] Lutz H D, Kuske P, Wussow K 1988 Solid State Ionics 28 1282
 Google Scholar
Google Scholar
[13] Steiner H J, Lutz H D 1992 Z. Anorg. Allg. Chem. 613 26
 Google Scholar
Google Scholar
[14] Asano T, Sakai A, Ouchi S, Sakaida M, Miyazaki A, Hasegawa S 2018 Adv. Mater. 30 1803075
 Google Scholar
Google Scholar
[15] Li X N, Liang J W, Luo J, et al. 2019 Energy Environ. Sci. 12 2665
 Google Scholar
Google Scholar
[16] Li X N, Liang J W, Chen N, et al. 2019 Angew. Chem. 131 16579
 Google Scholar
Google Scholar
[17] Wang S, Bai Q, Nolan A M, Liu Y S, Gong S, Sun Q, Mo Y F 2019 Angew. Chem. Int. Ed. 58 8039
 Google Scholar
Google Scholar
[18] Wang Y, Richards W D, Ong S P, Miara L J, Kim J C, Mo Y F, Ceder G 2015 Nat. Mater. 14 1026
 Google Scholar
Google Scholar
[19] Schlem R, Bernges T, Li C, Kraft M A, Minafra N, Zeier W G 2020 ACS Appl. Energy Mater. 3 3684
 Google Scholar
Google Scholar
[20] Wang K, Ren Q Y, Gu Z Q, Duan C M, Wang J Z, Zhu F, Fu Y Y, Hao J P, Zhu J F, He L H, Wang C W, Lu Y Y, Ma J, Ma C 2021 Nat. Commun. 12 4410
 Google Scholar
Google Scholar
[21] Liang J W, Li X N, Wang S, et al. 2020 J. Am. Chem. Soc. 142 7012
 Google Scholar
Google Scholar
[22] Schlem R, Muy S, Prinz N, Banik A, Shao-Horn Y, Zobel M, Zeier W G 2020 Adv. Energy Mater. 10 1903719
 Google Scholar
Google Scholar
[23] Liu Z T, Ma S, Liu J, Xiong S, Ma Y F, Chen H L 2021 ACS Energy Lett. 6 298
 Google Scholar
Google Scholar
[24] Li X N, Liang J W, Adair K R, et al. 2020 Nano Lett. 20 4384
 Google Scholar
Google Scholar
[25] He B, Chi S T, Ye A J, Mi P H, Zhang L W, Pu B W, Zou Z Y, Ran Y B, Zhao Q, Wang D, Zhang W Q, Zhao J T, Adams S, Avdeev M, Shi S Q 2020 Sci. Data 7 151
 Google Scholar
Google Scholar
[26] 钟淑琳, 仇家豪, 罗文葳, 吴木生 2021 70 158203
 Google Scholar
Google Scholar
Zhong S L, Qiu J H, Luo W W, Wu M S 2021 Acta Phys. Sin. 70 158203
 Google Scholar
Google Scholar
[27] Shi S Q, Gao J, Liu Y, Zhao Y, Wu Q, Ju W W, Ouyang C Y, Xiao R J 2016 Chin. Phys. B 25 018212
 Google Scholar
Google Scholar
[28] Kresse G, Furthmüeller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[29] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[30] Perdew J P, Ernzerhof M, Burke K 1996 J. Chem. Phys. 105 9982
 Google Scholar
Google Scholar
[31] Dudarev S L, Botton G A, Savrasov S Y, Humphreys C J, Sutton A P 1998 Phys. Rev. B 57 1505
 Google Scholar
Google Scholar
[32] Stevanović V, Lany S, Zhang X W, Zunger A 2012 Phys. Rev. B 85 115104
 Google Scholar
Google Scholar
[33] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[34] Henkelman G, Uberuaga B P, Jónsson H 2000 J. Chem. Phys. 113 9901
 Google Scholar
Google Scholar
[35] 郑路敏, 钟淑英, 徐波, 欧阳楚英 2019 68 138201
 Google Scholar
Google Scholar
Zheng L M, Zhong S Y, Xu B, Ouyang C Y 2019 Acta Phys. Sin. 68 138201
 Google Scholar
Google Scholar
[36] Ouyang C Y, Shi S Q, Wang Z X, Huang X J, Chen L Q 2004 Phys. Rev. B 69 104303
 Google Scholar
Google Scholar
[37] Wu M S, Xu B, Lei X L, Huang K L, Ouyang C Y 2018 J. Mater. Chem. A 6 1150
 Google Scholar
Google Scholar
[38] Wang Y C, Lü J, Zhu L, Ma Y M 2010 Phys. Rev. B 82 094116
 Google Scholar
Google Scholar
[39] Wang Y C, Lü J, Zhu L, Ma Y M 2012 Comput. Phys. Commun. 183 2063
 Google Scholar
Google Scholar
[40] Hongzhiwei Technology 2021 Device Studio (Version 2021A) China
[41] Sun W H, Dacek S T, Ong S P, Hautier G, Jain A, Richards W D, Gamst A C, Persson K A, Ceder G 2016 Sci. Adv. 2 e1600225
 Google Scholar
Google Scholar
[42] Bartel C J, Millican S L, Deml A M, Rumptz J R, Tumas W, Weimer A W, Lany S, Stevanović V, Musgrave C B, Holder A M 2018 Nat. Commun. 9 4168
 Google Scholar
Google Scholar
[43] Aykol M, Dwaraknath S S, Sun W H, Persson K A 2018 Sci. Adv. 4 eaaq0148
 Google Scholar
Google Scholar
[44] Zhao Q, Pan L, Li Y J, Chen L Q, Shi S Q 2018 Rare Met. 37 497
 Google Scholar
Google Scholar
[45] 任元, 邹喆乂, 赵倩, 王达, 喻嘉, 施思齐 2020 69 226601
 Google Scholar
Google Scholar
Ren Y, Zou Z Y, Zhao Q, Wang D, Yu J, Shi S Q 2020 Acta Phys. Sin. 69 226601
 Google Scholar
Google Scholar
[46] Qiu J H, Wu M S, Luo W W, Xu B, Liu G, Ouyang C Y, 2021 J. Phys. Chem. C 125 23510
 Google Scholar
Google Scholar
[47] Pan L, Zhang L W, Ye A J, Chi S T, Zou Z Y, He B, Chen L L, Zhao Q, Wang D, Shi S Q 2019 J. Materiomics 5 688
 Google Scholar
Google Scholar
[48] He B, Mi P H, Ye A J, Chi S T, Jiao Y, Zhang L W, Pu B W, Zou Z Y, Zhang W Q, Avdeev M, Adams S, Zhao J T, Shi S Q 2021 Acta Mater. 203 116490
 Google Scholar
Google Scholar
[49] Momma K, Izumi F 2011 J. Appl. Crystallogr. 44 1272
 Google Scholar
Google Scholar
[50] Chen H, Adams S 2017 IUCrJ 4 614
 Google Scholar
Google Scholar
计量
- 文章访问数: 9313
- PDF下载量: 197
- 被引次数: 0













 下载:
下载: