-
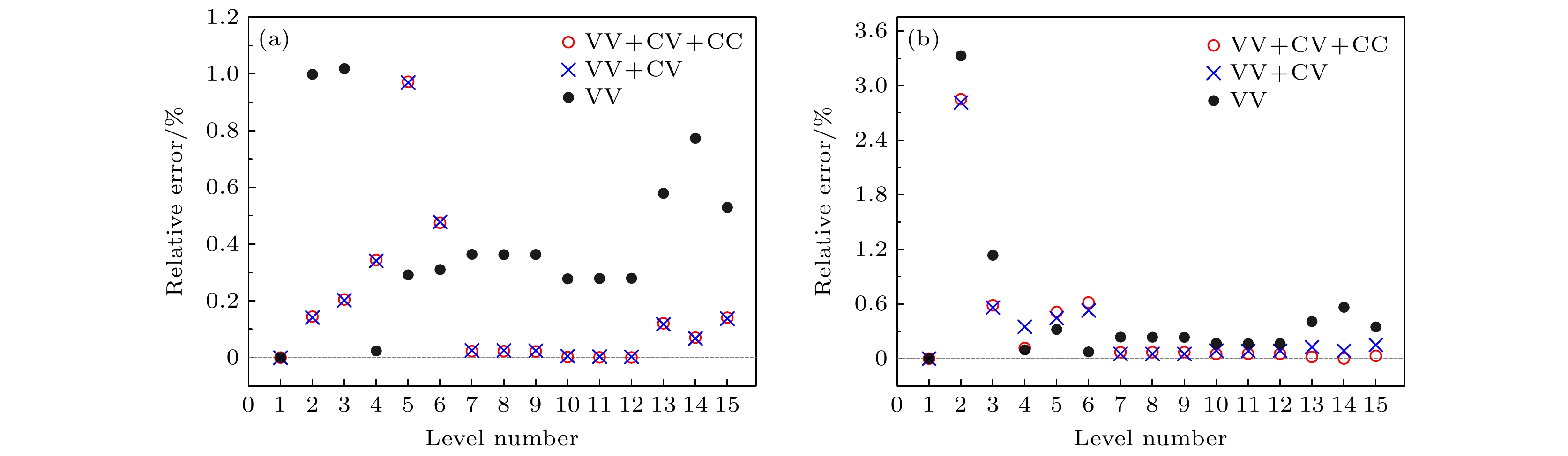
The atomic energy level structures and transition properties of 1s22s22p2 ground configuration and 1s22s2p3 excited configuration in carbon-like ions with Z = 10, 14, 32, 36, 50 are investigated theoretically using the fully relativistic multi-configuration Dirac-Hartree-Fock (MCDHF) method. Based on the wavefunction constructed with careful consideration of electron correlations, the theoretical calculations are completed by taking into account the Breit interaction, quantum electrodynamic effect and nuclear mass effect. Then the effects of three types of electron correlations, namely valence-valence, core-valence, and core-core correlations, on energy levels are studied in detail, and high-precision excitation energies are obtained. Compared with other theoretical results, the calculated excitation energies for Ne V ion are the closest to the NIST (National Institute of Standards and Technology) data, and the excitation energies of other ions also possess relatively high precision. Additionally, by combining the NIST data and the LS coupled atomic state compositions, the fuzziness in identifying atomic states generated from the code is analyzed, and the corresponding renamed atomic states are presented. For electric dipole transitions, the transition wavelengths of Ne V and Si IX ions reported in this work are in good agreement with the available NIST data, with the relative errors being less than 0.62%. Their transition ratesaccord well with other theoretical results. And for majority of electric dipole transitions, the electric dipole transition parameters calculated in Babushkin and Coulomb gauges are well consistent with each other, which demonstrates the feasibility and reliability of the MCDHF method for theoretically calculating the energy structures and spectral properties of 1s22s22p2 and 1s22s2p3 configurations in carbon-like ions. The results cover a wide range of levels and transitions for carbon-like ions, and the data are expected to enrich the fundamental database for carbon-like ions and provide valuable theoretical references for relevant studies. The datasets presented in this paper are openly available at https://doi.org/10.57760/sciencedb.j00213.00145. -
Keywords:
- carbon-like ion /
- MCDHF method /
- energy level /
- electric dipole transition
-
表 1 Ne V离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态在AS2—AS7活性空间的原子态能级
Table 1. Energies of atomic states for 1s22s22p2 and 1s22s2p3 configurations in Ne V ion (Z = 10, 14, 32, 36, 50) within AS2–AS7 active spaces.
Lv. No. States EAS2/a.u. EAS3/a.u. EAS4/a.u. EAS5/a.u. EAS6/a.u. EAS7/a.u. 1 1s22s22p2 3P0 –120.79410 –120.82751 –120.83972 –120.84535 –120.84811 –120.84951 2 1s22s22p2 3P1 –120.79224 –120.82564 –120.83784 –120.84347 –120.84623 –120.84763 3 1s22s22p2 3P2 –120.78908 –120.82246 –120.83465 –120.84028 –120.84304 –120.84444 4 1s22s22p2 1D2 –120.64690 –120.68647 –120.70016 –120.70634 –120.70949 –120.71101 5 1s22s22p2 1S0 –120.48406 –120.52863 –120.54349 –120.55022 –120.55385 –120.55545 6 1s22s2p3 5S2 –120.40336 –120.43148 –120.44190 –120.44575 –120.44802 –120.44863 7 1s22s2p3 3D3 –119.98436 –120.02513 –120.03869 –120.04426 –120.04727 –120.04851 8 1s22s2p3 3D2 –119.98402 –120.02480 –120.03837 –120.04394 –120.04695 –120.04819 9 1s22s2p3 3D1 –119.98391 –120.02469 –120.03826 –120.04383 –120.04684 –120.04808 10 1s22s2p3 3P2 –119.82820 –119.87432 –119.88934 –119.89610 –119.89966 –119.90109 11 1s22s2p3 3P1 –119.82817 –119.87430 –119.88932 –119.89607 –119.89963 –119.90107 12 1s22s2p3 3P0 –119.82801 –119.87414 –119.88916 –119.89592 –119.89948 –119.90092 13 1s22s2p3 1D2 –119.53581 –119.58668 –119.60269 –119.60989 –119.61344 –119.61526 14 1s22s2p3 3S1 –119.50144 –119.54851 –119.56397 –119.57073 –119.57426 –119.57567 15 1s22s2p3 1P1 –119.37379 –119.43100 –119.44855 –119.45719 –119.46129 –119.46322 表 2 Si IX离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态在AS2—AS7活性空间的原子态能级
Table 2. Energies of atomic states for 1s22s22p2 and 1s22s2p3 configurations in Si IX ion (Z = 10, 14, 32, 36, 50) within AS2–AS7 active spaces.
Lv. No. States EAS2/a.u. EAS3/a.u. EAS4/a.u. EAS5/a.u. EAS6/a.u. EAS7/a.u. 1 1s22s22p2 3P0 –252.15395 –252.18931 –252.20215 –252.20788 –252.21083 –252.21243 2 1s22s22p2 3P1 –252.14278 –252.17808 –252.19089 –252.19662 –252.19957 –252.20117 3 1s22s22p2 3P2 –252.12503 –252.16031 –252.17311 –252.17883 –252.18178 –252.18338 4 1s22s22p2 1D2 –251.90392 –251.94520 –251.95960 –251.96589 –251.96924 –251.97100 5 1s22s22p2 1S0 –251.64493 –251.69062 –251.70618 –251.71310 –251.71691 –251.71873 6 1s22s2p3 5S2 –251.48251 –251.51136 –251.52262 –251.52635 –251.52888 –251.52969 7 1s22s2p3 3D3 –250.81441 –250.85677 –250.87149 –250.87712 –250.88042 –250.88184 8 1s22s2p3 3D2 –250.81405 –250.85646 –250.87119 –250.87683 –250.88014 –250.88155 9 1s22s2p3 3D1 –250.81338 –250.85581 –250.87053 –250.87617 –250.87949 –250.88090 10 1s22s2p3 3P2 –250.57107 –250.61784 –250.63380 –250.64064 –250.64417 –250.64576 11 1s22s2p3 3P1 –250.57078 –250.61757 –250.63353 –250.64037 –250.64391 –250.64550 12 1s22s2p3 3P0 –250.57064 –250.61738 –250.63334 –250.64017 –250.64370 –250.64529 13 1s22s2p3 1D2 –250.12207 –250.17503 –250.19228 –250.19964 –250.20349 –250.20541 14 1s22s2p3 3S1 –250.09823 –250.14722 –250.16358 –250.17072 –250.17431 –250.17598 15 1s22s2p3 1P1 –249.87552 –249.93338 –249.95187 –249.96048 –249.96455 –249.96656 表 5 Sn XLV离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态在AS2—AS7活性空间的原子态能级
Table 5. Energies of atomic states for 1s22s22p2 and 1s22s2p3 configurations in Sn XLV ion (Z = 10, 14, 32, 36, 50) within AS2–AS7 active spaces.
Lv. No. States EAS2/a.u. EAS3/a.u. EAS4/a.u. EAS5/a.u. EAS6/a.u. EAS7/a.u. 1 1s22s22p2 3P0 –3718.67573 –3718.72761 –3718.75354 –3718.76491 –3718.77451 –3718.77886 2 1s22s22p2 3P1 –3710.09251 –3710.13935 –3710.16324 –3710.17387 –3710.18302 –3710.18713 3 1s22s22p2 1D2 –3709.54675 –3709.59733 –3709.62247 –3709.63358 –3709.64302 –3709.64729 4 1s22s2p3 $ {}^3{\text{P}}_{2}^{\text{a}} $
(1s22s2p3 5S2)–3704.00426 –3704.05462 –3704.08080 –3704.09038 –3704.09880 –3704.10330 5 1s22s2p3 3D1 –3701.92161 –3701.98187 –3702.01100 –3702.02220 –3702.03131 –3702.03627 6 1s22s22p2 3P2 –3700.55275 –3700.60034 –3700.62417 –3700.63477 –3700.64383 –3700.64792 7 1s22s22p2 1S0 –3699.20097 –3699.25462 –3699.28068 –3699.29246 –3699.30198 –3699.30638 8 1s22s2p3 3D2 –3695.86768 –3695.91436 –3695.93885 –3695.94773 –3695.95582 –3695.95981 9 1s22s2p3 3D3 –3694.68488 –3694.73762 –3694.76403 –3694.77380 –3694.78232 –3694.78666 10 1s22s2p3 3P0 –3693.45937 –3693.51678 –3693.54478 –3693.55576 –3693.56463 –3693.56911 11 1s22s2p3 3S1 –3692.98094 –3693.03801 –3693.06571 –3693.07645 –3693.08525 –3693.08974 12 1s22s2p3 1D2 –3692.60294 –3692.66348 –3692.69221 –3692.70334 –3692.71240 –3692.71708 13 1s22s2p3 3P1 –3692.45644 –3692.51749 –3692.54662 –3692.55827 –3692.56736 –3692.57210 14 1s22s2p3 $ {}^3{\text{P}}_{2}^{\text{b}} $
(1s22s2p3 3P2)–3684.80095 –3684.85516 –3684.88199 –3684.89234 –3684.90090 –3684.90516 15 1s22s2p3 1P1 –3682.88893 –3682.94955 –3682.97832 –3682.98984 –3682.99885 –3683.00340 表 3 Ge XXVII离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态在AS2—AS7活性空间的原子态能级
Table 3. Energies of atomic states for 1s22s22p2 and 1s22s2p3 configurations in Ge XXVII ion (Z = 10, 14, 32, 36, 50) within AS2–AS7 active spaces.
Lv. No. States EAS2/a.u. EAS3/a.u. EAS4/a.u. EAS5/a.u. EAS6/a.u. EAS7/a.u. 1 1s22s22p2 3P0 –1454.10079 –1454.14307 –1454.16034 –1454.16743 –1454.17263 –1454.17467 2 1s22s22p2 3P1 –1453.10195 –1453.14197 –1453.15849 –1453.16527 –1453.17032 –1453.17228 3 1s22s22p2 3P2 –1452.78799 –1452.83052 –1452.84779 –1452.85486 –1452.86009 –1452.86214 4 1s22s22p2 1D2 –1451.53539 –1451.57840 –1451.59571 –1451.60281 –1451.60804 –1451.61011 5 1s22s22p2 1S0 –1450.76903 –1450.81702 –1450.83602 –1450.84417 –1450.84969 –1450.85198 6 1s22s2p3 5S2 –1450.28531 –1450.31956 –1450.33517 –1450.34021 –1450.34444 –1450.34582 7 1s22s2p3 3D1 –1448.73688 –1448.78565 –1448.80540 –1448.81262 –1448.81780 –1448.81986 8 1s22s2p3 3D2 –1448.57279 –1448.61893 –1448.63791 –1448.64468 –1448.64969 –1448.65163 9 1s22s2p3 3D3 –1448.19745 –1448.24367 –1448.26262 –1448.26932 –1448.27433 –1448.27629 10 1s22s2p3 3P0 –1447.52469 –1447.57456 –1447.59476 –1447.60251 –1447.60777 –1447.60988 11 1s22s2p3 3P1 –1447.39793 –1447.44735 –1447.46733 –1447.47490 –1447.48012 –1447.48222 12 1s22s2p3 3P2 –1447.18261 –1447.23273 –1447.25287 –1447.26044 –1447.26570 –1447.26784 13 1s22s2p3 3S1 –1446.63584 –1446.69039 –1446.71173 –1446.72030 –1446.72580 –1446.72816 14 1s22s2p3 1D2 –1446.02983 –1446.08429 –1446.10553 –1446.11376 –1446.11928 –1446.12166 15 1s22s2p3 1P1 –1445.07774 –1445.13513 –1445.15726 –1445.16628 –1445.17197 –1445.17447 表 4 Kr XXXI离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态在AS2—AS7活性空间的原子态能级
Table 4. Energies of atomic states for 1s22s22p2 and 1s22s2p3 configurations in Kr XXXI ion (Z = 10, 14, 32, 36, 50) within AS2–AS7 active spaces.
Lv. No. States EAS2/a.u. EAS3/a.u. EAS4/a.u. EAS5/a.u. EAS6/a.u. EAS7/a.u. 1 1s22s22p2 3P0 –1861.56151 –1861.60560 –1861.62435 –1861.63204 –1861.63807 –1861.64038 2 1s22s22p2 3P1 –1859.75712 –1859.79831 –1859.81605 –1859.82334 –1859.82916 –1859.83136 3 1s22s22p2 1D2 –1859.37952 –1859.42371 –1859.44237 –1859.45002 –1859.45605 –1859.45838 4 1s22s22p2 3P2 –1857.30056 –1857.34410 –1857.36240 –1857.36994 –1857.37588 –1857.37817 5 1s22s22p2 1S0 –1856.41418 –1856.46294 –1856.48307 –1856.49168 –1856.49795 –1856.50049 6 1s22s2p3 5S2 –1856.21811 –1856.25577 –1856.27332 –1856.27915 –1856.28423 –1856.28593 7 1s22s2p3 3D1 –1854.57783 –1854.62889 –1854.65031 –1854.65819 –1854.66416 –1854.66649 8 1s22s2p3 3D2 –1854.02497 –1854.07072 –1854.09051 –1854.09748 –1854.10309 –1854.10518 9 1s22s2p3 3D3 –1853.42728 –1853.47463 –1853.49484 –1853.50197 –1853.50769 –1853.50987 10 1s22s2p3 3P0 –1852.64544 –1852.69654 –1852.71806 –1852.72627 –1852.73225 –1852.73458 11 1s22s2p3 3P1 –1852.43863 –1852.48938 –1852.51066 –1852.51867 –1852.52461 –1852.52694 12 1s22s2p3 ${}^1{\text{D}}_{2}^{\text{a}}$
(1s22s2p3 3P2)–1852.14350 –1852.19611 –1852.21786 –1852.22601 –1852.23208 –1852.23450 13 1s22s2p3 3S1 –1851.74091 –1851.79651 –1851.81920 –1851.82819 –1851.83442 –1851.83700 14 1s22s2p3 ${}^1{\text{D}}_{2}^{\text{b}}$
(1s22s2p3 1D2)–1850.47133 –1850.52479 –1850.54678 –1850.55518 –1850.56129 –1850.56378 15 1s22s2p3 1P1 –1849.27920 –1849.33674 –1849.35991 –1849.36921 –1849.37556 –1849.37824 表 6 Ne V离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态原子态的激发能
Table 6. Excitation energies of states for 1s22s22p2 and 1s22s2p3 configurations in Ne V ions (Z = 10, 14, 32, 36, 50).
Lv. No. State Ecal
/cm–1EMBPT[18]
/cm–1EHFR[27]
/cm–1ETFDA[28]
/cm–1EMCHF[31]
/cm–1EMCDHF1[22]
/cm–1EMCDHF2[23]
/cm–1ENIST[40]
/cm–1δ/% δ/cm–1 1 1s22s22p2 3P0 0 0 — — 0 0 — 0 0 0 2 1s22s22p2 3P1 411.82 410.6660 — — 413.52 411.4 412 411.227 0.1442 0.593 3 1s22s22p2 3P2 1111.74 1105.544 — — 1109.89 1108.6 1112 1109.467 0.2049 2.273 4 1s22s22p2 1D2 30394.93 29963.98 — — 30388.87 30428.1 — 30290.67 0.3442 104.26 5 1s22s22p2 1S0 64537.13 64660.65 — — 63932.41 64141.3 — 63915.4 0.9727 621.73 6 1s22s2p3 5S2 87979.45 89949.36 — — 89417.23 88176.6 87782 88399.5 0.4752 420.05 7 1s22s2p3 3D3 175793.08 175362.7 — — 177091.78 175906.6 — 175832.3 0.0223 39.22 8 1s22s2p3 3D2 175863.71 175431.2 — — 177161.79 175976.7 — 175902.7 0.0222 38.99 9 1s22s2p3 3D1 175887.35 175453.0 — — 177184.54 176000.3 — 175925.0 0.0214 37.65 10 1s22s2p3 3P2 208147.27 208283.8 — — 209479.97 208347.0 — 208151.3 0.0019 4.03 11 1s22s2p3 3P1 208151.98 208288.6 — — 209485.10 208351.9 — 208153.3 0.0006 1.32 12 1s22s2p3 3P0 208185.58 208321.7 — — 209519.18 208388.7 — 208185 0.0003 0.58 13 1s22s2p3 1D2 270878.89 269549.6 — — 271972.52 270855.6 — 270552.9 0.1205 325.99 14 1s22s2p3 3S1 279567.62 278211.2 — — 280689.94 279582.4 — 279371.2 0.0703 196.42 15 1s22s2p3 1P1 304246.43 302434.5 — — 305326.16 304289.6 — 303819.2 0.1406 427.23 表 10 Sn XLV离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态原子态的激发能
Table 10. Excitation energies of states for 1s22s22p2 and 1s22s2p3 configurations in Sn XLV ions (Z = 10, 14, 32, 36, 50).
Lv. No. State Ecal
/cm–1EMBPT[18]
/cm–1EHFR[27]
/cm–1ETFDA[28]
/cm–1EMCHF[31]
/cm–1EMCDHF1[22]
/cm–1EMCDHF2[23]
/cm–1ENIST[40]
/cm–1δ/% δ/cm–1 1 1s22s22p2 3P0 0 0 — — — — — — — — 2 1s22s22p2 3P1 1885654 1885837 — — — — 1885461 — — — 3 1s22s22p2 1D2 2004135 2004255 — — — — — — — — 4 1s22s2p3 $ {}^3{\text{P}}_{2}^{\text{a}} $
(1s22s2p3 5S2)3220891 3222265 — — — — 3220810 — — — 5 1s22s2p3 3D1 3674549 3675597 — — — — — — — — 6 1s22s22p2 3P2 3979255 3979184 — — — — 3979010 — — — 7 1s22s22p2 1S0 4273686 4273568 — — — — — — — — 8 1s22s2p3 3D2 5008169 5009638 — — — — — — — — 9 1s22s2p3 3D3 5265644 5266985 — — — — — — — — 10 1s22s2p3 3P0 5532862 5534116 — — — — — — — — 11 1s22s2p3 3S1 5638071 5639355 — — — — — — — — 12 1s22s2p3 1D2 5719867 5721043 — — — — — — — — 13 1s22s2p3 3P1 5751679 5752781 — — — — — — — — 14 1s22s2p3 $ {}^3{\text{P}}_{2}^{\text{b}} $
(1s22s2p3 3P2)7434367 7435689 — — — — — — — — 15 1s22s2p3 1P1 7851752 7852863 — — — — — — — — 表 7 Si IX离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态原子态的激发能
Table 7. Excitation energies of states for 1s22s22p2 and 1s22s2p3 configurations in Si IX ions (Z = 10, 14, 32, 36, 50).
Lv. No. State Ecal
/cm–1EMBPT[18]
/cm–1EHFR[27]
/cm–1ETFDA[28]
/cm–1EMCHF[31]
/cm–1EMCDHF1[22]
/cm–1EMCDHF2[23]
/cm–1ENIST[40]
/cm–1δ/% δ/cm–1 1 1s22s22p2 3P0 0 0 0 0 0 0 — 0 0 0 2 1s22s22p2 3P1 2472 2539.017 2533 2637 2582 2540 2473 2545.0 2.8684 73 3 1s22s22p2 3P2 6376 6404.025 6721 6753 6452 6411 6377 6414 0.5925 38 4 1s22s22p2 1D2 52987 52731.80 50726 56291 53076 53070 — 52925.9 0.1154 61.1 5 1s22s22p2 1S0 108352 108410.6 119027 132263 107826 108017 — 107799 0.5130 553 6 1s22s2p3 5S2 149841 151487.8 146529 128561 154077 150120 149624 150770 0.6162 929 7 1s22s2p3 3D3 292026 292093.6 286103 285832 296224 292323 — 292232 0.0705 206 8 1s22s2p3 3D2 292089 292151.7 285850 286020 296405 292384 — 292296 0.0708 207 9 1s22s2p3 3D1 292231 292290.4 285798 285866 296274 292525 — 292441 0.0718 210 10 1s22s2p3 3P1 343838 344277.7 332017 337703 348047 344313 — 344009 0.0497 171 11 1s22s2p3 3P0 343895 344330.1 332217 337800 348157 344202 — 344075 0.0523 180 12 1s22s2p3 3P2 343941 344390.6 331907 337756 348103 344256 — 344118 0.0514 177 13 1s22s2p3 1D2 440482 439823.7 431290 449098 444583 440751 — 440403 0.0179 79 14 1s22s2p3 3S1 446940 446248.8 436901 454797 451039 447194 — 446942 0.0004 2 15 1s22s2p3 1P1 492902 491959.4 477444 500967 497004 493218 — 492755 0.0298 147 表 8 Ge XXVII离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态原子态的激发能
Table 8. Excitation energies of states for 1s22s22p2 and 1s22s2p3 configurations in Ge XXVII ions (Z = 10, 14, 32, 36, 50).
Lv. No. State Ecal
/cm–1EMBPT[18]
/cm–1EHFR[27]
/cm–1ETFDA[28]
/cm–1EMCHF[31]
/cm–1EMCDHF1[22]
/cm–1EMCDHF2[23]
/cm–1ENIST[40]
/cm–1δ/% δ/cm–1 1 1s22s22p2 3P0 0 0 — — — — — 0 0 0 2 1s22s22p2 3P1 219998 220040.9 — — — — 219953 219880 0.0537 118 3 1s22s22p2 3P2 288064 288046.4 — — — — 288122 287736 0.1140 328 4 1s22s22p2 1D2 562851 562703.1 — — — — — — — — 5 1s22s22p2 1S0 729240 729004.9 — — — — — — — — 6 1s22s2p3 5S2 840329 841558.8 — — — — 840075 — — — 7 1s22s2p3 3D1 1175235 1175835 — — — — — — — — 8 1s22s2p3 3D2 1212157 1212816 — — — — — — — — 9 1s22s2p3 3D3 1294534 1295181 — — — — — — — — 10 1s22s2p3 3P0 1440794 1441490 — — — — — — — — 11 1s22s2p3 3P1 1468812 1469518 — — — — — — — — 12 1s22s2p3 3P2 1515861 1516612 — — — — — 1516690 0.0547 829 13 1s22s2p3 3S1 1634306 1634571 — — — — — 1633620 0.0420 686 14 1s22s2p3 1D2 1767417 1767693 — — — — — — — — 15 1s22s2p3 1P1 1975301 1975518 — — — — — — — — 表 9 Kr XXXI离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态原子态的激发能
Table 9. Excitation energies of states for 1s22s22p2 and 1s22s2p3 configurations in Kr XXXI ions (Z = 10, 14, 32, 36, 50).
Lv. No. State Ecal
/cm–1EMBPT[18]
/cm–1EHFR[27]
/cm–1ETFDA[28]
/cm–1EMCHF[31]
/cm–1EMCDHF1[22]
/cm–1EMCDHF2[23]
/cm–1ENIST[40]
/cm–1δ/% δ/cm–1 1 1s22s22p2 3P0 0 0 — — — — 0 0 0 2 1s22s22p2 3P1 397033 397090.1 — — — — 396962 396820 0.0537 213 3 1s22s22p2 1D2 478893 478885.2 — — — — — — — — 4 1s22s22p2 3P2 935443 935288.5 — — — — 478950 478200 95.6175 457243 5 1s22s22p2 1S0 1128073 1127837 — — — — — — — — 6 1s22s2p3 5S2 1175162 1176400 — — — — 1174960 — — — 7 1s22s2p3 3D1 1530587 1531276 — — — — — 1530200 0.0253 387 8 1s22s2p3 3D2 1653780 1654590 — — — — — 1653800 0.0012 20 9 1s22s2p3 3D3 1784435 1785211 — — — — — 1783500 0.0524 935 10 1s22s2p3 3P0 1954591 1955386 — — — — — 1955900 0.0669 1309 11 1s22s2p3 3P1 2000162 2000973 — — — — — 1999100 0.0531 1062 12 1s22s2p3 ${}^1{\text{D}}_{2}^{\text{a}}$
(1s22s2p3 3P2)2064345 2065150 — — — — — 2062900 0.0700 1445 13 1s22s2p3 3S1 2151587 2152024 — — — — — 2151900 0.0145 313 14 1s22s2p3 ${}^1{\text{D}}_{2}^{\text{b}}$
(1s22s2p3 1D2)2431025 2431535 — — — — — — — — 15 1s22s2p3 1P1 2691220 2691625 — — — — — — — — 表 11 Ne V离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态的混合系数和寿命
Table 11. Mixing coefficients and lifetimes of 1s22s22p2 and 1s22s2p3 configurations in Ne V ion with Z = 10, 14, 32, 36, 50.
Lv. No. State LS-composition τl/ns τv/ns τ[17]/ns τ[20]/ns τ[41]/ns 1 1s22s22p2 3P0 0.98 — — — — — 2 1s22s22p2 3P1 0.98 — — 7.8711 — — 3 1s22s22p2 3P2 0.98 — — 2.2011 — — 4 1s22s22p2 1D2 0.97 — — 1.999 — — 5 1s22s22p2 1S0 0.94+0.05 2p4(${}_0^1{\text{S}}$)1S — — 1.438 — — 6 1s22s2p3 5S2 1 1.225 7.944 1.155 — — 7 1s22s2p3 3D3 0.99 8.71–1 8.70–1 8.70–1 — — 8 1s22s2p3 3D2 0.99 8.60–1 8.61–1 8.59–1 — 1.08 9 1s22s2p3 3D1 0.99 8.53–1 8.55–1 8.52–1 — — 10 1s22s2p3 3P2 0.99 3.22–1 3.20–1 3.15–1 — 3.80–1 11 1s22s2p3 3P1 0.99 3.20–1 3.18–1 3.14–1 — — 12 1s22s2p3 3P0 0.99 3.19–1 3.18–1 3.13–1 — — 13 1s22s2p3 1D2 0.98 1.03–1 1.05–1 1.04–1 — 1.44–1 14 1s22s2p3 3S1 0.98 4.59–2 4.71–2 4.59–2 — 6.10–2 15 1s22s2p3 1P1 0.98 6.09–2 6.20–2 6.01–2 — 8.90–2 注: 表中a×10b表示为ab. 表 15 Sn XLV离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态的混合系数和寿命
Table 15. Mixing coefficients and lifetimes of 1s22s22p2 and 1s22s2p3 configurations in Sn XLV ion with Z = 10, 14, 32, 36, 50.
Lv. No. State LS-composition τl/ns τv/ns τ[17]/ns τ[20]/ns τ[41]/ns 1 1s22s22p2 3P0 0.72+0.28 2s2 2p2(${}_0^1{\text{S}}$) 1S — — — — — 2 1s22s22p2 3P1 1 — — — — — 3 1s22s22p2 1D2 0.61+0.38 2s22p2(${}_2^3{\text{P}}$) 3P — — — — — 4 1s22s2p3 $ {}^3{\text{P}}_{2}^{\text{a}} $
(1s22s2p3 5S2)0.43+0.38 2s 2S 2p3(${}_3^4{\text{P}}$) 5S) p+0.38 22S 2p3(${}_3^2{\text{D}}$) 3D) 9.11–2 8.31–2 — — — 5 1s22s2p3 3D1 0.39+0.24 2s 2S 2p3(${}_1^2{\text{P}}$) 1P) p+0.24 22S 2p3(${}_1^2{\text{P}}$) 3P) 2.08–3 2.07–3 — — — 6 1s22s22p2 3P2 0.61+0.38 2s2 2p2(${}_2^1{\text{D}}$) 1D — — — 7 1s22s22p2 1S0 0.70+0.28 2s2 2p2(${}_2^3{\text{P}}$) 3P — — — 8 1s22s2p3 3D2 0.49+0.45 2s 2S 2p3(${}_3^4{\text{S}}$) 5S) p+0.45 22S 2p3(${}_3^2{\text{D}}$) 1D) 9.44–3 9.33–3 — — — 9 1s22s2p3 3D3 1 1.01–2 1.00–2 — — — 10 1s22s2p3 3P0 1 2.51–3 2.50–3 — — — 11 1s22s2p3 3S1 0.40+0.39 2s 2S 2p3(${}_3^2{\text{D}}$) 3D) p+0.39 22S 2p3(${}_1^2{\text{P}}$) 3P) 1.37–3 1.37–3 — — — 12 1s22s2p3 1D2 0.71+0.22 2s 2S 2p3(${}_3^2{\text{D}}$) 3D) p+0.22 22S 2p3(${}_3^4{\text{S}}$) 5S) 1.36–3 1.35–3 — — — 13 1s22s2p3 3P1 0.48+0.31 2s 2S 2p3(${}_1^2{\text{P}}$) 1P) p+0.31 22S 2p3(${}_3^4{\text{S}}$) 3S) 1.56–3 1.56–3 — — — 14 1s22s2p3 $ {}^3{\text{P}}_{2}^{\text{b}} $
(1s22s2p3 3P2)0.51+0.21 2s 2S 2p3(${}_3^2{\text{D}}$) 1D) p+0.21 22S 2p3(${}_3^2{\text{D}}$) 3D) 2.52–3 2.51–3 — — — 15 1s22s2p3 1P1 0.43+0.27 2s 2S 2p3(${}_3^4{\text{S}}$) 3S) p+0.27 22S 2p3(${}_3^2{\text{D}}$) 3D) 9.42–4 9.40–4 — — — 注: 表中a×10b表示为ab. 表 12 Si IX离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态的混合系数和寿命
Table 12. Mixing coefficients and lifetimes of 1s22s22p2 and 1s22s2p3 configurations in Si IX ion with Z = 10, 14, 32, 36, 50.
Lv. No. State LS-composition τl/ns τv/ns τ[17]/ns τ[20]/ns τ[41]/ns 1 1s22s22p2 3P0 0.98 — — — — — 2 1s22s22p2 3P1 0.98 — — — — — 3 1s22s22p2 3P2 0.98 — — — — — 4 1s22s22p2 1D2 0.98 — — — — — 5 1s22s22p2 1S0 0.95 + 0.05 2p4(${}_0^1{\text{S}}$)1S — — — — — 6 1s22s2p3 5S2 1 4.773 3.653 — — — 7 1s22s2p3 3D3 0.99 4.42–1 4.43–1 — — — 8 1s22s2p3 3D2 0.99 4.23–1 4.26–1 — — — 9 1s22s2p3 3D1 0.99 4.12–1 4.15–1 — — — 10 1s22s2p3 3P1 0.99 1.66–1 1.66–1 — — — 11 1s22s2p3 3P0 1 1.65–1 1.64–1 — — — 12 1s22s2p3 3P2 0.99 1.70–1 1.69–1 — — — 13 1s22s2p3 1D2 0.99 5.59–2 5.66–2 — — — 14 1s22s2p3 3S1 0.99 2.71–2 2.75–2 — — — 15 1s22s2p3 1P1 0.99 3.48–2 3.51–2 — — — 注: 表中a×10b表示为ab. 表 13 Ge XXVII离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态的混合系数和寿命
Table 13. Mixing coefficients and lifetimes of 1s22s22p2 and 1s22s2p3 configurations in Ge XXVII ion with Z = 10, 14, 32, 36, 50.
Lv. No. State LS-composition τl/ns τv/ns τ[17]/ns τ[20]/ns τ[41]/ns 1 1s22s22p2 3P0 0.82 + 0.17 2s2 2p2(${}_0^1{\text{S}}$) 1S — — — — — 2 1s22s22p2 3P1 0.99 — — — — — 3 1s22s22p2 3P2 0.56 + 0.43 2s2 2p2(${}_2^1{\text{D}}$) 1D — — — — — 4 1s22s22p2 1D2 0.56 + 0.43 2s2 2p2(${}_2^3{\text{P}}$) 3P — — — — — 5 1s22s22p2 1S0 0.80 + 0.17 2s2 2p2(${}_2^3{\text{P}}$) 3P+0.03 2p4(${}_0^1{\text{S}}$) 1S — — — — — 6 1s22s2p3 5S2 0.86 + 0.12 2s 2S 2p3(${}_1^2{\text{P}}$) 3P) 1.67 1.56 — — — 7 1s22s2p3 3D1 0.68 + 0.22 2s 2S 2p3(${}_1^2{\text{P}}$) 3P) p + 0.222S 2p3(${}_1^2{\text{P}}$) 1P) 3.03–2 3.03–2 — — — 8 1s22s2p3 3D2 0.70 + 0.20 2s 2S 2p3(${}_1^2{\text{P}}$) 3P) p + 0.202S 2p3(${}_3^4{\text{S}}$) 5S) 6.20–2 6.14–2 — — — 9 1s22s2p3 3D3 1 8.34–2 8.24–2 — — — 10 1s22s2p3 3P0 1 2.39–2 2.39–2 — — — 11 1s22s2p3 3P1 0.63 + 0.24 2s 2S 2p3(${}_3^2{\text{D}}$) 3D) p + 0.242S 2p3(${}_3^4{\text{S}}$) 3S) 1.91–2 1.91–2 — — — 12 1s22s2p3 3P2 0.43 + 0.28 2s 2S 2p3(${}_3^2{\text{D}}$) 3D) p + 0.s 2S 2p3(${}_3^2{\text{D}}$) 3D) 1.91–2 1.91–2 — — — 13 1s22s2p3 3S1 0.60 + 0.26 2s 2S 2p3(${}_1^2{\text{P}}$) 1P) p + 0.262S 2p3(${}_1^2{\text{P}}$) 3P) 6.95–3 6.95–3 — — — 14 1s22s2p3 1D2 0.70 + 0.25 2s 2S 2p3(${}_1^2{\text{P}}$) 3P) p + 0.252S 2p3(${}_3^2{\text{D}}$) 3D) 1.26–2 1.26–2 — — — 15 1s22s2p3 1P1 0.66 + 0.25 2s 2S 2p3(${}_3^4{\text{S}}$) 3S) p + 0.252S 2p3(${}_3^2{\text{D}}$) 3D) 6.61–3 6.61–3 — — — 注: 表中a×10b表示为ab. 表 14 Kr XXXI离子(Z = 10, 14, 32, 36, 50)1s22s22p2和1s22s2p3组态的混合系数和寿命
Table 14. Mixing coefficients and lifetimes of 1s22s22p2 and 1s22s2p3 configurations in Kr XXXI ion with Z = 10, 14, 32, 36, 50.
Lv. No. State LS-composition τl/ns τv/ns τ[17]/ns τ[20]/ns τ[41]/ns 1 1s22s22p2 3P0 0.79 + 0.20 2s2 2p2(${}_0^1{\text{S}}$) 1S — — — — 2 1s22s22p2 3P1 0.99 — — — 1.1443 — 3 1s22s22p2 1D2 0.51 + 0.49 2s2 2p2(${}_2^3{\text{P}}$) 3P — — — 2.4135 — 4 1s22s22p2 3P2 0.50 + 0.49 2s2 2p2(${}_2^1{\text{D}}$) 1D — — — 4.9962 — 5 1s22s22p2 1S0 0.77 + 0.21 2s22p2(${}_2^3{\text{P}}$) 3P+0.02 2p4(${}_0^1{\text{S}}$) 1S — — — 2.3002 — 6 1s22s2p3 5S2 0.73 + 0.21 2s 2S2p3(${}_1^2{\text{P}}$) 3P) 3 +
0.212S 2p3(${}_3^2{\text{D}}$) 3D)5.57–1 5.24–1 — 5.797–1 — 7 1s22s2p3 3D1 0.58 + 0.24 2s 2S 2p3(${}_1^2{\text{P}}$) 3P) p +
0.242S 2p3(${}_1^2{\text{P}}$) 1P)1.60–2 1.60–2 — 1.569–2 — 8 1s22s2p3 3D2 0.63 + 0.19 2s 2S 2p3(${}_3^4{\text{S}}$) 5S) p +
0.192S 2p3(${}_1^2{\text{P}}$) 3P)4.40–2 4.36–2 — 4.246–2 — 9 1s22s2p3 3D3 1 5.64–2 5.56–2 — 5.481–2 — 10 1s22s2p3 3P0 1 1.51–2 1.51–2 — 1.461–2 — 11 1s22s2p3 3P1 0.51 + 0.30 2s 2S 2p3(${}_3^2{\text{D}}$) 3D) p +
0.302S 2p3(${}_3^4{\text{S}}$) 3S)1.07–2 1.07–2 — 1.048–2 — 12 1s22s2p3 ${}^1{\text{D}}_{2}^{\text{a}}$
(1s22s2p3 3P2)0.46 + 0.25 2s 2S 2p3(${}_3^2{\text{D}}$) 3D) p +
0.252S 2p3(${}_1^2{\text{P}}$) 3P)9.95–3 9.94–3 — 9.784–3 — 13 1s22s2p3 3S1 0.48 + 0.29 2s 2S 2p3(${}_1^2{\text{P}}$) 1P) p +
0.292S 2p3(${}_1^2{\text{P}}$) 3P)5.31–3 5.30–3 — 5.100–3 — 14 1s22s2p3 ${}^1{\text{D}}_{2}^{\text{b}}$
(1s22s2p3 1D2)0.51 + 0.38 2s 2S 2p3(${}_1^2{\text{P}}$) 3P) p +
0.382S 2p3(${}_3^2{\text{D}}$) 3D)1.01–2 1.00–2 — 9.607–3 — 15 1s22s2p3 1P1 0.58 + 0.28 2s 2S 2p3(${}_3^4{\text{S}}$) 3S) p +
0.282S 2p3(${}_3^2{\text{D}}$) 3D)4.52–3 4.52–3 — 4.369–3 — 注: 表中a×10b表示为ab. 表 16 Ne V离子(Z = 10, 14, 32, 36, 50)1s22s2p3-1s22s22p2间E1跃迁谱线波长、跃迁速率、加权振子强度
Table 16. The E1 transition wavelength, rate, weighted oscillator strength, and line strength between the 1s22s2p3 and 1s22s22p2 configurations in Ne V ions with Z = 10, 14, 32, 36, 50.
Transition A—B λ/Å AC/s–1 AB/s–1 gfC gfB SC/a.u. SB/a.u. AC/AB D1—3P0 568.53 6.7218 6.7298 9.770–2 9.782–2 1.829–1 1.831–1 1.00 — 6.6208[22] 6.6158[22] — — — — — 568.424[40] — 7.708[40] — — — 2.10–1[40] — 3P1—3P0 480.40 1.0329 1.0299 1.072–1 1.068–1 1.695–1 1.689–1 1.00 — 1.0279[22] 1.0319[22] — — — — — 480.415[40] — 1.399[40] — — — 2.10–1[40] — 3S1—3P0 357.68 2.3479 2.4109 1.351–1 1.387–1 1.590–1 1.633–1 0.97 — 2.3789[22] 2.3859[22] — — — — — 357.947[40] — 2.509[40] — — — 1.70–1[40] — 1P1—3P0 328.67 8.7564 8.5104 4.254–6 4.135–6 4.603–6 4.474–6 1.03 — 9.0874[22] 8.4884[22] — — — — —
3D1—1S0898.04 1.7794 1.6794 6.452–6 6.090–6 1.908–5 1.801–5 1.06 — 1.7584[22] 1.7304[22] — — — — — 3P1—1S0 696.29 1.0835 9.2444 2.361–5 2.016–5 5.411–5 4.620–5 1.17 — 1.1145[22] 9.4744[22] — — — — — 3S1—1S0 465.04 4.0825 4.1365 3.971–5 4.023–5 6.079–5 6.159–5 0.99 — 3.9415[22] 3.9085[22] — — — — — 1P1—1S0 417.16 3.0809 3.1369 2.411–1 2.455–1 3.311–1 3.371–1 0.98 416.834[40] 3.0719[22] 3.0649[22] — — — — — 3P0—3P1 481.28 3.1449 3.1349 1.092–1 1.088–1 1.730–1 1.725–1 1.00 — 3.1249[22] 3.1409[22] — — — — — 481.293[40] — 4.009[40] — — — 2.20–1[40] — 3D1—3P1 569.86 4.7008 4.7118 6.864–2 6.881–2 1.288–1 1.291–1 1.00 — 4.6298[22] 4.6308[22] — — — — — 569.756[40] — 5.808[40] — — — 1.60–1[40] — 3P1—3P1 481.36 8.2898 8.2648 8.638–2 8.612–2 1.369–1 1.365–1 1.00 — 8.2388[22] 8.2758[22] — — — — — 481.366[40] — 1.009[40] — — — 1.70–1[40] — 3S1—3P1 358.21 7.0479 7.2359 4.067–1 4.175–1 4.796–1 4.924–1 0.97 — 7.1429[22] 7.1599[22] — — — — — 358.474[40] — 7.309[40] — — — 5.00–1[40] — 1P1—3P1 329.12 4.7316 4.9086 2.305–4 2.391–4 2.497–4 2.591–4 0.96 — 4.7936[22] 4.8516[22] — — — — — 5S2—3P1 1141.94 3.5183 2.3293 3.439–6 2.277–6 1.293–5 8.558–6 1.51 — 3.4853[22] 2.3403[22] — — — — — 1136.515[40] — — — — — — — 3D2—3P1 569.94 9.0008 9.0028 2.191–1 2.192–1 4.112–1 4.113–1 1.00 — 8.8668[22] 8.8508[22] — — — — — 569.828[40] — 1.009[40] — — — 4.60–1[40] — 3P2—3P1 481.37 7.4148 7.3798 1.288–1 1.282–1 2.041–1 2.031–1 1.00 — 7.3738[22] 7.3978[22] — — — — — 481.371[40] — 1.009[40] — — — 2.80–1[40] — 1D2—3P1 369.72 1.1735 1.2745 1.202–5 1.306–5 1.463–5 1.589–5 0.92 — 1.1595[22] 1.2735[22] — — — — — 3D1—3P2 572.15 2.7367 2.7507 4.029–3 4.049–3 7.589–3 7.626–3 1.00 — 2.6947[22] 2.7007[22] — — — — — 3P1—3P2 482.98 1.2739 1.2709 1.336–1 1.332–1 2.124–1 2.118–1 1.00 — 1.2659[22] 1.2729[22] — — — — — 482.990[40] — 1.709[40] — — — 2.80–1[40] — 3S1—3P2 359.11 1.18010 1.21110 6.842–1 7.021–1 8.089–1 8.301–1 0.97 — 1.19610[22] 1.19810[22] — — — — — 359.374[40] — 1.2010[40] — — — 8.25–1[40] — 1P1—3P2 329.88 5.0775 5.5065 2.485–5 2.695–5 2.699–5 2.927–5 0.92 — 1.2659[22] 1.2729[22] — — — — — 3D1—1D2 687.30 3.1894 2.3384 6.776–6 4.966–6 1.533–5 1.124–5 1.36 — 3.3774[22] 2.3984[22] — — — — — 3P1—1D2 562.55 2.9235 2.7955 4.161–5 3.979–5 7.706–5 7.368–5 1.05 — 2.8875[22] 2.7895[22] — — — — — 3S1—1D2 401.32 6.6595 6.5885 4.823–5 4.772–5 6.372–5 6.304–5 1.01 — 6.7655[22] 6.8915[22] — — — — — 1P1—1D2 365.15 1.30310 1.32610 7.815–1 7.951–1 9.394–1 9.558–1 0.98 — 1.32010[22] 1.33010[22] — — — — — 365.593[40] — 1.3510[40] — — — 9.77–1[40] — 5S2—3P2 1151.14 9.0753 5.8393 9.014–6 5.800–6 3.416–5 2.198–5 1.55 1145.606[40] 9.2073[22] 5.8773[22] — — — — — 3D2—3P2 572.22 2.6138 2.6208 6.413–2 6.431–2 1.208–1 1.212–1 1.00 — 2.5738[22] 2.5748[22] — — — — — 572.105[40] — 3.508[40] — — — 1.60–1[40] — 3P2—3P2 482.99 2.3769 2.3689 4.155–1 4.140–1 6.607–1 6.584–1 1.00 — 2.3619[22] 2.3729[22] — — — — — 482.994[40] — 3.009[40] — — — 8.30–1[40] — 1D2—3P2 370.68 2.6736 2.8156 2.753–4 2.899–4 3.360–4 3.538–4 0.95 — 2.7336[22] 2.7336[22] — — — — — 5S2—1D2 1736.53 6.487–1 3.006–1 1.466–9 6.796–10 8.383–9 3.885–9 2.16 — 9.084–1[22] 3.080–1[22] — — — — — 3D2—1D2 687.41 5.1194 4.6994 1.813–5 1.664–5 4.103–5 3.766–5 1.09 — 5.0484[22] 4.5634[22] — — — — — 3P2—1D2 562.56 4.7204 4.8524 1.120–5 1.151–5 2.074–5 2.132–5 0.97 — 4.2144[22] 4.8644[22] — — — — — 1D2—1D2 415.82 9.4719 9.6719 1.228 1.253 1.680 1.716 0.98 — 9.5079[22] 9.5239[22] — — — — — 416.212[40] — 1.1010[40] — — — 1.96[40] — 3D3—3P2 572.45 1.1489 1.1489 3.949–1 3.946–1 7.442–1 7.437–1 1.00 — 1.1319[22] 1.1289[22] — — — — — 572.335[40] — 1.409[40] — — — 9.10–1[40] — 3D3—1D2 687.75 2.4225 2.2425 1.202–4 1.113–4 2.722–4 2.520–4 1.08 — 2.3925[22] 2.1825[22] — — — — — 注: 表中A, B分别为1s22s2p3和1s22s22p2组态; a×10b表示为ab. 表 20 Sn XLV离子(Z = 10, 14, 32, 36, 50)1s22s2p3-1s22s22p2间E1跃迁谱线波长、跃迁速率、加权振子强度
Table 20. The E1 transition wavelength, rate, weighted oscillator strength, and line strength between the 1s22s2p3 and 1s22s22p2 configurations in Sn XLV ions with Z = 10, 14, 32, 36, 50.
Transition A—B λ/Å AC/s–1 AB/s–1 gfC gfB SC/a.u. SB/a.u. AC/AB 3D1—3P0 27.21 4.58911 4.57211 1.529–1 1.523–1 1.370–2 1.364–2 1.00 3S1—3P0 17.74 1.64610 1.64810 2.329–3 2.332–3 1.360–4 1.362–4 1.00 3P1—3P0 17.39 8.0239 8.0429 1.091–3 1.093–3 6.243–5 6.258–5 1.00 1P1—3P0 12.74 1.5027 1.5207 1.096–6 1.109–6 4.595–8 4.650–8 0.99 3D1—1S0 166.91 6.3747 7.3987 2.662–4 3.090–4 1.463–4 1.698–4 0.86 3S1—1S0 73.29 9.9528 8.8788 2.404–3 2.145–3 5.802–4 5.175–4 1.12 3P1—1S0 67.66 9.0119 8.2659 1.855–2 1.702–2 4.133–3 3.790–3 1.09 1P1—1S0 27.95 2.27711 2.26211 7.999–2 7.945–2 7.360–3 7.310–3 1.01 3P0—3P1 27.42 3.99211 3.98411 4.499–2 4.490–2 4.061–3 4.053–3 1.00 3D1—3P1 55.90 3.9669 3.7039 5.574–3 5.204–3 1.026–3 9.578–4 1.07 3S1—3P1 26.65 6.92211 6.90211 2.211–1 2.205–1 1.940–2 1.934–2 1.00 3P1—3P1 25.87 1.94310 1.92810 5.845–3 5.801–3 4.978–4 4.940–4 1.01 1P1—3P1 16.76 2.73810 2.74610 3.460–3 3.469–3 1.909–4 1.914–4 1.00 5S2—3P1 74.89 7.6479 7.0119 3.215–2 2.948–2 7.927–3 7.268–3 1.09 3D2—3P1 32.03 1.03411 1.02311 7.951–2 7.864–2 8.383–3 8.291–3 1.01 1D2—3P1 26.08 3.70610 3.70910 1.890–2 1.891–2 1.623–3 1.624–3 1.00 3P2—3P1 18.02 2.7659 2.7719 6.731–4 6.747–4 3.993–5 4.003–5 1.00 3D1—1D2 59.86 1.90710 1.78610 3.074–2 2.878–2 6.059–3 5.673–3 1.07 3S1—1D2 27.52 1.06910 1.05510 3.641–3 3.593–3 3.298–4 3.255–4 1.01 3P1—1D2 26.68 5.99911 5.98211 1.921–1 1.916–1 1.688–2 1.683–2 1.00 1P1—1D2 17.10 4.1919 4.2079 5.513–4 5.534–4 3.104–5 3.115–5 1.00 3D1—3P2 328.18 3.2766 4.3876 2.645–4 3.542–4 2.858–4 3.826–4 0.75 3S1—3P2 60.28 7.7769 7.1419 1.271–2 1.167–2 2.522–3 2.316–3 1.09 3P1—3P2 56.42 4.0529 3.8089 5.801–3 5.452–3 1.077–3 1.013–3 1.06 1P1—3P2 25.82 8.04511 8.03211 2.413–1 2.409–1 2.051–2 2.048–2 1.00 5S2—1D2 82.19 4.3289 3.9079 2.191–2 1.978–2 5.929–3 5.352–3 1.11 3D2—1D2 33.29 2.9829 2.9209 2.477–3 2.425–3 2.715–4 2.658–4 1.02 1D2—1D2 26.91 6.88911 6.86611 3.740–1 3.728–1 3.314–2 3.303–2 1.00 3P2—1D2 18.42 7.7389 7.7549 1.967–3 1.971–3 1.193–4 1.195–4 1.00 5S2—3P2 131.86 4.7977 5.4727 6.252–4 7.133–4 2.714–4 3.096–4 0.88 3D2—3P2 97.19 7.2928 6.2768 5.163–3 4.443–3 1.652–3 1.422–3 1.16 1D2—3P2 57.45 1.10210 1.02410 2.725–2 2.534–2 5.155–3 4.793–3 1.08 3P2—3P2 28.94 3.87511 3.85011 2.433–1 2.418–1 2.318–2 2.303–2 1.01 3D3—1D2 30.66 9.15210 9.06810 9.028–2 8.946–2 9.113–3 9.030–3 1.01 3D3—3P2 77.74 8.1689 7.3709 5.180–2 4.674–2 1.326–2 1.196–2 1.11 注: 表中A, B分别为1s22s2p3和1s22s22p2组态; a×10b表示为ab. 表 17 Si IX离子(Z = 10, 14, 32, 36, 50)1s22s2p3-1s22s22p2间E1跃迁谱线波长、跃迁速率、加权振子强度
Table 17. The E1 transition wavelength, rate, weighted oscillator strength, and line strength between the 1s22s2p3 and 1s22s22p2 configurations in Si IX ions with Z = 10, 14, 32, 36, 50.
Transition A—B λ/Å AC/s–1 AB/s–1 gfC gfB SC/a.u. SB/a.u. AC/AB 3D1—3P0 342.19 1.4939 1.5029 7.861–2 7.910–2 8.855–2 8.911–2 0.99 — 1.4949[22] 1.4949[22] — — — — — 342.36[26] — 1.4689[26] — — — 8.724–2[26] — 341.949[40] — 1.499[40] — — — 8.81–2[40] — 3P1—3P0 290.83 1.9169 1.9079 7.29–2 7.255–2 6.980–2 6.947–2 1.00 — 1.9179[22] 1.9199[22] — 6.35–2[27] — — — 290.93[26] — 1.8859[26] — 6.27–2[28] — 6.871–2[26] — 290.69[40] — 1.949[40] — 7.37–2[31] — 7.05–2[40] — 3S1—3P0 223.74 3.9759 4.0379 8.950–2 9.089–2 6.592–2 6.695–2 0.98 — 4.0089[22] 4.0159[22] — 1.22–1[27] — — — 223.93[26] — 3.9549[26] — 1.23–1[28] — 6.574–2[26] — 223.743[40] — 4.049[40] — 9.12–2[31] — 6.70–2[40] — 1P1—3P0 202.88 4.4955 4.1145 8.320–6 7.616–6 5.557–6 5.086–6 1.09 — 4.3005[22] 3.9415[22] — — — — — 203.22[26] — 4.4775[26] — — — 5.563–6[26] — 202.941[40] — 3.525[40] — — — 4.36–6[40] — 3D1—1S0 543.82 4.0795 3.9575 5.426–5 5.263–5 9.714–5 9.423–5 1.03 — 4.0795[22] 4.0295[22] — — — — — 542.99[26] — 3.9445[26] — — — 9.349–5[26] — 541.589[40] — 3.645[40] — — — 8.56–5[40] — 3P1—1S0 424.65 2.1906 1.9776 1.776–4 1.603–4 2.483–4 2.242–4 1.11 — 2.2166[22] 2.0056[22] — — — — — 424.08[26] — 1.9706[26] — — — 2.225–4[26] — 423.352[40] — 1.876[40] — — — 2.10–4[40] — 3S1—1S0 295.34 5.9576 6.0536 2.337–4 2.375–4 2.272–4 2.309–4 0.98 — 5.8746[22] 5.8286[22] — — — — — 295.3[26] — 6.0306[26] — — — 2.299–4[26] — 294.861[40] — 5.156[40] — — — 1.95–4[40] — 1P1—1S0 260.04 5.8019 5.8899 1.764–1 1.791–1 1.510–1 1.533–1 0.99 — 5.8399[22] 5.8299[22] — 2.96–1[27] — — — 260.31[26] — 5.7229[26] — 2.92–1[28] — 1.494–1[26] — 259.770[40] — 5.809[40] — 1.79–1[31] — 1.51–1[40] — 3P0—3P1 292.89 6.0699 6.0549 7.804–2 7.785–2 7.525–2 7.506–2 1.00 — 6.0649[22] 6.0839[22] — 7.01–2[27] — — — 292.99[26] — 1.1969[26] — 6.89–2[28] — 7.422–2[26] — 292.800[40] — 6.079[40] — 7.90–2[31] — 7.53–2[40] — 3D1—3P1 345.11 8.7448 8.8308 4.684–2 4.730–2 5.321–2 5.374–2 0.99 — 8.7448[22] 8.7668[22] — — — — — 345.37[26] — 8.6068[26] — — — 5.249–2[26] — 344.951[40] — 8.778[40] — — — 5.33–2[40] — 3P1—3P1 292.94 1.8359 1.8329 7.084–2 7.069–2 6.831–2 6.817–2 1.00 — 1.8349[22] 1.8389[22] — 6.71–2[27] — — — 293.1[26] — 1.8069[26] — 6.51–2[28] — 6.733–2[26] — 292.857[40] — 1.849[40] — 7.16–2[31] — 6.85–2[40] — 3S1—3P1 224.98 1.19410 1.21210 2.719–1 2.760–1 2.014–1 2.044–1 0.99 — 1.20410[22] 1.20610[22] — 3.70–1[27] — — — 225.21[26] — 1.18710[26] — 3.72–1[28] — 2.008–1[26] — 225.024[40] — 1.2110[40] — 2.77–1[31] — 2.05–1[40] — 1P1—3P1 203.90 7.2337 7.3967 1.352–3 1.383–3 9.078–4 9.284–4 0.98 — 7.2367[22] 7.2987[22] — — — — — 204.27[26] — 7.3717[26] — — — 9.302–4[26] — 203.994[40] — 6.707[40] — — — 8.43–4[40] — 5S2—3P1 678.56 7.9144 6.1994 2.731–5 2.139–5 6.102–5 4.779–5 1.28 — 8.0524[22] 6.3304[22] — — — — — 673.72[26] — 6.4814[26] — — — 4.891–5[26] — 674.65[40] — 6.194[40] — — — 4.69–5[40] — 3D2—3P1 345.28 1.9509 1.9599 1.743–1 1.751–1 1.981–1 1.990–1 1.00 — 1.9519[22] 1.9479[22] — — — — — 345.5[26] — 1.9149[26] — — — 1.948–1[26] — 345.124[40] — 1.949[40] — — — 1.97–1[40] — 3P2—3P1 292.85 1.2199 1.2109 7.834–2 7.778–2 7.553–2 7.499–2 1.01 — 1.2199[22] 1.2199[22] — 6.47–2[27] — — — 293.04[26] — 5.9749[26] — 6.46–2[28] — 7.420–2[26] — 292.763[40] — 1.239[40] — 7.91–2[31] — 7.64–2[40] — 1D2—3P1 228.30 2.8346 2.9586 1.107–4 1.156–4 8.323–5 8.685–5 0.96 — 2.8206[22] 2.9646[22] — — — — — 228.73[26] — 2.9476[26] — — — 8.703–5[26] — 228.385[40] — 2.886[40] — — — 8.48–5[40] — 3D1—3P2 349.82 3.5887 3.6547 1.9745–3 2.011–3 2.274–3 2.316–3 0.98 — 3.6257[22] 3.6447[22] — — — — — 350.05[26] — 3.5717[26] — — — 2.268–3[26] — 349.617[40] — 3.697[40] — — — 2.34–3[40] — 3P1—3P2 296.32 2.2619 2.2569 8.930–2 8.909–2 8.712–2 8.691–2 1.00 — 2.2599[22] 2.2699[22] — 7.90–2[27] — — — 296.46[26] — 2.2279[26] — 7.80–2[28] — 8.592–2[26] — 296.213[40] — 2.289[40] — 9.04–2[31] — 8.79–2[40] — 3S1—3P2 226.98 2.04010 2.06810 4.726–1 4.792–1 3.532–1 3.581–1 0.99 — 2.05710[22] 2.05810[22] — — — — — 227.19[26] — 2.02610[26] — — — 3.517–1[26] — 227.000[40] — 2.0710[40] — — — 3.58–1[40] — 1P1—3P2 205.53 2.5766 2.8666 4.895–5 5.446–5 3.312–5 3.685–5 0.90 — 2.5606[22] 2.6456[22] — — — — — 205.9[26] — 2.9486[26] — — — 3.811–5[26] — 205.617[40] — 2.066[40] — — — 2.66–5[40] — 3D1—1D2 417.97 8.6325 7.1145 6.782–5 5.590–5 9.332–5 7.692–5 1.21 — 8.6765[22] 7.1575[22] — — — — — 417.85[26] — 7.1235[26] — — — 7.694–5[26] — 417.510[40] — 7.115[40] — — — 7.66–5[40] — 3P1—1D2 343.81 6.2936 6.1236 3.346–4 3.255–4 3.787–4 3.684–4 1.03 — 6.2116[22] 6.1076[22] — — — — — 343.7[26] — 6.1146[26] — — — 3.675–4[26] — 343.545[40] — 5.766[40] — — — 3.46–4[40] — 3S1—1D2 253.83 3.7936 3.6476 1.099–4 1.057–4 9.186–5 8.832–5 1.04 — 3.7346[22] 3.7956[22] — — — — — 253.94[26] — 3.9866[26] — — — 9.664–5[26] — 253.797[40] — 3.306[40] — — — 8.00–5[40] — 1P1—1D2 227.31 2.26010 2.27310 5.251–1 5.281–1 3.930–1 3.952–1 0.99 — 2.27610[22] 2.28410[22] — 4.78–1[27] — — — 227.63[26] — 2.23510[26] — 4.83–1[28] — 3.902–1[26] — 227.361[40] — 2.3110[40] — 5.36–1[31] — 4.02–1[40] — 5S2—3P2 697.02 1.9435 1.4735 7.077–5 5.363–5 1.624–4 1.231–4 1.32 — 1.9155[22] 1.4665[22] — — — — — 691.75[26] — 1.5055[26] — — — 1.229–4[26] — 692.73[40] — 1.435[40] — — — 1.18–4[40] — 3D2—3P2 350.00 3.9498 3.9998 3.626–2 3.672–2 4.178–2 4.231–2 0.99 — 3.9618[22] 3.9728[22] — — — — — 350.18[26] — 3.8958[26] — — — 4.128–2[26] — 349.795[40] — 4.008[40] — — — 4.23–2[40] — 3P2—3P2 296.23 4.6859 4.6699 3.082–1 3.071–1 3.005–1 2.995–1 1.00 — 4.6829[22] 4.6919[22] — 2.83–1[27] — — — 296.35[26] — 4.6089[26] — 2.76–1[28] — 2.959–1[26] — 296.117[40] — 4.719[40] — 3.12–1[31] — 3.02–1[40] — 1D2—3P2 230.35 5.8127 6.0027 2.312–3 2.388–3 1.753–3 1.811–3 0.97 — 5.8077[22] 5.9117[22] — — — — — 230.78[26] — 5.8747[26] — — — 1.782–3[26] — 230.421[40] — 5.467[40] — — — 1.65–3[40] — 5S2—1D2 1032.47 2.5842 1.3382 2.065–7 1.069–7 7.018–7 3.633–7 1.93 — 1.6812[22] 1.0802[22] — — — — — 1018.31[26] — 1.1972[26] — — — 3.120–7[26] — 3D2—1D2 418.22 1.1186 1.0746 1.466–4 1.408–4 2.019–4 1.939–4 1.04 — 1.1246[22] 1.0596[22] — — — — — 418.05[26] — 1.0526[26] — — — 1.897–4[26] — 417.763[40] — 9.825[40] — — — 1.77–4[40] — 3P2—1D2 343.69 9.5375 9.6695 8.445–5 8.561–5 9.555–5 9.686–5 0.99 — 9.0185[22] 9.8575[22] — — — — — 343.55[26] — 9.6775[26] — — — 9.683–5[26] — 343.416[40] — 8.275[40] — — — 8.26–5[40] — 1D2—1D2 258.06 1.75910 1.78110 8.781–1 8.891–1 7.460–1 7.554–1 0.99 — 1.77010[22] 1.77010[22] — 1.21[27] — — — 258.42[26] — 1.73810[26] — 1.20[28] — 7.403–1[26] — 258.08[40] — 1.7710[40] — 8.93–1[31] — 7.52–1[40] — 3D3—3P2 350.07 2.2479 2.2559 2.890–1 2.900–1 3.331–1 3.342–1 1 — 2.2509[22] 2.2419[22] — — — — — 350.25[26] — 2.2019[26] — — — 3.268–1[26] — 349.873[40] — 2.249[40] — — — 3.31–1[40] — 3D3—1D2 418.33 5.8086 5.5756 1.067–3 1.024–3 1.469–3 1.410–3 1.04 — 5.7996[22] 5.4926[22] — — — — — 418.15[26] — 5.4836[26] — — — 1.385–3[26] — 417.875[40] — 5.196[40] — — — 1.31–3[40] — 注: 表中A, B分别为1s22s2p3和1s22s22p2组态; a×10b表示为ab. 表 18 Ge XXVII 离子(Z = 10, 14, 32, 36, 50)1s22s2p3-1s22s22p2间E1跃迁谱线波长、跃迁速率、加权振子强度
Table 18. The E1 transition wavelength, rate, weighted oscillator strength, and line strength between the 1s22s2p3 and 1s22s22p2 configurations in Ge XXVII ions with Z = 10, 14, 32, 36, 50.
Transition A—B λ/Å AC/s–1 AB/s–1 gfC gfB SC/a.u. SB/a.u. AC/AB 3D1—3P0 85.09 3.14510 3.14810 1.024–1 1.025–1 2.869–2 2.872–2
1.0085.07[26] — 3.14310[26] — — — 2.865–2[26] — 3P1—3P0 68.08 5.2709 5.2559 1.099–2 1.095–2 2.462–3 2.455–3 1.00 68.07[26] — 5.2609[26] — — — 2.456–3[26] — 3S1—3P0 61.19 1.12210 1.12910 1.890–2 1.902–2 3.806–3 3.831–3 0.99 61.18[26] — 1.12510[26] — — — 3.813–3[26] — 1P1—3P0 50.62 4.6717 4.6197 5.384–5 5.324–5 8.973–6 8.873–6 1.01 50.62[26] — 4.6367[26] — — — 8.902–6[26] — 3D1—1S0 224.22 5.6477 5.2747 1.277–3 1.193–3 9.424–4 8.803–4 1.07 223.88[26] — 5.3297[26] — — — 8.854–4[26] — 3P1—1S0 135.21 4.1148 3.8488 3.383–3 3.164–3 1.506–3 1.408–3 1.07 135.08[26] — 3.8518[26] — — — 1.405–3[26] — 3S1—1S0 110.49 1.9019 1.8589 1.044–2 1.020–2 3.796–3 3.710–3 1.02 110.41[26] — 1.8509[26] — — — 3.688–3[26] — 1P1—1S0 80.25 2.85210 2.84910 8.260–2 8.253–2 2.182–2 2.180–2 1.00 80.21[26] — 2.83910[26] — — — 2.169–2[26] — 3P0—3P1 81.91 4.17310 4.17310 4.198–2 4.198–2 1.132–2 1.132–2 1.00 81.89[26] — 4.17410[26] — — — 1.131–2[26] — 3D1—3P1 104.69 2.6217 3.1337 1.292–4 1.544–4 4.452–5 5.322–5 0.84 104.66[26] — 3.0567[26] — — — 5.188–5[26] — 3P1—3P1 80.08 4.48610 4.49110 1.294–1 1.295–1 3.411–2 3.414–2 1.00 80.06[26] — 4.48510[26] — — — 3.408–2[26] — 3S1—3P1 70.71 2.94110 2.94510 6.613–2 6.621–2 1.539–2 1.541–2 1.00 70.70[26] — 2.93510[26] — — — 1.535–2[26] — 1P1—3P1 56.97 1.05410 1.06010 1.539–2 1.547–2 2.886–3 2.902–3 0.99 56.96[26] — 1.05810[26] — — — 2.895–3[26] — 5S2—3P1 161.20 4.0058 3.7728 7.802–3 7.348–3 4.1405–3 3.900–3 1.06 161.01[26] — 3.7928[26] — — — 3.906–3[26] — 3D2—3P1 100.79 1.58010 1.56710 1.203–1 1.193–1 3.991–2 3.958–2 1.01 100.75[26] — 1.56410[26] — — — 3.948–2[26] — 3P2—3P1 77.17 9.9147 1.0708 4.425–4 4.774–4 1.124–4 1.213–4 0.93 77.15[26] 1.0548[26] 1.194–4[26] 1D2—3P1 64.62 1.1549 1.1599 3.611–3 3.629–3 7.683–4 7.721–4 0.99 64.62[26] — 1.1639[26] — — — 7.741–4[26] — 3D1—3P2 112.72 1.1049 1.0599 6.310–3 6.050–3 2.341–3 2.245–3 1.04 112.68[26] 1.0589[26] 2.241–3[26] 3P1—3P2 84.69 1.5779 1.5939 5.087–3 5.140–3 1.418–3 1.433–3 0.99 84.67[26] — 1.5999[26] — — — 1.437–3[26] — 3S1—3P2 74.28 9.93410 9.91910 2.465–1 2.462–1 6.029–2 6.020–2 1.00 74.27[26] — 9.90510[26] — — — 6.007–2[26] — 1P1—3P2 59.27 1.1069 1.0959 1.748–3 1.730–3 3.410–4 3.376–4 1.01 59.26[26] — 1.1049[26] — — — 3.403–4[26] — 3D1—1D2 163.29 3.2148 2.9908 3.855–3 3.586–3 2.072–3 1.928–3 1.07 163.19[26] — 3.0108[26] — — — 1.937–3[26] — 3P1—1D2 110.38 8.6246 1.1257 4.726–5 6.167–5 1.717–5 2.241–5 0.77 110.33[26] — 1.1687[26] — — — 2.322–5[26] — 3S1—1D2 93.33 1.9719 1.9409 7.721–3 7.599–3 2.372–3 2.335–3 1.02 93.30[26] — 1.9219[26] — — — 2.310–3[26] — 1P1—1D2 70.80 1.10911 1.11011 2.501–1 2.503–1 5.829–2 5.835–2 1.00 70.78[26] — 1.10911[26] — — — 5.826–2[26] — 5S2—3P2 181.07 2.3298 2.1398 5.723–3 5.258–3 3.412–3 3.134–3 1.09 180.81[26] — 2.1538[26] — — — 3.140–3[26] — 3D2—3P2 108.21 4.0698 3.8918 3.572–3 3.416–3 1.273–3 1.217–3 1.05 108.17[26] — 3.9078[26] — — — 1.220–3[26] — 3P2—3P2 81.45 5.19210 5.19510 2.582–1 2.583–1 6.922–2 6.927–2 1.00 81.42[26] — 5.19110[26] — — — 6.915–2[26] — 1D2—3P2 67.60 1.42110 1.43010 4.866–2 4.898–2 1.083–2 1.090–2 0.99 67.59[26] — 1.42210[26] — — — 1.083–2[26] — 5S2—1D2 360.39 6.5476 5.3496 6.374–4 5.207–4 7.563–4 6.178–4 1.22 359.24[26] — 5.4336[26] — — — 6.215–4[26] — 3D2—1D2 154.01 6.5817 6.2307 1.170–3 1.108–3 5.933–4 5.616–4 1.06 153.89[26] — 6.1507[26] — — — 5.531–4[26] — 3P2—1D2 104.93 7.7217 6.9777 6.372–4 5.758–4 2.201–4 1.989–4 1.11 104.88[26] — 6.9087[26] — — — 1.967–4[26] — 1D2—1D2 83.02 6.36210 6.34010 3.287–1 3.275–1 8.982–2 8.951–2 1.00 82.99[26] — 6.32510[26] — — — 8.923–2[26] — 3D3—3P2 99.36 9.4929 9.4419 9.833–2 9.781–2 3.216–2 3.199–2 1.01 99.31[26] — 9.4189[26] — — — 3.187–2[26] — 3D3—1D2 136.67 2.6409 2.5459 5.174–2 4.988–2 2.328–2 2.244–2 1.04 136.58[26] — 2.5399[26] — — — 2.235–2[26] — 注: 表中A, B分别为1s22s2p3和1s22s22p2组态; a×10b表示为ab. 表 19 Kr XXXI离子(Z = 10, 14, 32, 36, 50)1s22s2p3-1s22s22p2间E1跃迁谱线波长、跃迁速率、加权振子强度
Table 19. The E1 transition wavelength, rate, weighted oscillator strength, and line strength between the 1s22s2p3 and 1s22s22p2 configurations in Kr XXXI ions with Z = 10, 14, 32, 36, 50.
Transition A—B λ/Å AC/s–1 AB/s–1 gfC gfB SC/a.u. SB/a.u. AC/AB 3D1—3P0 65.33 5.90010 5.90010 1.133–1 1.133–1 2.436–2 2.436–2 1.00 65.32[26] — 5.89410[26] — — — 2.432–2[26] — 3P1—3P0 50.00 6.4679 6.4599 7.270–3 7.261–3 1.197–3 1.195–3 1.00 49.98[26] — 6.4689[26] — — — 1.196–3[26] — 3S1—3P0 46.48 1.13010 1.13610 1.098–2 1.103–2 1.680–3 1.688–3 0.99 46.47[26] — 1.13210[26] — — — 1.681–3[26] — 1P1—3P0 37.16 4.2727 4.2627 2.653–5 2.646–5 3.245–6 3.237–6 1.00 37.15[26] — 4.2727[26] — — — 3.244–6[26] — 3D1—1S0 248.44 3.2157 2.9087 8.925–4 8.073–4 7.300–4 6.603–4 1.11 248.00[26] — 2.9437[26] — — — 6.646–4[26] — 3P1—1S0 114.67 6.3048 5.8788 3.728–3 3.476–3 1.407–3 1.312–3 1.07 114.57[26] — 5.8808[26] — — — 1.309–3[26] — 3S1—1S0 97.70 3.0619 2.9609 1.314–2 1.271–2 4.227–3 4.087–3 1.03 97.63[26] — 2.9529[26] — — — 4.067–3[26] — 1P1—1S0 63.97 4.19010 4.17810 7.712–2 7.691–2 1.624–2 1.620–2 1.00 63.94[26] — 4.16810[26] — — — 1.614–2[26] — 3P0—3P1 64.20 6.62510 6.62510 4.094–2 4.094–2 8.654–3 8.653–3 1.00 64.19[26] — 6.62710[26] — — — 8.650–3[26] — 3D1—3P1 88.22 1.6408 1.4828 5.741–4 5.187–4 1.667–4 1.506–4 1.11 88.19[26] — 1.4938[26] — — — 1.516–4[26] — 3P1—3P1 62.38 8.55710 8.56010 1.498–1 1.498–1 3.075–2 3.076–2 1.00 62.36[26] — 8.55310[26] — — — 3.072–2[26] — 3S1—3P1 56.99 3.10910 3.10710 4.542–2 4.539–2 8.522–3 8.517–3 1.00 56.98[26] — 3.10010[26] — — — 8.494–3[26] — 1P1—3P1 43.59 1.40910 1.41510 1.204–2 1.209–2 1.727–3 1.735–3 1.00 43.58[26] — 1.41210[26] — — — 1.731–3[26] — 5S2—3P1 128.51 1.2669 1.1999 1.567–2 1.483–2 6.629–3 6.276–3 1.06 128.39[26] — 1.2029[26] — — — 6.280–3[26] — 3D2—3P1 79.57 2.20010 2.17910 1.044–1 1.034–1 2.735–2 2.709–2 1.01 79.54[26] — 2.17710[26] — — — 2.703–2[26] — 3P2—3P1 59.98 1.4089 1.4359 3.795–3 3.870–3 7.494–4 7.641–4 0.98 59.96[26] — 1.4309[26] — — — 7.607–4[26] — 1D2—3P1 49.16 1.5769 1.5819 2.856–3 2.865–3 4.622–4 4.637–4 1.00 49.16[26] — 1.5879[26] — — — 4.650–4[26] — 3D1—3P2 95.08 3.2419 3.1119 1.318–2 1.265–2 4.125–3 3.960–3 1.04 95.05[26] — 3.1119[26] — — — 3.955–3[26] — 3P1—3P2 65.73 7.9468 8.0868 1.544–3 1.571–3 3.342–4 3.401–4 0.98 65.72[26] — 8.1398[26] — — — 3.420–4[26] — 3S1—3P2 59.78 1.40211 1.39911 2.254–1 2.250–1 4.437–2 4.423–2 1.00 59.77[26] — 1.39811[26] — — — 4.421–2[26] — 1P1—3P2 45.20 1.6789 1.6729 1.542–3 1.537–3 2.295–4 2.287–4 1.00 45.19[26] — 1.6849[26] — — — 2.302–4[26] — 3D1—1D2 168.03 2.2558 2.0738 2.863–3 2.632–3 1.584–3 1.456–3 1.09 167.89[26] — 2.0908[26] — — — 1.465–3[26] — 3P1—1D2 93.92 2.2238 1.9978 8.821–4 7.921–4 2.728–4 2.449–4 1.11 93.88[26] — 1.9758[26] — — — 2.419–4[26] — 3S1—1D2 82.23 3.1199 3.0439 9.483–3 9.254–3 2.567–3 2.505–3 1.02 82.20[26] — 3.0259[26] — — — 2.487–3[26] — 1P1—1D2 56.95 1.63411 1.63511 2.384–1 2.385–1 4.469–2 4.473–2 1.00 56.94[26] 1.63411[26] 4.466–2[26] 5S2—3P2 143.62 6.3928 5.9288 9.884–3 9.166–3 4.673–3 4.334–3 1.08 143.46[26] — 5.9558[26] — — — 4.339–3[26] — 3D2—3P2 85.11 8.3678 8.0968 4.544–3 4.396–3 1.273–3 1.232–3 1.03 85.07[26] — 8.1068[26] — — — 1.232–3[26] — 3P2—3P2 63.07 9.77910 9.78010 2.916–1 2.916–1 6.055–2 6.056–2 1.00 63.06[26] — 9.77310[26] — — — 6.047–2[26] — 1D2—3P2 51.23 1.28210 1.28810 2.522–2 2.534–2 4.253–3 4.274–3 1.00 51.21[26] — 1.28210[26] — — — 4.249–3[26] — 5S2—1D2 417.15 5.0226 3.8886 6.550–4 5.072–4 8.996–4 6.966–4 1.29 415.63[26] — 3.9576[26] — — — 7.011–4[26] — 3D2—1D2 139.21 1.2658 1.1698 1.838–3 1.699–3 8.422–4 7.785–4 1.08 139.08[26] 1.1608[26] 7.705–4[26] 3P2—1D2 88.58 1.3719 1.3089 8.066–3 7.691–3 2.352–3 2.243–3 1.05 88.54[26] — 1.3039[26] — — — 2.232–3[26] — 1D2—1D2 66.86 8.53010 8.49010 2.859–1 2.845–1 6.293–2 6.263–2 1.00 66.84[26] — 8.47910[26] — — — 6.249–2[26] — 3D3—3P2 76.60 1.42010 1.41110 8.740–2 8.686–2 2.204–2 2.190–2 1.01 76.57[26] — 1.40910[26] — — — 2.184–2[26] — 3D3—1D2 117.79 3.7969 3.6279 5.527–2 5.280–2 2.143–2 2.048–2 1.05 117.70[26] — 3.6219[26] — — — 2.040–2[26] — 注: 表中A, B分别为1s22s2p3和1s22s22p2组态; a×10b表示为ab. -
[1] Arav N, Edmonds D, Borguet B, Kriss G A, Kaastra J S, Behar E, Bianchi S, Cappi M, Costantini E, Detmers R G, Ebrero J, Mehdipour M, Paltani S, Petrucci P O, Pinto C, Ponti G, Steenbrugge K C, De Vries C P 2012 Astron. Astrophys. 544 A33
 Google Scholar
Google Scholar
[2] Mao J, Kaastra J S, Mehdipour M, Raassen A J J, Gu L, Miller J M 2017 Astron. Astrophys. 607 A100
 Google Scholar
Google Scholar
[3] Feldman U, Doschek G A 2007 At. Data Nucl. Data Tables 93 779
 Google Scholar
Google Scholar
[4] Zatsarinny O, Gorczyca T W, Korista K T, Badnell N R, Savin D W 2004 Astron. Astrophys. 417 1173
 Google Scholar
Google Scholar
[5] Curdt W, Landi E, Feldman U 2004 Astron. Astrophys. 427 1045
 Google Scholar
Google Scholar
[6] Si R, Li S, Wang K, Guo X L, Chen Z B, Yan J, Chen, C Y, Brage T, Zou Y M 2017 Astron. Astrophys. 600 A85
 Google Scholar
Google Scholar
[7] Ramsbottom C, Ballance C, Smyth R, Conroy A, Fernández-Menchero L, Turkington M, Keenan F 2018 Galaxies 6 90
 Google Scholar
Google Scholar
[8] Belmonte M T, Pickering J C, Clear C P, Concepción Mairey F, Liggins F 2018 Galaxies 6 109
 Google Scholar
Google Scholar
[9] Raju P K, Dwivedi B N, Gupta A K 1994 Sol. Phys. 149 289
 Google Scholar
Google Scholar
[10] Lyubimkov L S 2013 Astrophysics 56 472
 Google Scholar
Google Scholar
[11] Koutchmy S, Baudin F, Abdi S, Golub L, Sèvre F 2019 Astron. Astrophys. 632 A86
 Google Scholar
Google Scholar
[12] Feldman U, Doschek G A, Mariska J T, Bhatia A K, Mason H E 1978 Astrophys. J. 226 674
 Google Scholar
Google Scholar
[13] Vilkas M J, Martinson I, Merkelis G, Gaigalas G, Kisielius R 1996 Phys. Scr. 54 281
 Google Scholar
Google Scholar
[14] Safronova U I, Shlyaptseva A S 1999 Phys. Scr. 60 36
 Google Scholar
Google Scholar
[15] Aggarwal K M, Keenan F P, Msezane A Z 2003 Astron. Astrophys. 401 377
 Google Scholar
Google Scholar
[16] Tachiev G, Froese Fischer C 2001 Can. J. Phys. 79 955
 Google Scholar
Google Scholar
[17] Froese Fischer C, Tachiev G 2004 At. Data Nucl. Data Tables 87 1
 Google Scholar
Google Scholar
[18] Gu M F 2005 At. Data Nucl. Data Tables 89 267
 Google Scholar
Google Scholar
[19] Safronova U I, Ralchenko Y, Murakami I, Kato T, Kato D 2006 Phys. Scr. 73 143
 Google Scholar
Google Scholar
[20] Aggarwal K M, Keenan F P, Lawson K D 2008 At. Data Nucl. Data Tables 94 323
 Google Scholar
Google Scholar
[21] Jönsson P, Bieroń J 2010 J. Phys. B: At. Mol. Opt. Phys. 43 074023
 Google Scholar
Google Scholar
[22] Jönsson P, Rynkun P, Gaigalas G 2011 At. Data Nucl. Data Tables 97 648
 Google Scholar
Google Scholar
[23] Liu H, Jiang G, Hu F, Wang C K, Wang Z B, Yang J M 2013 Chin. Phys. B 22 073202
 Google Scholar
Google Scholar
[24] Ekman J, Jönsson P, Gustafsson S, Hartman H, Gaigalas G, Godefroid M R, Froese Fischer C 2014 Astron. Astrophys. 564 A24
 Google Scholar
Google Scholar
[25] Nazé C, Verdebout S, Rynkun P, Gaigalas G, Godefroid M, Jönsson P 2014 At. Data Nucl. Data Tables 100 1197
 Google Scholar
Google Scholar
[26] Wang K, Li D F, Liu H T, Han X Y, Duan B, Li C Y, Li J G, Guo X L, Chen C Y, Yan J 2014 Astrophys. J. Suppl. Ser. 215 26
 Google Scholar
Google Scholar
[27] Alwadie N, Almodlej A, Ben Nessib N, Dimitrijević M S 2020 Contrib. Astron. Obs. Skalnaté Pleso 50 86
[28] Almodlej A, Alrashed H, Ben Nessib N, Dimitrijević M S 2021 Mon. Not. R. Astron. Soc. 507 3228
 Google Scholar
Google Scholar
[29] Tang W, Deng B L, Zhang G S, Meng B, Yang R, Wang S 2025 Indian J. Phys. 99 793
 Google Scholar
Google Scholar
[30] Fricke B 1984 Phys. Scr. 1984 129
[31] Fritzsche S 2002 Phys. Scr. 2002 37
[32] Grant I P 2007 Relativistic Quantum Theory of Atoms and Molecules: Theory and Computation (New York: Springer) pp384-431
[33] Froese Fischer C, Gaigalas G, Jönsson P, Bieroń J 2019 Comput. Phys. Commun. 237 184
 Google Scholar
Google Scholar
[34] Zeng J Y 1981 Advanced Quantum Mechanics VI (Beijing: Science Press) pp389-407
[35] 曹铭欣 2019 硕士学位论文 (兰州: 西北师范大学)
Cao M X 2019 M. S. Thesis (Lanzhou: Northwest Normal University
[36] Borschevsky A, Pašteka L F, Pershina V, Eliav E, Kaldor U 2015 Phys. Rev. A 91 020501
 Google Scholar
Google Scholar
[37] Gaston N, Schwerdtfeger P, Nazarewicz W 2002 Phys. Rev. A 66 062505
 Google Scholar
Google Scholar
[38] Glushkov A V, Ambrosov S V, Loboda A, Chernyakova Y G, Khetselius O Y, Svinarenko A A 2004 Nucl. Phys. A 734 E21
 Google Scholar
Google Scholar
[39] Grant I P 1974 J. Phys. B: Atom. Mol. Phys. 7 1458
 Google Scholar
Google Scholar
[40] Kramida A, Ralchenko Y, Reader J, NIST ASD Team (2024) https://www.nist.gov/pml/atomic-spectra-database [2024-6-5]
[41] McIntyre Jr L C, Donahue D J, Bernstein E M 1978 Phys. Scr. 17 5
 Google Scholar
Google Scholar
[42] Kramida A 2024 Eur. Phys. J. D 78 36
 Google Scholar
Google Scholar
[43] Froese Fischer C, Tachiev G 2004 At. Data Nucl. Data Tables 87 1
 Google Scholar
Google Scholar
[44] Chen M H, Reed K J, McWilliams D M, Guo D S, Barlow L, Lee M, Walker V 1997 At. Data Nucl. Data Tables 65 289
 Google Scholar
Google Scholar
Metrics
- Abstract views: 101
- PDF Downloads: 1
- Cited By: 0














 DownLoad:
DownLoad:
