-
锆合金(如: 锆铌(Zr-Nb)合金)的辐照损伤问题是裂变堆结构材料和燃料棒包壳材料设计的关键, 而深入理解辐照损伤的物理机制, 往往需借助于原子尺度的计算模拟, 如: 分子动力学和第一性原理等. 针对随机置换固溶体合金的模拟, 首先需构建能反映合金元素随机分布特征的大尺寸超胞, 然而第一性原理计算量大, 不宜使用过大(如 ≥ 200原子)超胞. 通常第一性原理计算使用的是特殊准随机(SQS)超胞, SQS超胞可部分反映合金元素的随机分布特性, 但对于特定组分只对应一种构型, 这种模型是否能反映真实随机置换固溶体中多种局域构型的统计平均还有待进一步研究验证. 分子动力学可在更大的尺度上进行计算模拟, 能够通过随机取代(RSS)模型研究更多的合金构型, 因此, 本文基于RSS超胞模型及SQS扩展超胞模型, 运用分子动力学方法对Zr-Nb合金进行了研究. 首先通过构型误差分析确定了能真实反映固溶体合金性能统计性的RSS超胞的临界尺寸; 然后计算比较了Zr-Nb合金SQS扩展超胞和一系列RSS超胞的晶格常数、形成能和能量-体积关系. 研究表明, 利用SQS超胞模拟得到的固溶体的晶格常数、形成能和能量体积曲线与一系列RSS超胞的对应统计值接近, 因而SQS超胞可用于研究随机置换固溶体合金.Irradiation damage to zirconium alloys (e.g., zirconium niobium (Zr-Nb) alloy) is the key to the design of fission-reactor structural materials and fuel rod cladding materials. Atomic scale computational simulations such as molecular dynamics and first principles are often needed to understand the physical mechanism of irradiation damage. For the simulation of randomly substitutional solid solution, it is necessary to construct large-sized supercells that can reflect the random distribution characteristics of alloy elements. However, it is not suitable to use large-size supercells (such as ≥ 200 atoms) for first principle calculation, due to the large computational cost. Special quasirandom supercells (SQS) are usually used for first principles calculation. The SQS can partly reflect the random distribution characteristics of alloy elements, but it only corresponds to one configuration for specific components, hence whether this model can reflect the statistical average of multiple local configurations in a real randomly substitutional solid solution is still an open question, and needs further studying and verifying. Molecular dynamics (MD) simulation can be carried out on the randomly substitutional solid solution with a larger scale based on random substitution (RSS) method, these supercells include more local configurations. Therefore, the MD studies of Zr-Nb alloy are carried out for the RSS and SQS-extended supercells. The critical size of RSS supercell which can truly reflect the statistical properties of solid solution alloy is determined. Then the lattice constant, formation energy and energy-volume relationship of SQS-extended supercell of Zr-Nb alloy and a series of RSS supercells are calculated and compared. The results show that the lattice constants, the formation energy and energy volume curves of the solid solution obtained by SQS supercell simulation are close to a series of corresponding statistical values of the physical properties of RSS supercells, so the SQS supercells can be used to study the random substitution of solid solution alloys.
-
Keywords:
- Zr-Nb alloy /
- molecular dynamics /
- special quasirandom supercells model /
- critical supercell
[1] Silva C, Leonard K, Trammel M, Bryan C 2018 Mater. Sci. Eng., A 716 296
 Google Scholar
Google Scholar
[2] Smirnova D E, Starikov S V 2017 Comput. Mater. Sci. 129 259
 Google Scholar
Google Scholar
[3] Kharchenko V O, Kharchenko D O 2013 Condens. Matter Phys. 16 13801
 Google Scholar
Google Scholar
[4] Sabol G P, Moan G D 2000 Zirconium in the Nuclear Industry: 12th International Symposium (West Conshohocken, PA: ASTM) p505
[5] Bradley E R, Sabol G P 1996 Zirconium in the Nuclear Industry: 11th International Symposium (West Conshohocken, PA: ASTM) p710
[6] Novikov V V, Markelov V A, Tselishchev A V, Konkov V F, Sinelnikov L P, Panchenko V L 2006 J. Nucl. Sci. Technol. 43 991
 Google Scholar
Google Scholar
[7] Hohenberg P, Kohn W 1964 Phys. Rev. 136 864
 Google Scholar
Google Scholar
[8] Alder B J, Wainwright T E 1957 J. Chem. Phys. 27 1208
 Google Scholar
Google Scholar
[9] Wei S H, Ferreira L G, Bernard J E, Zunger A 1990 Phys. Rev. B 42 9622
 Google Scholar
Google Scholar
[10] Jiang C, Wolverton C, Sofo J, Chen L Q, Liu Z K 2004 Phys. Rev. B 69 214202
 Google Scholar
Google Scholar
[11] Shin D, Arróyave R, Liu Z K, van de Walle A 2006 Phys. Rev. B 74 024204
 Google Scholar
Google Scholar
[12] Hu Y L, Bai L H, Tong Y G, Deng D Y, Liang X B, Zhang J, Li Y J, Chen Y X 2020 J. Alloys Compd. 827 153963
 Google Scholar
Google Scholar
[13] Daw M S, Baskes M I 1984 Phys. Rev. B 29 6443
 Google Scholar
Google Scholar
[14] Wadley H N G, Zhou X, Johnson R A, Neurock M 2001 Prog. Mater. Sci. 46 329
 Google Scholar
Google Scholar
[15] Zhou X W, Wadley H N G, Filhol J S, Neurock M N 2004 Phys. Rev. B 69 14413
 Google Scholar
Google Scholar
[16] Johnson R A 1989 Phys. Rev. B 39 12554
 Google Scholar
Google Scholar
[17] Lin D Y, Wang S S, Peng D L, Li M, Hui X D 2013 J. Phys. Condens. Matter 25 209501
 Google Scholar
Google Scholar
[18] Lide D R 2009 CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data (Boca Raton, FL: CRC Press) p1
[19] Hou Q, Li M, Zhou Y, Cui J, Cui Z, Wang J 2013 Comput. Phys. Commun. 184 2091
 Google Scholar
Google Scholar
[20] Jacob K T, Raj S, Rannesh L 2007 J. Mater. Res. 98 776
 Google Scholar
Google Scholar
[21] Stukowski A 2010 Modell. Simul. Mater. Sci. Eng. 18 015012
 Google Scholar
Google Scholar
[22] Benites G M, Fernández Guillermet A, Cuello G J, Campo J 2000 J. Alloys Compd. 299 183
 Google Scholar
Google Scholar
[23] Grad G B, Pieres J J, Guillermet A F, Cuello G J, Granada J R, Mayer R E 1995 Physica B 213-214 433
 Google Scholar
Google Scholar
[24] Lichter B D 1960 Trans. Met. Soc. AIME 218 1015
[25] Barannikova S A, Zharmukhambetova A M, Nikonov A Y, Dmitriev A V, Ponomareva A V, Abrikosov I A 2015 IOP Conf. Ser.: Mater. Sci. Eng. 71 012078
 Google Scholar
Google Scholar
[26] Okamoto H 1992 J. Phase Equilib. 13 577
 Google Scholar
Google Scholar
[27] Rose J H, Smith J R, Guinea F, Ferrante J 1984 Phys. Rev. B 29 2963
 Google Scholar
Google Scholar
-
图 1
$ {A}_{x}{B}_{1-x} $ 随机置换固溶体SQS模型超胞 (a) x = 0.5, SQS-16, bcc晶格; (b) x = 0.25或x = 0.75, SQS-16, bcc晶格; (c) x = 0.5, SQS-8, hcp晶格; (d) x = 0.25或x = 0.75, SQS-16, hcp晶格Fig. 1. Supercells of the
$ {A}_{x}{B}_{1-x} $ random solid solution: (a) x = 0.5, SQS-16, bcc lattice; (b) x = 0.25 or x = 0.75, SQS-16, bcc lattice; (c) x = 0.5, SQS-8, hcp lattice; (d) x = 0.25 or x = 0.75, SQS-16, hcp lattice.图 4 由SQS和RSS模型得到的合金晶格常数与Nb浓度的关系 (a) bcc晶格; (b) hcp晶格; (a)中实验值取自文献[22, 23], ADP势函数计算的晶格常数取自Smirnova和Starikov[2]
Fig. 4. Relationships between the alloy lattice constant and Nb concentration obtained from SQS and RSS models: (a) The bcc lattice; (b) the hcp lattice. In Fig. 4(a), the experimental values were obtained from literatures[22,23], and the lattice constant calculated from the ADP potential function was taken from Smirnova and Starikov[2].
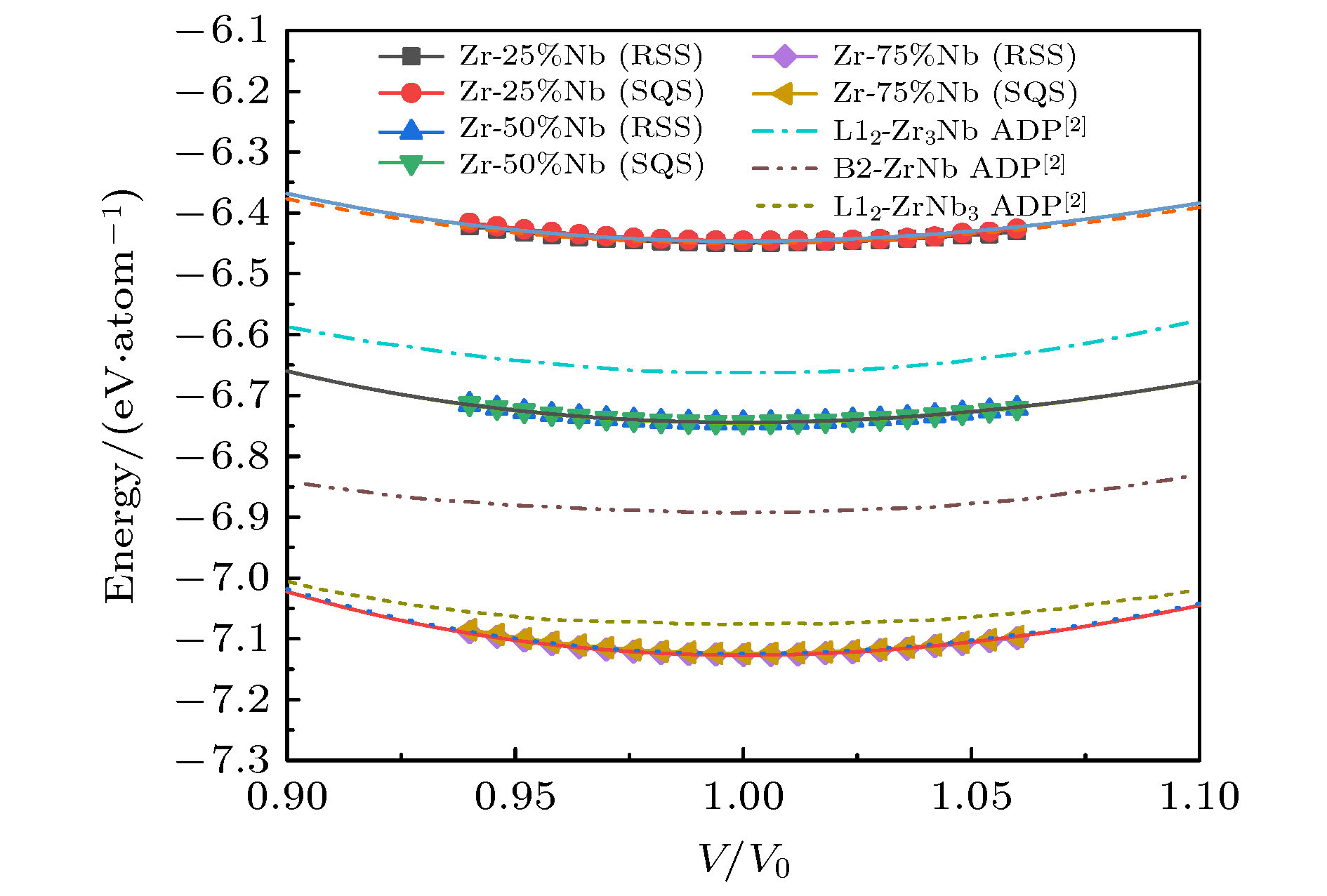
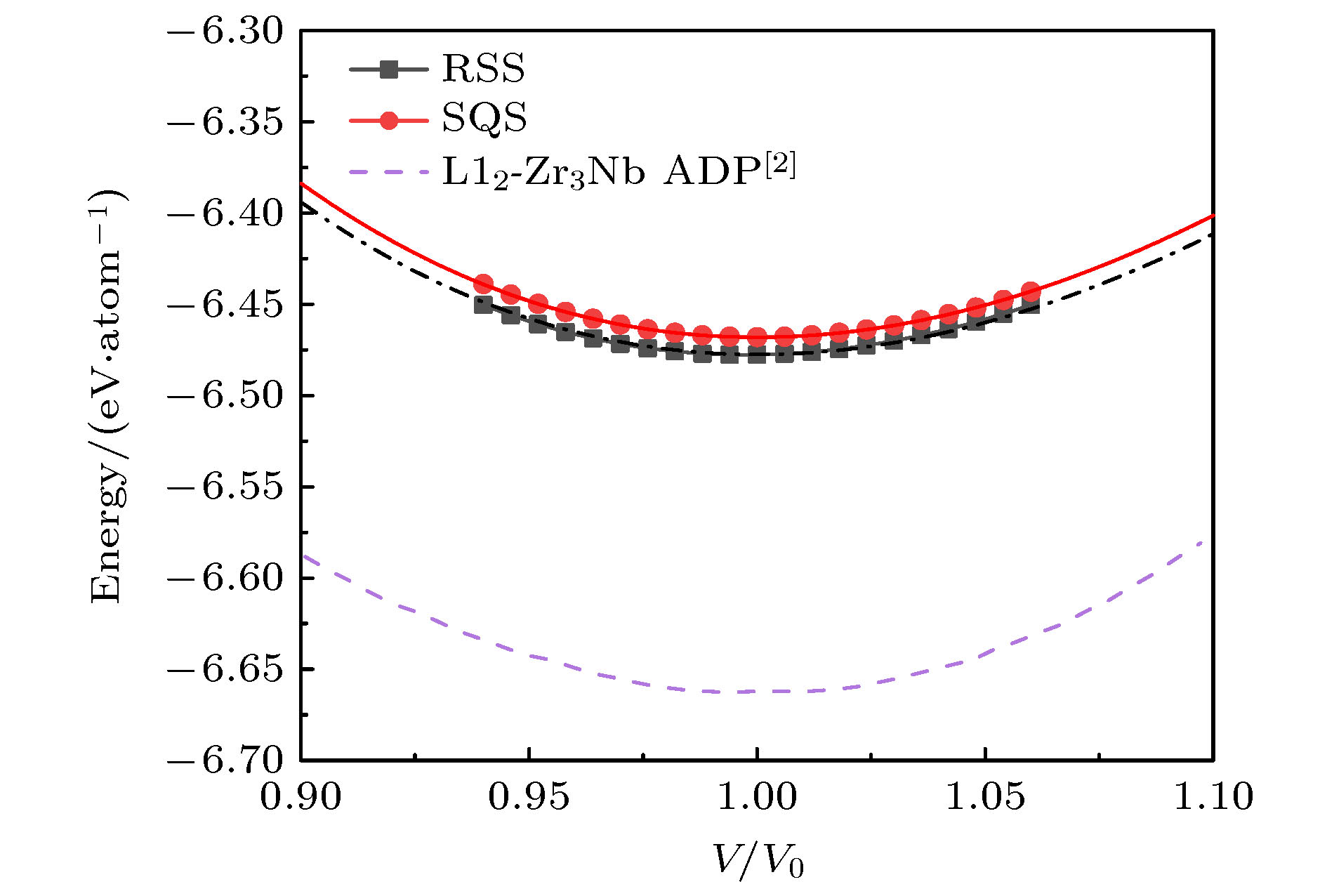
图 6 bcc晶格RSS超胞和SQS超胞的E-V曲线, 以及ADP势的计算结果, 其中, 多边形和圆形图标为对应的SQS和RSS模型的能量计算值, 对应的曲线是用EOS方程[27]拟合得到的E-V曲线; 单点划线、双点划线和短划线是Smirnova和Starikov[2]得到的ADP势模拟结果
Fig. 6. Energy-volume curves of RSS and SQS supercells in bcc lattice, and the calculation results of ADP potential. The polygon and circular icons are the energy calculation values of the corresponding SQS and RSS structure, and the corresponding curves are the E-V curves obtained by fitting EOS equation[27]. Single dotted line, double dotted line and short dotted line are the calculated results of ADP potential obtained by Smirnova and Starikov[2].
表 1 由EOS方程拟合得到的Zr-Nb合金性质(带“*”的为文献[17]的拟合结果; 第一行对应RSS超胞, 第二行对应SQS超胞)
Table 1. Properties of Zr-Nb alloy obtained by fitting EOS equation, and the “ * ” is the fitting result of literature[17]. The first line corresponds RSS structure, and the second line corresponds SQS structure.
Alloy a/Å c/Å Ec/(eV·atom–1) B0/GPa Zr0.75Nb0.25(bcc) 3.527 6.450 96 3.531 6.447 100 Zr0.75Nb0.25(hcp) 3.200 5.097 6.468 144 3.202 5.100 6.477 142 *L12-Zr3Nb 4.49 6.45 107 Zr0.5Nb0.5(bcc) 3.442 6.745 118 3.441 6.744 117 *B2-ZrNb 3.48 6.69 116 Zr0.25Nb0.75(bcc) 3.366 7.127 153 3.367 7.124 156 *L12-ZrNb3 4.34 6.96 100 -
[1] Silva C, Leonard K, Trammel M, Bryan C 2018 Mater. Sci. Eng., A 716 296
 Google Scholar
Google Scholar
[2] Smirnova D E, Starikov S V 2017 Comput. Mater. Sci. 129 259
 Google Scholar
Google Scholar
[3] Kharchenko V O, Kharchenko D O 2013 Condens. Matter Phys. 16 13801
 Google Scholar
Google Scholar
[4] Sabol G P, Moan G D 2000 Zirconium in the Nuclear Industry: 12th International Symposium (West Conshohocken, PA: ASTM) p505
[5] Bradley E R, Sabol G P 1996 Zirconium in the Nuclear Industry: 11th International Symposium (West Conshohocken, PA: ASTM) p710
[6] Novikov V V, Markelov V A, Tselishchev A V, Konkov V F, Sinelnikov L P, Panchenko V L 2006 J. Nucl. Sci. Technol. 43 991
 Google Scholar
Google Scholar
[7] Hohenberg P, Kohn W 1964 Phys. Rev. 136 864
 Google Scholar
Google Scholar
[8] Alder B J, Wainwright T E 1957 J. Chem. Phys. 27 1208
 Google Scholar
Google Scholar
[9] Wei S H, Ferreira L G, Bernard J E, Zunger A 1990 Phys. Rev. B 42 9622
 Google Scholar
Google Scholar
[10] Jiang C, Wolverton C, Sofo J, Chen L Q, Liu Z K 2004 Phys. Rev. B 69 214202
 Google Scholar
Google Scholar
[11] Shin D, Arróyave R, Liu Z K, van de Walle A 2006 Phys. Rev. B 74 024204
 Google Scholar
Google Scholar
[12] Hu Y L, Bai L H, Tong Y G, Deng D Y, Liang X B, Zhang J, Li Y J, Chen Y X 2020 J. Alloys Compd. 827 153963
 Google Scholar
Google Scholar
[13] Daw M S, Baskes M I 1984 Phys. Rev. B 29 6443
 Google Scholar
Google Scholar
[14] Wadley H N G, Zhou X, Johnson R A, Neurock M 2001 Prog. Mater. Sci. 46 329
 Google Scholar
Google Scholar
[15] Zhou X W, Wadley H N G, Filhol J S, Neurock M N 2004 Phys. Rev. B 69 14413
 Google Scholar
Google Scholar
[16] Johnson R A 1989 Phys. Rev. B 39 12554
 Google Scholar
Google Scholar
[17] Lin D Y, Wang S S, Peng D L, Li M, Hui X D 2013 J. Phys. Condens. Matter 25 209501
 Google Scholar
Google Scholar
[18] Lide D R 2009 CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data (Boca Raton, FL: CRC Press) p1
[19] Hou Q, Li M, Zhou Y, Cui J, Cui Z, Wang J 2013 Comput. Phys. Commun. 184 2091
 Google Scholar
Google Scholar
[20] Jacob K T, Raj S, Rannesh L 2007 J. Mater. Res. 98 776
 Google Scholar
Google Scholar
[21] Stukowski A 2010 Modell. Simul. Mater. Sci. Eng. 18 015012
 Google Scholar
Google Scholar
[22] Benites G M, Fernández Guillermet A, Cuello G J, Campo J 2000 J. Alloys Compd. 299 183
 Google Scholar
Google Scholar
[23] Grad G B, Pieres J J, Guillermet A F, Cuello G J, Granada J R, Mayer R E 1995 Physica B 213-214 433
 Google Scholar
Google Scholar
[24] Lichter B D 1960 Trans. Met. Soc. AIME 218 1015
[25] Barannikova S A, Zharmukhambetova A M, Nikonov A Y, Dmitriev A V, Ponomareva A V, Abrikosov I A 2015 IOP Conf. Ser.: Mater. Sci. Eng. 71 012078
 Google Scholar
Google Scholar
[26] Okamoto H 1992 J. Phase Equilib. 13 577
 Google Scholar
Google Scholar
[27] Rose J H, Smith J R, Guinea F, Ferrante J 1984 Phys. Rev. B 29 2963
 Google Scholar
Google Scholar
计量
- 文章访问数: 15398
- PDF下载量: 356
- 被引次数: 0
















 下载:
下载: