-
SnTe相比于PbTe在热电领域更具有应用潜力, 原因是可以减少Pb对环境的毒性作用. 但SnTe化合物带隙宽度小, 本征Sn空位浓度(nv(Sn))大, 因此, 本征 SnTe具有太大的载流子浓度(~1021 cm–3). 改善SnTe材料的热电性能有多种方法, 但本次工作以熵工程技术为指导分步设计材料成分. 第一步, 与5%的GeTe形成Sn0.95Ge0.05Te合金以增大外加元素或化合物的固溶度; 然后, Sn0.95Ge0.05Te与5% n-型Ag2Se固溶以进一步降低SnTe的p-型载流子浓度; 第三步, 采用Bi/Sn等摩尔置换形成(Sn0.95-xGe0.05BixTe)0.95(Ag2Se)0.05 (x = 0—0.1)合金, 以进一步增大固溶体的构型熵(ΔS ). 经过多组元固溶后, 共增加构型熵ΔS = 5.67 J·mol–1·K–1(x = 0.075), 从而大大降低了载流子浓度和热导率, 使得最大热电优值(ZT )从本征SnTe的约0.22提高到约0.80 (x = 0.075). 证实了熵工程技术是一种能改善SnTe化合物热电性能的有效机制. 但实验结果也说明, 虽然熵增对材料的热电输运机制有较大影响, 但熵增机制需要与其他机制协调才能大幅提高材料的热电优值.SnTe is a good alternative to PbTe in the thermoelectric (TE) applications, in that it is a compound with no toxic element Pb. Besides, the compound SnTe has a relatively narrow bandgap (0.3–0.4 eV) and high Sn vacancy concentration (Snv) as well. Accordingly, it gives a high carrier concentration (1021 cm–3) at room temperature (RT), which is not favorable for thermoelectrics, therefore the regulation of both the electronic and phonon scattering mechanisms is strongly required. Up to date, there have been many approaches to improving its TE performance. The typical examples are those involving the valence band convergence, nanostructuring, substitutional and interstitial defects, and lattice softening, which are all practical and effective to improve the TE performance of SnTe. However, in this work the entropy is taken as an indicator to design the SnTe-based TE material with multicomponents and then optimize its TE performance. The detailed scheme involves the chemical composition design step by step. At first, SnTe alloys with 5% GaTe to form a solid solution Sn0.95Ge0.05Te, aiming to increase the solubility of the foreign species. The second step is to form another solid solution (Sn0.95Ge0.05Te)0.95(Ag2Se)0.05 via the alloying Sn0.95Ge0.05Te with 5% Ag2Se. The purpose of this step is to reduce the p-type carrier concentration of the system, for the species Ag2Se is a typical n-type semiconductor. The last step is to form a series of solid solutions (Sn0.95–xGe0.05BixTe)0.95(Ag2Se)0.05 by substituting different amounts of Bi on Sn in (Sn0.95Ge0.05Te)0.95(Ag2Se)0.05, to further enhance the configurational entropy (ΔS). Because of the above approaches, both the carrier concentration and thermal conductivity decrease while the highest TE figure of merit (ZT) increases from 0.22 for the pristine SnTe to ~0.8 for the alloy (Sn0.95–xGe0.05BixTe)0.95(Ag2Se)0.05 (x = 0.075). This result proves that the entropy engineering is a practical way to improve the TE performance of SnTe, and at the same time it illustrates that it is very important to harmonize the entropy engineering with other electronic and phonon scattering mechanisms, in order to improve the TE performance of SnTe effectively.
-
Keywords:
- thermoelectric performance /
- entropy engineering /
- SnTe /
- thermal conductivity /
- electronic property /
- carrier concentration
[1] Zhang Q, Liao B, Lan Y C, Lukas K, Liu W, Esfarjani K, Opeil C, Broido D, Chen G, Ren Z 2013 PNAS 110 13261
 Google Scholar
Google Scholar
[2] Rogers L 1968 J. Phys. D:Appl. Phys. 1 845
 Google Scholar
Google Scholar
[3] Brebrick R 1963 J. Phys. Chem. Solids 24 27
 Google Scholar
Google Scholar
[4] Li M, Ying P, Du Z, Liu X, Li X, Fang T, Cui J 2022 ACS Appl. Mater. Interfaces 14 8171
 Google Scholar
Google Scholar
[5] Banik A, Shenoy U S, Saha S, Waghmare U V, Biswas K 2016 J. Am. Chem. Soc. 138 13068
 Google Scholar
Google Scholar
[6] Tan G, Shi F, Hao S, Chi H, Bailey T P, Zhao L, Uher C, Wolverton C, Dravid V P, Kanatzidis M G 2015 J. Am. Chem. Soc. 137 11507
 Google Scholar
Google Scholar
[7] Zhou M, Gibbs Z M, Wang H, Han Y, Xin C, Li L 2014 Phys. Chem. Chem. Phys. 16 20741
 Google Scholar
Google Scholar
[8] Pei Y, Shi X, LaLonde A, Wang H, Chen L, Snyder G J 2011 Nature 473 66
 Google Scholar
Google Scholar
[9] Pei Y, Wang H, Snyder G J 2012 Adv. Mater. 24 6125
 Google Scholar
Google Scholar
[10] Moshwan R, Yang L, Zou J, Chen Z G 2017 Adv. Funct. Mater. 27 1703278
 Google Scholar
Google Scholar
[11] Wang L, Tan X, Liu G, Xu J, Shao H, Yu B, Jiang H, Yue S, Jiang J 2017 ACS Energy Lett. 2 1203
 Google Scholar
Google Scholar
[12] Tan G, Hao S, Hanus R C, Zhang X, Anand S, Bailey T P, Rettie A J E, Su X, Uher C, Dravid V P, Snyder G J, Wolverton C, Kanatzidis M G 2018 ACS Energy Lett. 3 705
 Google Scholar
Google Scholar
[13] Banik A, Vishal B, Perumal S, Datta R, Biswas K 2016 Energy Environ. Sci. 9 2011
 Google Scholar
Google Scholar
[14] Zhao L D, Lo S H, Zhang Y, Sun H, Tan G, Uher C, Wolverton C, Dravid V P, Kanatzidis M G 2014 Nature 508 373
 Google Scholar
Google Scholar
[15] Zheng L, Li W, Lin S, Li J, Chen Z, Pei Y 2017 ACS Energy Lett. 2 563
 Google Scholar
Google Scholar
[16] Tang J, Gao B, Lin S, Li J, Chen Z, Xiong F, Li W, Chen Y, Pei Y 2018 Adv. Funct. Mater. 28 1803586
 Google Scholar
Google Scholar
[17] Roychowdhury S, Biswas R K, Dutta M, Pati S K, Biswas K 2019 ACS Energy Lett. 4 1658
 Google Scholar
Google Scholar
[18] Tan G, Zeier W G, Shi F, Wang P, Snyder G J, Dravid V P, Kanatzidis M G 2015 Chem. Mater. 27 7801
 Google Scholar
Google Scholar
[19] Tang J, Gao B, Lin S, Wang X, Zhang X, Xiong F, Li W, Chen Y, Pei Y 2018 ACS Energy Lett. 3 1969
 Google Scholar
Google Scholar
[20] Yao Z, Li W, Tang J, Chen Z, Lin S, Biswas K, Burkov A, Pei Y 2019 InfoMat 1 571
 Google Scholar
Google Scholar
[21] Liu R, Chen H, Zhao K, Qin Y, Jiang B, Zhang T, Sha G, Shi X, Uher C, Zhang W, Chen L 2017 Adv. Mater. 29 1702712
 Google Scholar
Google Scholar
[22] Hu L, Zhang Y, Wu H, Li J, Li Y, Mckenna M, He J, Liu F, Pennycook S, Zeng X 2018 Adv. Energy Mater. 8 1802116
 Google Scholar
Google Scholar
[23] Acharya S, Anwar S, Mori T, Soni A 2018 J. Mater. Chem. C 6 6489
 Google Scholar
Google Scholar
[24] Sarkar D, Ghosh T, Banik A, Roychowdhury S, Sanyal D, Biswas K 2020 Angew Chem. Int. Ed. 59 11115
 Google Scholar
Google Scholar
[25] Jood P, Ohta M 2020 ACS Appl. Energy Mater. 3 2160
[26] Mi W, Qiu P, Zhang T, Lv Y, Shi X, Chen L 2014 Appl. Phys. Lett. 104 133903
 Google Scholar
Google Scholar
[27] Snyder G J, Toberer E S 2008 Nat. Mater. 7 105
 Google Scholar
Google Scholar
[28] Kim H, Gibbs Z, Tang Y, Wang H, Snyder G J 2015 APL Mater. 3 041506
 Google Scholar
Google Scholar
[29] Roychowdhury S, Shenoy U S, Waghmare U V. Biswas K 2017 J. Mater. Chem. C 5 5737
 Google Scholar
Google Scholar
[30] Song S, Lo C, Aminzare M, Tseng Y, Valiyaveettil S, Mozharivskyj Y 2020 Dalton Trans. 49 6135
 Google Scholar
Google Scholar
[31] Tan G, Shi F, Sun H, Zhao L, Uher C, Dravid V P, Kanatzidis M G 2014 J. Mater. Chem. A 2 20849
 Google Scholar
Google Scholar
[32] Guo X, Chen Z, Tang J 2020 Appl. Phys. Lett. 116 103901
 Google Scholar
Google Scholar
[33] Chen Z, Guo X, Zhang F, Shi Q, Tang M, Ang R 2020 J. Mater. Chem. A 8 16790
 Google Scholar
Google Scholar
[34] Chen R, Qiu P F, Jiang B B, Hu P, Zhang Y M, Yang J, Ren D, Shi X, Chen L 2018 J. Mater. Chem. A 6 6493
 Google Scholar
Google Scholar
[35] Qiu Y, Jin Y, Wang D Y, Guan M, He W, Peng S, Liu R, Gao X, Zhao L 2019 J. Mater. Chem. A 7 26393
 Google Scholar
Google Scholar
[36] Jiang B, Qiu P, Chen H, Huang J, Mao T, Wang Y, Song Q, Ren D, Shi X, Chen L 2018 Mater. Today Phys. 5 20
 Google Scholar
Google Scholar
[37] Orabi R A R A, Mecholsky N A, Hwang J, Kim W, Rhyee J, Wee D, Fornari M 2016 Chem. Mater. 28 376
 Google Scholar
Google Scholar
[38] Cahill D G, Watson S K, Pohl R O 1992 Phys. Rev. B 46 6131
 Google Scholar
Google Scholar
[39] You L, Zhang J, Pan S, Jiang Y, Wang K, Yang J, Pei Y, Zhu Q, Agne M T, Snyder G. J, Ren Z, Zhang W, Luo J 2019 Energy Environ. Sci. 12 3089
 Google Scholar
Google Scholar
[40] Zhu H, Zhao T, Zhang B, An Z, Mao S, Wang G, Han X, Lu X, Zhang J, Zhou X 2021 Adv. Energy Mater. 11 2003304
 Google Scholar
Google Scholar
[41] Li S, Li J, Yang L, Liu F, Ao W, Li Y 2016 Mater. Des. 108 51
 Google Scholar
Google Scholar
[42] Guo F, Cui B, Geng H, Zhang Y, Wu H, Zhang Q, Yu B, Pennycook S J, Cai W, Sui J 2019 Small 15 1902493
 Google Scholar
Google Scholar
-
图 1 (Sn0.95-xGe0.05BixTe)0.95(Ag2Se)0.05 (x = 0—0.1)的构型熵(ΔS)与Bi含量(x)的关系. ΔS1和ΔS2代表添加摩尔分数5% Ge和5% Ag2Se后的熵增
Fig. 1. Dependence of the configurational entropy (ΔS) on the Bi content (x) in (Sn0.95-xGe0.05BixTe)0.95(Ag2Se)0.05 (x = 0–0.1), where the ΔS1 and ΔS2 represent the increased entropy after addition of 5% Ge and 5% Ag2Se (mole fraction), respectively.
图 3 样品 (x = 0.05)的透射电镜图 (a) 低倍TEM图; (b) 高倍透射电镜图(HRTEM); (c) 选区电子衍射花样(SAED); (d) 能谱分析图(EDS)
Fig. 3. Transmission electron microscopy (TEM) images observed on the sample (Sn0.95-xGe0.05BixTe)0.95(Ag2Se)0.05 (x = 0.05): (a) TEM image; (b) HRTEM image; (c) the selected area electron diffraction (SAED) pattern; (d) EDS analysis.
图 4 室温条件下本征SnTe, Sn0.95Ge0.05Te和(Sn0.95–xGe0.05BixTe)0.95(Ag2Se)0.05 (x = 0, 0.025, 0.050, 0.075, 0.100 样品的(a)载流子浓度(nH); (b)载流子迁移率(μ)
Fig. 4. (a) Hall hole concentration (nH) and (b) carrier mobility (μ) of the pristine SnTe, Sn0.95Ge0.05Te, (Sn0.95–xGe0.05BixTe)0.95(Ag2Se)0.05 (x = 0, 0.025, 0.050, 0.075, 0.100) at room temperature (RT).
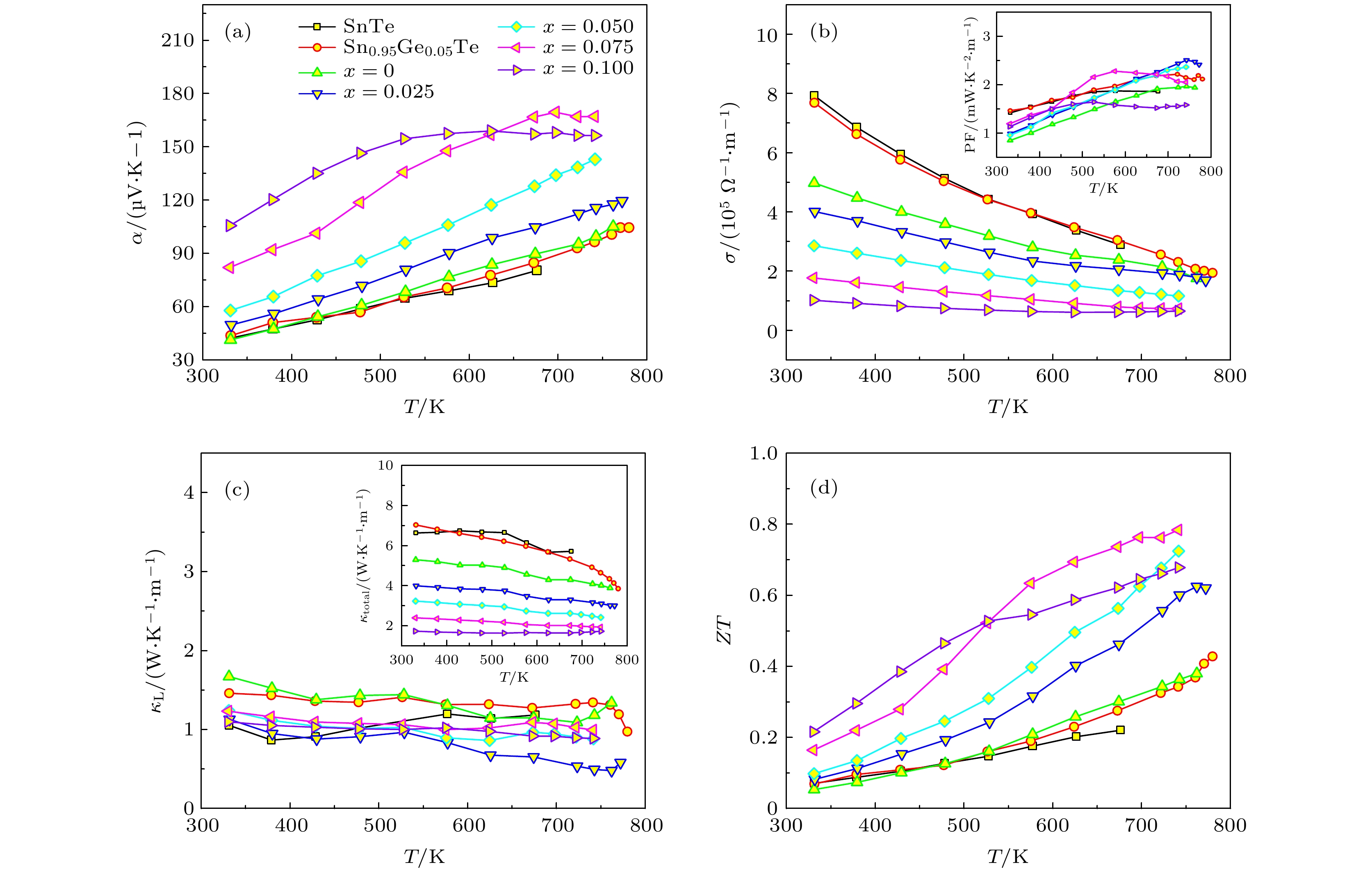
图 5 SnTe, Sn0.95Ge0.05Te和(Sn0.95-xGe0.05BixTe)0.95(Ag2Se)0.05 (x = 0, 0.025, 0.050, 0.075, 0.100)热电性能随温度的变化 (a) Seebeck系数(α); (b) 电导率 (σ), 插图为功率因子 (PF); (c) 晶格热导率 (κL), 插图为总热导率 (κtotal); (d) 热电优值(ZT )
Fig. 5. Temperature dependence of the TE properties for SnTe, Sn0.95Ge0.05Te and (Sn0.95-xGe0.05BixTe)0.95(Ag2Se)0.05 (x = 0, 0.025, 0.050, 0.075, 0.100): (a) Seebeck coefficients (α); (b) electrical conductivities (σ), the insert is the power factors (PF); (c) lattice thermal conductivities. (κL), the insert is the κtotal; (d) Figure of the merit ZT
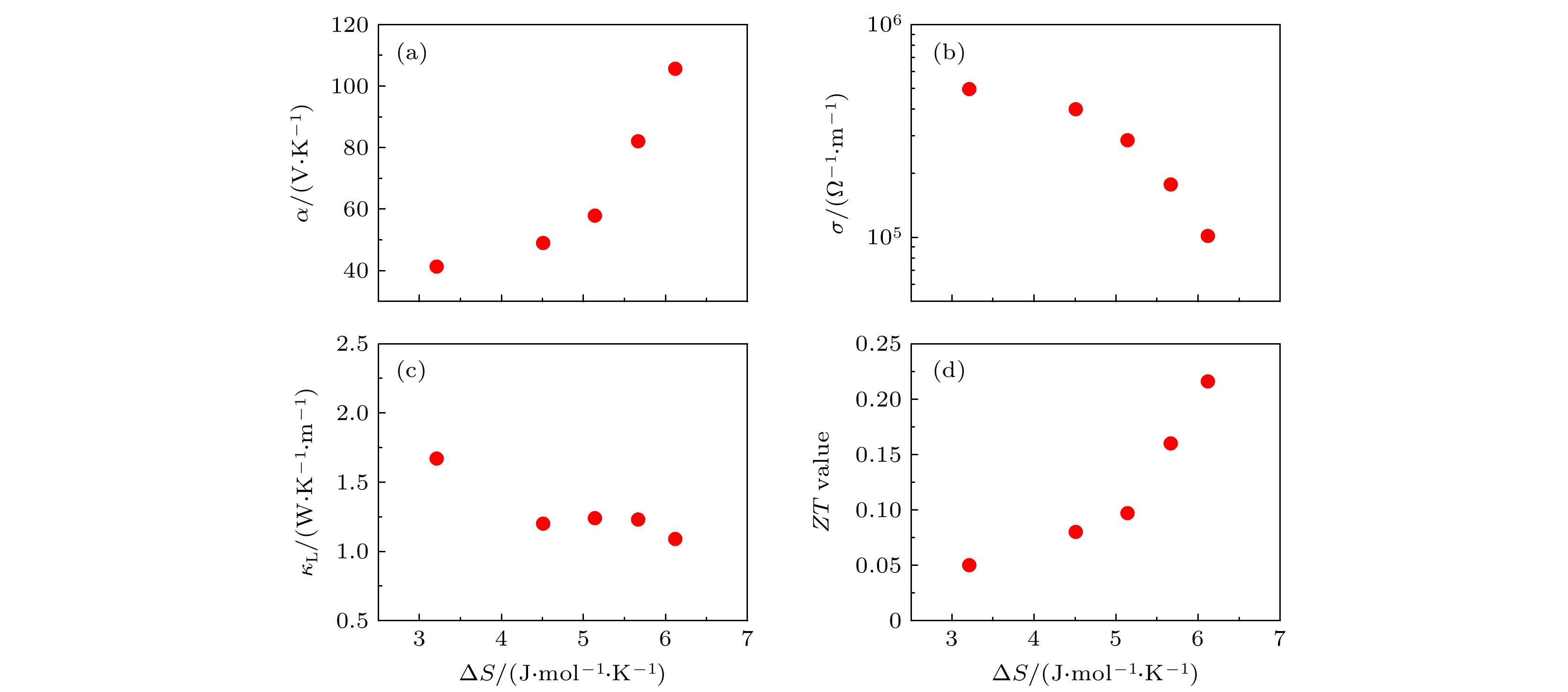
图 6 室温下各热电参数与(Sn0.95-xGe0.05BixTe)0.95(Ag2Se)0.05 (x = 0—0.1)构型熵(ΔS)的关系 (a) Seebeck系数与ΔS的关系; (b) 电导率与ΔS的关系; (c) 晶格热导率与ΔS的关系; (d) 热电优值与ΔS的关系
Fig. 6. Dependence of the TE performance on the configurational entropy (ΔS) of (Sn0.95-xGe0.05BixTe)0.95(Ag2Se)0.05 (x = 0–0.1): (a) Dependence of the Seebeck coefficient (α) on the configurational entropy (ΔS); (b) dependence of the electrical conductivity (σ) on the configurational entropy (ΔS); (c) dependence of the lattice thermal conductivity (κL) on the configurational entropy (ΔS); (d) dependence of the ZT value on the configurational entropy (ΔS).
-
[1] Zhang Q, Liao B, Lan Y C, Lukas K, Liu W, Esfarjani K, Opeil C, Broido D, Chen G, Ren Z 2013 PNAS 110 13261
 Google Scholar
Google Scholar
[2] Rogers L 1968 J. Phys. D:Appl. Phys. 1 845
 Google Scholar
Google Scholar
[3] Brebrick R 1963 J. Phys. Chem. Solids 24 27
 Google Scholar
Google Scholar
[4] Li M, Ying P, Du Z, Liu X, Li X, Fang T, Cui J 2022 ACS Appl. Mater. Interfaces 14 8171
 Google Scholar
Google Scholar
[5] Banik A, Shenoy U S, Saha S, Waghmare U V, Biswas K 2016 J. Am. Chem. Soc. 138 13068
 Google Scholar
Google Scholar
[6] Tan G, Shi F, Hao S, Chi H, Bailey T P, Zhao L, Uher C, Wolverton C, Dravid V P, Kanatzidis M G 2015 J. Am. Chem. Soc. 137 11507
 Google Scholar
Google Scholar
[7] Zhou M, Gibbs Z M, Wang H, Han Y, Xin C, Li L 2014 Phys. Chem. Chem. Phys. 16 20741
 Google Scholar
Google Scholar
[8] Pei Y, Shi X, LaLonde A, Wang H, Chen L, Snyder G J 2011 Nature 473 66
 Google Scholar
Google Scholar
[9] Pei Y, Wang H, Snyder G J 2012 Adv. Mater. 24 6125
 Google Scholar
Google Scholar
[10] Moshwan R, Yang L, Zou J, Chen Z G 2017 Adv. Funct. Mater. 27 1703278
 Google Scholar
Google Scholar
[11] Wang L, Tan X, Liu G, Xu J, Shao H, Yu B, Jiang H, Yue S, Jiang J 2017 ACS Energy Lett. 2 1203
 Google Scholar
Google Scholar
[12] Tan G, Hao S, Hanus R C, Zhang X, Anand S, Bailey T P, Rettie A J E, Su X, Uher C, Dravid V P, Snyder G J, Wolverton C, Kanatzidis M G 2018 ACS Energy Lett. 3 705
 Google Scholar
Google Scholar
[13] Banik A, Vishal B, Perumal S, Datta R, Biswas K 2016 Energy Environ. Sci. 9 2011
 Google Scholar
Google Scholar
[14] Zhao L D, Lo S H, Zhang Y, Sun H, Tan G, Uher C, Wolverton C, Dravid V P, Kanatzidis M G 2014 Nature 508 373
 Google Scholar
Google Scholar
[15] Zheng L, Li W, Lin S, Li J, Chen Z, Pei Y 2017 ACS Energy Lett. 2 563
 Google Scholar
Google Scholar
[16] Tang J, Gao B, Lin S, Li J, Chen Z, Xiong F, Li W, Chen Y, Pei Y 2018 Adv. Funct. Mater. 28 1803586
 Google Scholar
Google Scholar
[17] Roychowdhury S, Biswas R K, Dutta M, Pati S K, Biswas K 2019 ACS Energy Lett. 4 1658
 Google Scholar
Google Scholar
[18] Tan G, Zeier W G, Shi F, Wang P, Snyder G J, Dravid V P, Kanatzidis M G 2015 Chem. Mater. 27 7801
 Google Scholar
Google Scholar
[19] Tang J, Gao B, Lin S, Wang X, Zhang X, Xiong F, Li W, Chen Y, Pei Y 2018 ACS Energy Lett. 3 1969
 Google Scholar
Google Scholar
[20] Yao Z, Li W, Tang J, Chen Z, Lin S, Biswas K, Burkov A, Pei Y 2019 InfoMat 1 571
 Google Scholar
Google Scholar
[21] Liu R, Chen H, Zhao K, Qin Y, Jiang B, Zhang T, Sha G, Shi X, Uher C, Zhang W, Chen L 2017 Adv. Mater. 29 1702712
 Google Scholar
Google Scholar
[22] Hu L, Zhang Y, Wu H, Li J, Li Y, Mckenna M, He J, Liu F, Pennycook S, Zeng X 2018 Adv. Energy Mater. 8 1802116
 Google Scholar
Google Scholar
[23] Acharya S, Anwar S, Mori T, Soni A 2018 J. Mater. Chem. C 6 6489
 Google Scholar
Google Scholar
[24] Sarkar D, Ghosh T, Banik A, Roychowdhury S, Sanyal D, Biswas K 2020 Angew Chem. Int. Ed. 59 11115
 Google Scholar
Google Scholar
[25] Jood P, Ohta M 2020 ACS Appl. Energy Mater. 3 2160
[26] Mi W, Qiu P, Zhang T, Lv Y, Shi X, Chen L 2014 Appl. Phys. Lett. 104 133903
 Google Scholar
Google Scholar
[27] Snyder G J, Toberer E S 2008 Nat. Mater. 7 105
 Google Scholar
Google Scholar
[28] Kim H, Gibbs Z, Tang Y, Wang H, Snyder G J 2015 APL Mater. 3 041506
 Google Scholar
Google Scholar
[29] Roychowdhury S, Shenoy U S, Waghmare U V. Biswas K 2017 J. Mater. Chem. C 5 5737
 Google Scholar
Google Scholar
[30] Song S, Lo C, Aminzare M, Tseng Y, Valiyaveettil S, Mozharivskyj Y 2020 Dalton Trans. 49 6135
 Google Scholar
Google Scholar
[31] Tan G, Shi F, Sun H, Zhao L, Uher C, Dravid V P, Kanatzidis M G 2014 J. Mater. Chem. A 2 20849
 Google Scholar
Google Scholar
[32] Guo X, Chen Z, Tang J 2020 Appl. Phys. Lett. 116 103901
 Google Scholar
Google Scholar
[33] Chen Z, Guo X, Zhang F, Shi Q, Tang M, Ang R 2020 J. Mater. Chem. A 8 16790
 Google Scholar
Google Scholar
[34] Chen R, Qiu P F, Jiang B B, Hu P, Zhang Y M, Yang J, Ren D, Shi X, Chen L 2018 J. Mater. Chem. A 6 6493
 Google Scholar
Google Scholar
[35] Qiu Y, Jin Y, Wang D Y, Guan M, He W, Peng S, Liu R, Gao X, Zhao L 2019 J. Mater. Chem. A 7 26393
 Google Scholar
Google Scholar
[36] Jiang B, Qiu P, Chen H, Huang J, Mao T, Wang Y, Song Q, Ren D, Shi X, Chen L 2018 Mater. Today Phys. 5 20
 Google Scholar
Google Scholar
[37] Orabi R A R A, Mecholsky N A, Hwang J, Kim W, Rhyee J, Wee D, Fornari M 2016 Chem. Mater. 28 376
 Google Scholar
Google Scholar
[38] Cahill D G, Watson S K, Pohl R O 1992 Phys. Rev. B 46 6131
 Google Scholar
Google Scholar
[39] You L, Zhang J, Pan S, Jiang Y, Wang K, Yang J, Pei Y, Zhu Q, Agne M T, Snyder G. J, Ren Z, Zhang W, Luo J 2019 Energy Environ. Sci. 12 3089
 Google Scholar
Google Scholar
[40] Zhu H, Zhao T, Zhang B, An Z, Mao S, Wang G, Han X, Lu X, Zhang J, Zhou X 2021 Adv. Energy Mater. 11 2003304
 Google Scholar
Google Scholar
[41] Li S, Li J, Yang L, Liu F, Ao W, Li Y 2016 Mater. Des. 108 51
 Google Scholar
Google Scholar
[42] Guo F, Cui B, Geng H, Zhang Y, Wu H, Zhang Q, Yu B, Pennycook S J, Cai W, Sui J 2019 Small 15 1902493
 Google Scholar
Google Scholar
计量
- 文章访问数: 6999
- PDF下载量: 87
- 被引次数: 0
















 下载:
下载: