-
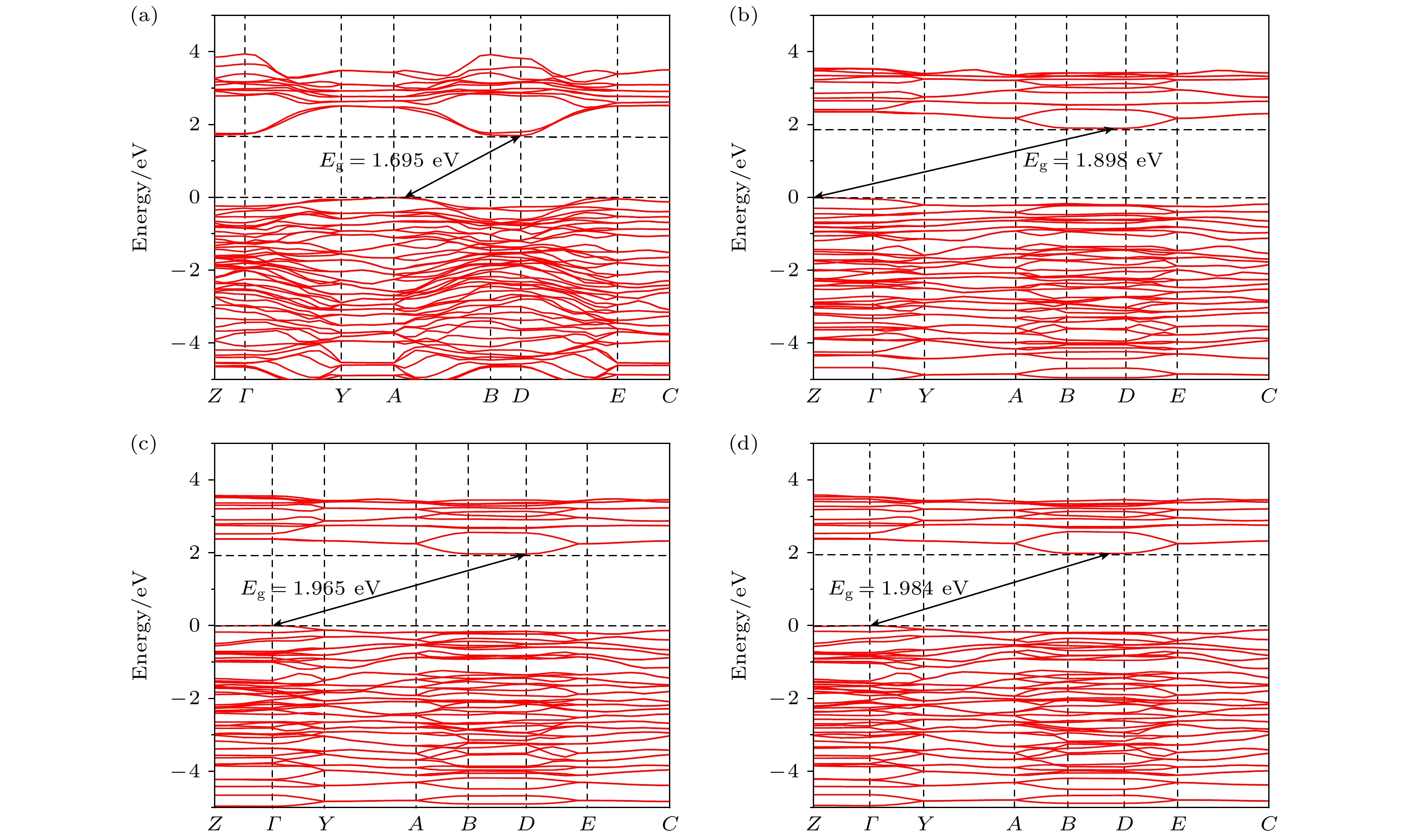
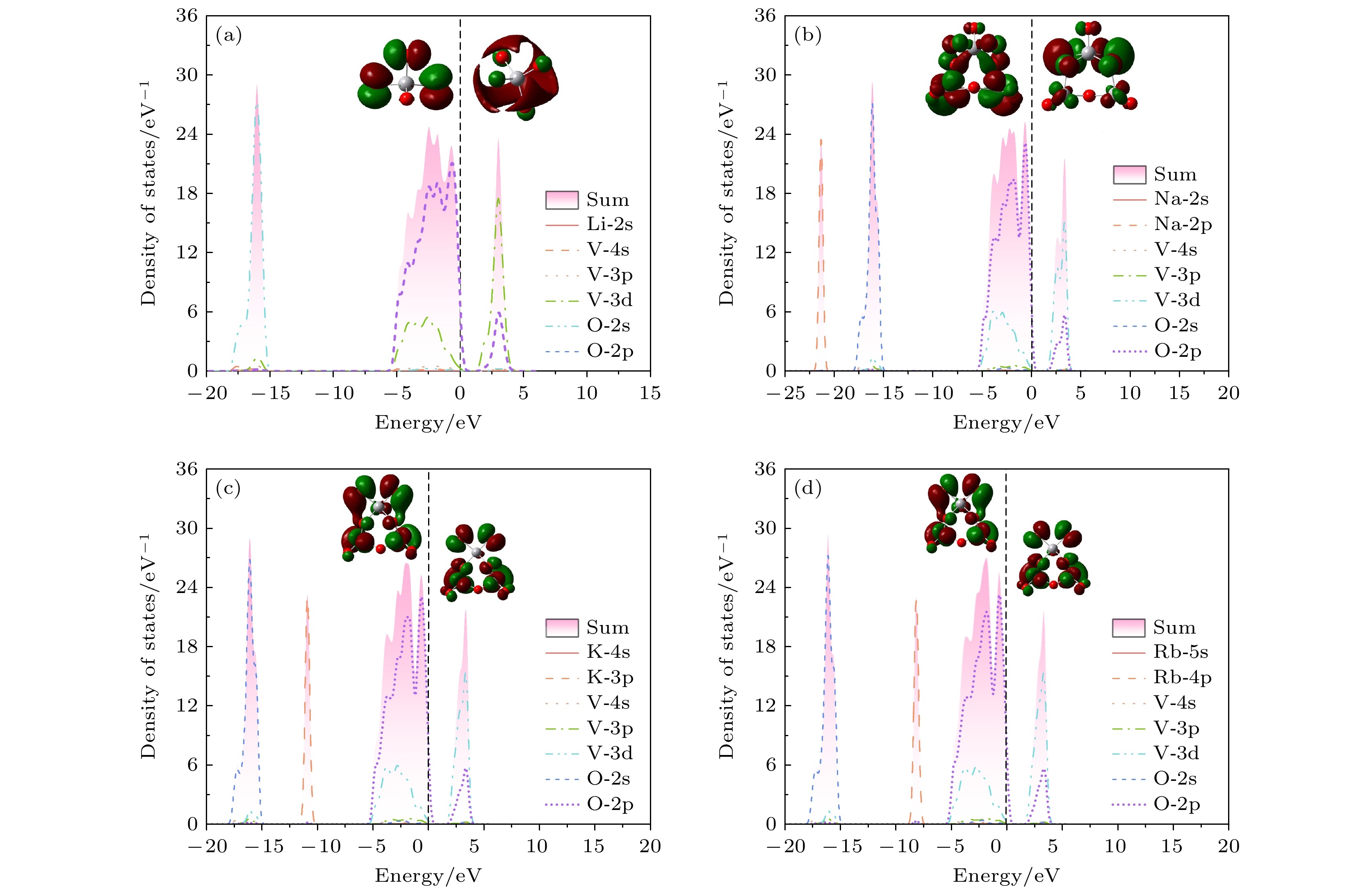
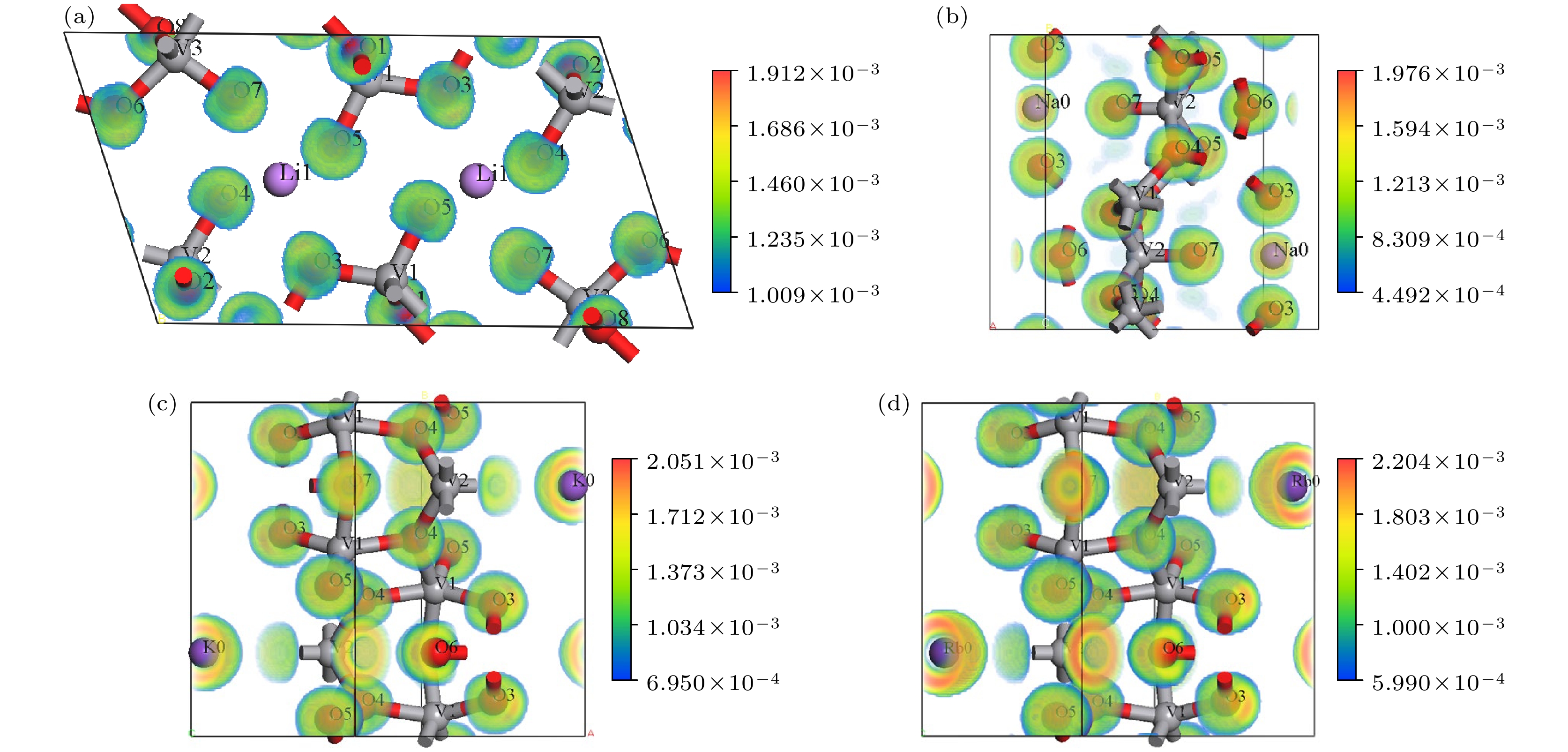
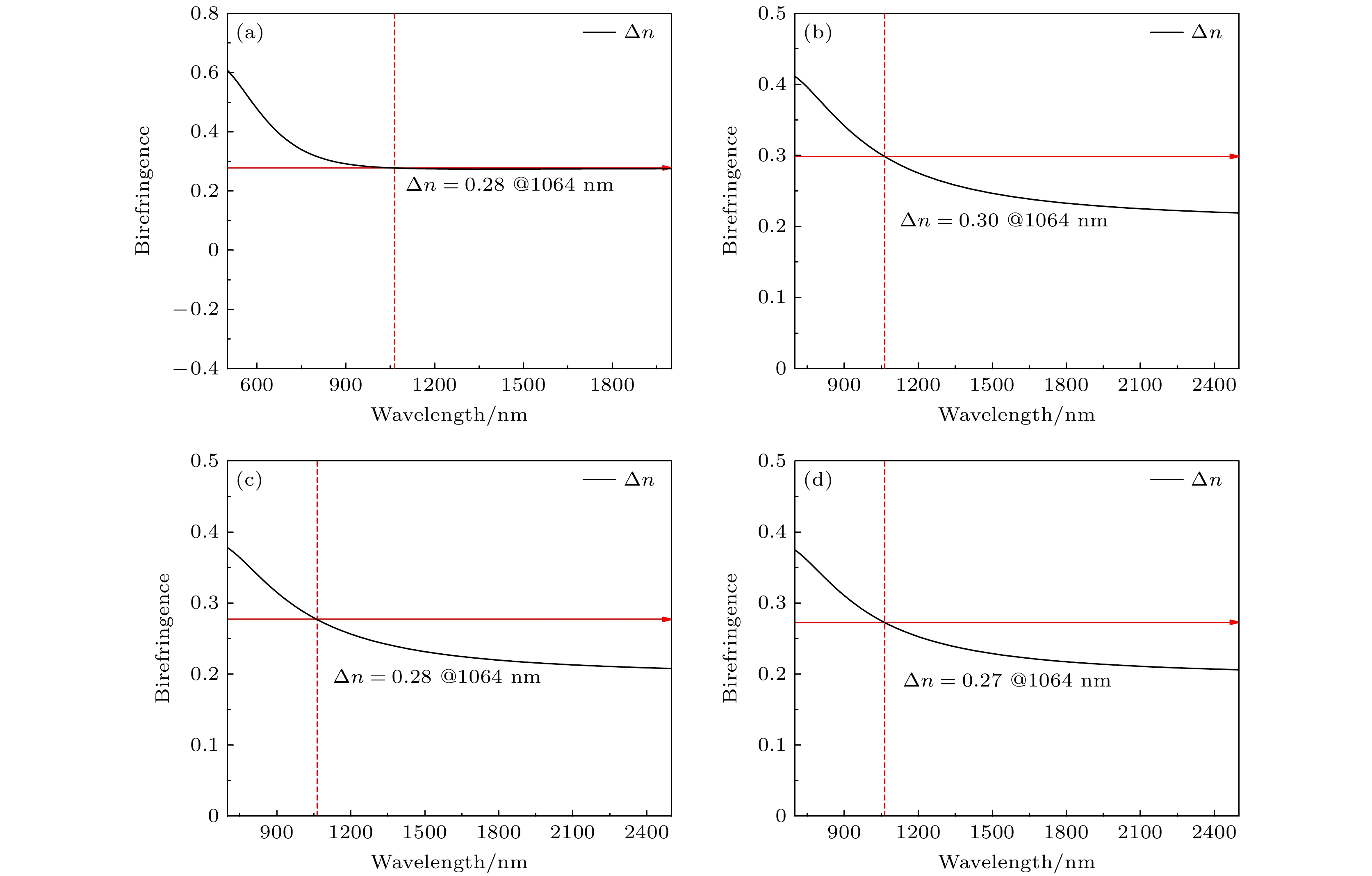
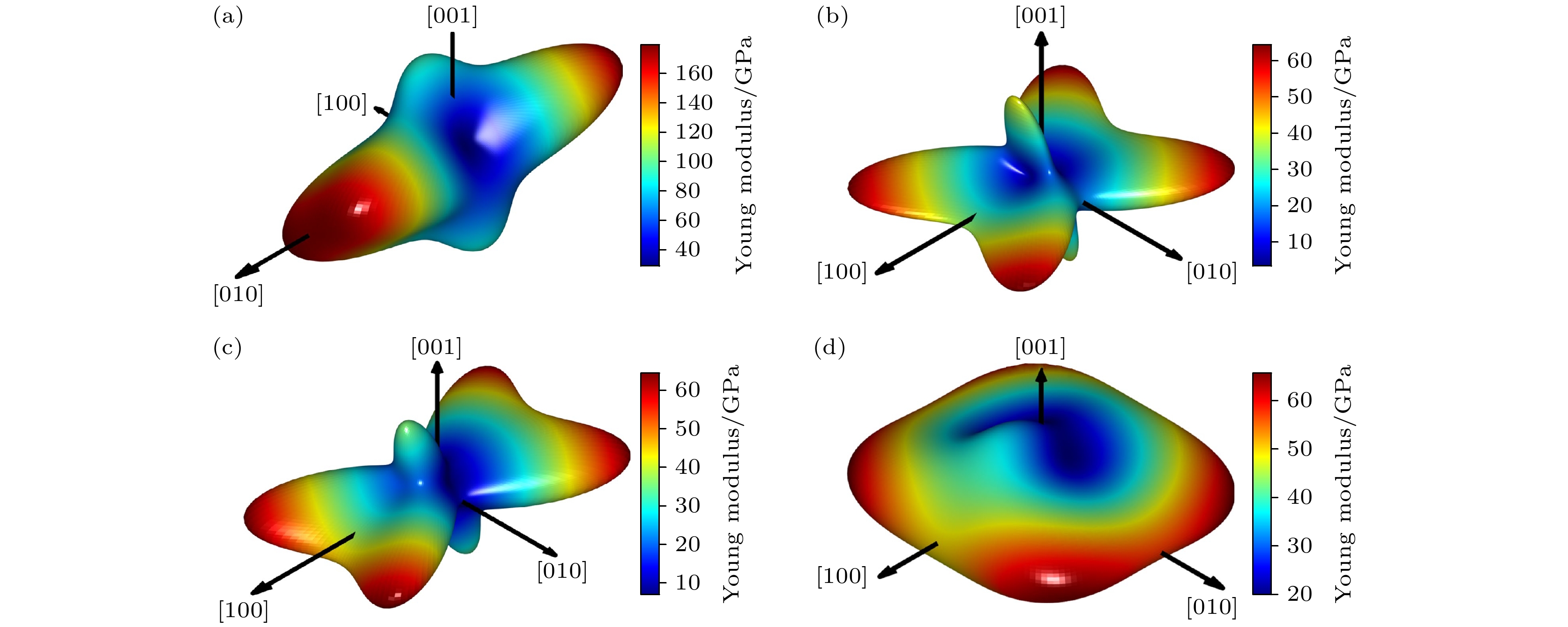
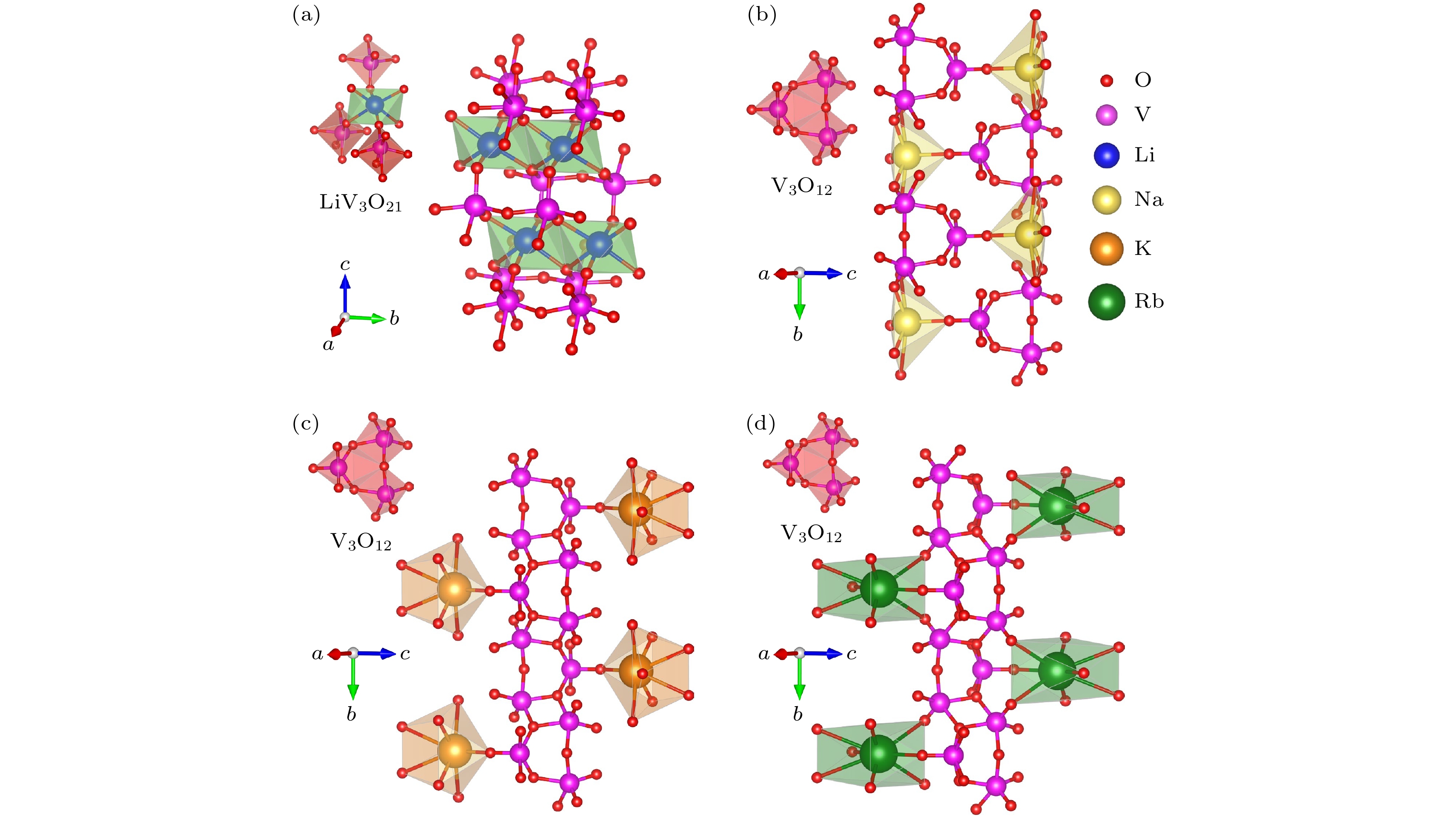
Birefringence, as a fundamental parameter of optical crystals, plays a vital role in numerous optical applications such as phase modulation, light splitting, and polarization, thereby making them key materials in laser science and technology. The significant birefringence of vanadate polyhedra provides a new approach for developing birefringent materials. In this study, first-principles calculations are used to investigate the band structures, density of states (DOS), electron localization functions (ELFs), and birefringence behaviors of four alkali metal vanadate crystals AV3O8 (A = Li, Na, K, Rb). The computational results show that all AV3O8 crystals have indirect band gaps, whose values are 1.695, 1.898, 1.965, and 1.984 eV for LiV3O8, NaV3O8, KV3O8, and RbV3O8, respectively. The DOS analysis reveals that near the Fermi level, the conduction band minima (CBM) in these vanadates are predominantly occupied by the outermost orbitals of V atoms, while the valence band maxima (VBM) are primarily contributed by O-2p orbitals. The O-2p orbitals also exhibit strong localization near the Fermi level. Combined with highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO-LUMO) analysis and population analysis, the bonding interactions in all four crystals mainly arise from the hybridization between V-3p and O-2p orbitals, indicating strong covalent bonding in V—O bonds. Through the analysis of structure-property relationships, the large birefringence is primarily attributed to the pronounced structural anisotropy, high anisotropy index of responsive electron distribution, unique arrangement of anionic groups, and d-p orbital hybridization between V-3d and O-2p orbitals. The calculated birefringence values at a wavelength of 1064 nm for LiV3O8, NaV3O8, KV3O8, and RbV3O8 are 0.28, 0.30, 0.28, and 0.27, respectively.
-
Keywords:
- alkali metal vanadate /
- first principles /
- electronic structure /
- birefringence
[1] Pedrotti F L, Pedrotti L M, Pedrotti L S 2018 Introduction to optics (England: Cambridge University Press) pp333–360
[2] Li X Z, Wang C, Chen X L, Li H, Jia L S, Wu L, Du Y X, Xu Y P 2004 Inorg. Chem. 43 8555
 Google Scholar
Google Scholar
[3] Nomura H, Furutono Y 2008 Microelectron. Eng. 85 1671
 Google Scholar
Google Scholar
[4] Aoki K, Miyazaki H T, Hirayama H 2003 Nat. Mater. 2 117
 Google Scholar
Google Scholar
[5] Lancry M, Desmarchelier R, Cook K, Poumellec B, Canning J 2014 Micromachines-Basel 5 825
 Google Scholar
Google Scholar
[6] Li R 2013 Z. Krist-Cryst. Mater. 228 526
 Google Scholar
Google Scholar
[7] Levy M, Jalali A A, Huang X 2009 J. Mater. Sci. Mater. El. 20 43
 Google Scholar
Google Scholar
[8] Zhang H, Zhang M, Pan S L, Yang Z H, Wang Z, Bian Q, Hou X L, Yu H W, Zhang F F, Wu K, Yang F, Peng Q J, Xu Z Y, Chang K B, Poeppelmeier K R 2015 Cryst. Growth Des. 15 523
 Google Scholar
Google Scholar
[9] Ghosh G 1999 Opt. Commun. 163 95
 Google Scholar
Google Scholar
[10] Luo H, Tkaczyk T, Sampson R, Dereniak E L 2006 Proc. SPIE 6119 136
 Google Scholar
Google Scholar
[11] Guoqing Z, Jun X, Xingda C, Heyu Z, Siting W, Ke X, Fuxi G 1998 J. Cryst. Growth 191 517
 Google Scholar
Google Scholar
[12] Appel R, Dyer C D, Lockwood J N 2002 Appl. Opt. 41 2470
 Google Scholar
Google Scholar
[13] Cyranoski D 2009 Nature 457 953
 Google Scholar
Google Scholar
[14] Krainer L, Paschotta R, Lecomte S, Moser M, Weingarten K J, Keller U 2002 IEEE J. Quantum Electron. 38 1331
 Google Scholar
Google Scholar
[15] Lisinetskii V A, Grabtchiko A S, Demidovich A A, Burakevich V N, Orlovich V A, Titov A N 2007 Appl. Phys. B 88 499
 Google Scholar
Google Scholar
[16] Vodchits A I, Orlovich V A, Apanasevich P A 2012 J. Appl. Spectrosc. 78 918
 Google Scholar
Google Scholar
[17] Yu H, Liu J, Zhang H, Kaminskii A A, Wang Z, Wang J 2014 Laser Photonics Rev. 8 847
 Google Scholar
Google Scholar
[18] Lei B H, Kong Q, Yang Z H, Yang Y, Wang Y J, Pan S L 2016 J. Mater. Chem. C 4 6295
 Google Scholar
Google Scholar
[19] Li K X, Zhang X Y, Chai B Q, Yu H W, Hu Z G, Wang J Y, Wu Y C, Wu H P 2025 Chem. Eur. J. 31 e202403515
 Google Scholar
Google Scholar
[20] Li K X, Wu H P, Yu H W, Hu Z G, Wang J Y, Wu Y C 2024 Chem. Commun. 60 12734
 Google Scholar
Google Scholar
[21] Lei B H, Yang Z H, Pan S L 2017 Chem. Commun. 53 2818
 Google Scholar
Google Scholar
[22] Huang Y, Zhang X Y, Zhao S G, Mao J G, Yang B P 2024 J. Mater. Chem. C 12 7286
 Google Scholar
Google Scholar
[23] Zhang S Z, Dong L F, Xu B H, Chen H G, Huo H, Liang F, Wu R, Gong P F, Lin Z S 2024 Inorg. Chem. Front. 11 5528
 Google Scholar
Google Scholar
[24] Cheng J L, Xu D, Lu J, Zhang F F, Hou X L 2023 Inorg. Chem. 62 20340
 Google Scholar
Google Scholar
[25] Chen Z X, Xu F, Cao S N, Li Z F, Yang H X, Ai X P, Cao Y L 2017 Small 13 1603148
 Google Scholar
Google Scholar
[26] Cao X Y, Yang Q, Zhu L M, Xie L L 2018 Ionics 24 943
 Google Scholar
Google Scholar
[27] Yang H, Li J, Zhang X G, Jin Y L 2008 J. Mater. Process. Technol. 207 265
 Google Scholar
Google Scholar
[28] Zhu L M, Li W X, Xie L L, Yang Q, Cao X Y 2019 Chem. Eng. J. 372 1056
 Google Scholar
Google Scholar
[29] Feng L L, Zhang W, Xu L N, Li D Z, Zhang Y Y 2020 Solid State Sci. 103 106187
 Google Scholar
Google Scholar
[30] Kim H J, Jo J H, Choi J U, Voronina N, Myung S T 2020 J. Power Sources 478 229072
 Google Scholar
Google Scholar
[31] Shchelkanova M, Shekhtman G, Pershina S, Vovkotrub E 2021 Materials 14 6976
 Google Scholar
Google Scholar
[32] Wu W Z, Ding J, Peng H R, Li G C 2011 Mater. Lett. 65 2155
 Google Scholar
Google Scholar
[33] Zhu J Z, Li X L, Chen S Y, Huang C M, Feng J J, Kuang Q, Fan Q H, Dong Y Z, Zhao Y M 2020 Electrochim. Acta 355 136799
 Google Scholar
Google Scholar
[34] Wadsley A D 1957 Acta Crystallogr. 10 261
 Google Scholar
Google Scholar
[35] Bachmann H G, Barnes W H 1962 Can Mineral 7 219
[36] Baddour-Hadjean R, Boudaoud A, Bach S, Emery N, Pereira-Ramos J P 2014 Inorg. Chem. 53 1764
 Google Scholar
Google Scholar
[37] Oka Y, Yao T, Yamamoto N 1997 Mater. Res. Bull. 32 1201
 Google Scholar
Google Scholar
[38] Segall M D, Lindan P J D, Probert M J 2002 J. Phys. : Condens. Matter 14 2717
 Google Scholar
Google Scholar
[39] Payne M C, Teter M P, Allan D C, Arias T A, Joannopoulos A J 1992 Rev. Mod. Phys. 64 1045
 Google Scholar
Google Scholar
[40] Srivastava G P, Weaire D 1987 Adv. Phys. 36 463
 Google Scholar
Google Scholar
[41] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[42] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[43] Hamann D R, Schlüter M, Chiang C 1979 Phys. Rev. Lett. 43 1494
 Google Scholar
Google Scholar
[44] Kong Q R, Yang Y, Liu L L, Bian Q, Lei B H, Li L P, Yang Z H, Su Z, Pan S L 2016 J. Mater. Res. 31 488
 Google Scholar
Google Scholar
[45] 阎守胜 2011 固体物理基础(北京: 北京大学出版社)
Yan S S 2011 Fundamentals of Solid-state Physics (Beijing: Peking University Press
[46] Hiscocks J, Frisch M J 2009 Gaussian 09: IOps Reference 9 (USA: Gaussian
[47] Riffet V, Contreras-Garcıa J, Carrasco J, Calatayud M 2016 J. Phys. Chem. C 120 4259
 Google Scholar
Google Scholar
[48] Mulliken R S 1931 Chem. Rev. 9 347
 Google Scholar
Google Scholar
[49] 张博, 王云杰, 齐亚杰, 和志豪, 丁家福, 苏欣 2024 人工晶体学报 53 999
 Google Scholar
Google Scholar
Zhang B, Wang Y J, Qi Y J, He Z H, Ding J F, Su X 2024 J. Synth. Cryst. 53 999
 Google Scholar
Google Scholar
[50] Su X, Wang Y J, Yang Z H, Huang X C, Pan S L, Li F, Lee M H 2013 J. Phys. Chem. C 117 14149
 Google Scholar
Google Scholar
[51] Tudi A, Han S, Yang Z H, Pan S L 2022 Coord. Chem. Rev. 459 214380
 Google Scholar
Google Scholar
[52] Wang X Y, Zhang B B, Yang D Q, Wang Y 2022 Dalton Trans. 51 14059
 Google Scholar
Google Scholar
[53] Bai S, Yang D Q, Zhang B B, Li L, Wang Y 2022 Dalton Trans. 51 3421
 Google Scholar
Google Scholar
[54] Chu Y, Wang H S, Chen Q, Su X, Chen Z X, Yang Z H, Li J J, Pan S L 2024 Adv. Funct. Mater. 34 2314933
 Google Scholar
Google Scholar
[55] Ding Y Y, Zhu M M, Wang J B, Li B, Qi H X, Liu L L, Chu Y Q 2024 Inorg. Chem. 63 20003
 Google Scholar
Google Scholar
[56] Lin L, Jiang X X, Wu C, Lin Z S, Huang Z P, Humphrey M G, Zhang C 2021 Dalton Trans. 50 7238
 Google Scholar
Google Scholar
[57] Su X, Chu Y, Yang Z H, Lei B H, Cao C, Wang Y, Pan S L 2020 J. Phys. Chem. C 124 24949
 Google Scholar
Google Scholar
[58] Bai S, Zhang X, Zhang B B, Li L, Wang Y J 2021 Inorg. Chem. 60 10006
 Google Scholar
Google Scholar
[59] Li S, Dou D, Chen C, Shi Q, Zhang B B, Wang Y J 2024 Inorg. Chem. 63 24076
 Google Scholar
Google Scholar
[60] Chu Y, Wang H S, Abutukadi T, Li Z, Mutailipu M, Su X, Yang Z H, Li J J, Pan S L 2023 Small 19 2305074
 Google Scholar
Google Scholar
[61] Liu H J, Liang C W, Liang W I, Chen H J, Yang J C, Peng C Y, Chu Y H 2012 Phys. Rev. B Condens. Matter Mater. Phys. 85 014104
 Google Scholar
Google Scholar
[62] 王云杰, 和志豪, 丁家福, 苏欣 2025 人工晶体学报 54 85
 Google Scholar
Google Scholar
Wang Y J, He Z H, Ding J F, Su X 2025 J. Synth. Cryst. 54 85
 Google Scholar
Google Scholar
[63] 丁家福, 和志豪, 王云杰, 苏欣 2025 人工晶体学报 54 95
 Google Scholar
Google Scholar
Ding J F, He Z H, Wang Y J, Su X 2025 J. Synth. Cryst. 54 95
 Google Scholar
Google Scholar
[64] 储冬冬, 杨志华, 潘世烈 2024 人工晶体学报 53 1475
 Google Scholar
Google Scholar
Chu D D, Yang Z H, Pan S L 2024 J. Synth. Cryst. 53 1475
 Google Scholar
Google Scholar
-
表 1 结构优化前后的AV3O8 (A = Li, Na, K, Rb)晶格参数
Table 1. Lattice parameters of AV3O8 (A = Li, Na, K, Rb) before and after geometry optimization.
Compounds a/nm b/nm c/nm β/(°) b/c Error V/nm3 LiV3O8 Before 0.668 0.360 1.203 107.830 0.299 1.67% 0.275 After 0.695 0.358 1.219 108.397 0.294 0.288 NaV3O8 Before 0.512 0.855 0.744 101.993 1.148 3.92% 0.319 After 0.531 0.877 0.795 113.173 1.103 0.350 KV3O8 Before 0.500 0.839 0.767 98.135 1.094 4.84% 0.319 After 0.516 0.865 0.831 103.812 1.041 0.361 RbV3O8 Before 0.501 0.842 0.791 96.943 1.064 2.16% 0.331 After 0.516 0.865 0.831 100.581 1.041 0.365 表 2 AV3O8 (A = Li, Na, K, Rb)的布居数 (Mulliken)
Table 2. Population of AV3O8 (A = Li, Na, K, Rb) (Mulliken).
Substance Species Atomic population Total Charge/e Bond Bond
populationLength/Å s p d LiV3O8 Li –0.20 0.00 0.00 –0.20 1.20 Li—O –0.06 2.16 V 0.20 0.31 3.20 3.71 1.29 O—V 0.01 2.86 O 1.90 4.68 0.00 6.59 –0.59 O—V 0.92 1.66 NaV3O8 Na 2.07 5.95 0.00 8.01 0.99 Na—O 0.02 2.38 V 0.15 0.33 3.18 3.66 1.34 O—V 0.92 1.64 O 1.89 4.72 0.00 6.62 –0.62 O—V 0.33 1.99 KV3O8 K 2.03 5.91 0.00 7.95 1.05 K—O 0.04 2.72 V 0.15 0.34 3.32 3.72 1.28 O—V 0.97 1.64 O 1.89 4.74 0.00 6.63 –0.63 O—V 0.33 1.98 RbV3O8 Rb 2.04 5.86 0.00 7.90 1.10 Rb—O 0.04 2.97 V 0.16 0.35 3.23 3.74 1.26 O—V 0.98 1.64 O 1.89 4.74 0.00 6.64 –0.64 O—V 0.34 1.98 表 3 AV3O8 (A = Li, Na, K, Rb)的响应电子分布各向异性指数(REDA)
Table 3. REDA of AV3O8 (A = Li, Na, K, Rb) of the electron distribution.
Compounds Groups δ Δn LiV3O8 VO4 0.011 0.28 NaV3O8 V3O8 0.019 0.30 KV3O8 V3O8 0.013 0.28 RbV3O8 V3O8 0.012 0.27 -
[1] Pedrotti F L, Pedrotti L M, Pedrotti L S 2018 Introduction to optics (England: Cambridge University Press) pp333–360
[2] Li X Z, Wang C, Chen X L, Li H, Jia L S, Wu L, Du Y X, Xu Y P 2004 Inorg. Chem. 43 8555
 Google Scholar
Google Scholar
[3] Nomura H, Furutono Y 2008 Microelectron. Eng. 85 1671
 Google Scholar
Google Scholar
[4] Aoki K, Miyazaki H T, Hirayama H 2003 Nat. Mater. 2 117
 Google Scholar
Google Scholar
[5] Lancry M, Desmarchelier R, Cook K, Poumellec B, Canning J 2014 Micromachines-Basel 5 825
 Google Scholar
Google Scholar
[6] Li R 2013 Z. Krist-Cryst. Mater. 228 526
 Google Scholar
Google Scholar
[7] Levy M, Jalali A A, Huang X 2009 J. Mater. Sci. Mater. El. 20 43
 Google Scholar
Google Scholar
[8] Zhang H, Zhang M, Pan S L, Yang Z H, Wang Z, Bian Q, Hou X L, Yu H W, Zhang F F, Wu K, Yang F, Peng Q J, Xu Z Y, Chang K B, Poeppelmeier K R 2015 Cryst. Growth Des. 15 523
 Google Scholar
Google Scholar
[9] Ghosh G 1999 Opt. Commun. 163 95
 Google Scholar
Google Scholar
[10] Luo H, Tkaczyk T, Sampson R, Dereniak E L 2006 Proc. SPIE 6119 136
 Google Scholar
Google Scholar
[11] Guoqing Z, Jun X, Xingda C, Heyu Z, Siting W, Ke X, Fuxi G 1998 J. Cryst. Growth 191 517
 Google Scholar
Google Scholar
[12] Appel R, Dyer C D, Lockwood J N 2002 Appl. Opt. 41 2470
 Google Scholar
Google Scholar
[13] Cyranoski D 2009 Nature 457 953
 Google Scholar
Google Scholar
[14] Krainer L, Paschotta R, Lecomte S, Moser M, Weingarten K J, Keller U 2002 IEEE J. Quantum Electron. 38 1331
 Google Scholar
Google Scholar
[15] Lisinetskii V A, Grabtchiko A S, Demidovich A A, Burakevich V N, Orlovich V A, Titov A N 2007 Appl. Phys. B 88 499
 Google Scholar
Google Scholar
[16] Vodchits A I, Orlovich V A, Apanasevich P A 2012 J. Appl. Spectrosc. 78 918
 Google Scholar
Google Scholar
[17] Yu H, Liu J, Zhang H, Kaminskii A A, Wang Z, Wang J 2014 Laser Photonics Rev. 8 847
 Google Scholar
Google Scholar
[18] Lei B H, Kong Q, Yang Z H, Yang Y, Wang Y J, Pan S L 2016 J. Mater. Chem. C 4 6295
 Google Scholar
Google Scholar
[19] Li K X, Zhang X Y, Chai B Q, Yu H W, Hu Z G, Wang J Y, Wu Y C, Wu H P 2025 Chem. Eur. J. 31 e202403515
 Google Scholar
Google Scholar
[20] Li K X, Wu H P, Yu H W, Hu Z G, Wang J Y, Wu Y C 2024 Chem. Commun. 60 12734
 Google Scholar
Google Scholar
[21] Lei B H, Yang Z H, Pan S L 2017 Chem. Commun. 53 2818
 Google Scholar
Google Scholar
[22] Huang Y, Zhang X Y, Zhao S G, Mao J G, Yang B P 2024 J. Mater. Chem. C 12 7286
 Google Scholar
Google Scholar
[23] Zhang S Z, Dong L F, Xu B H, Chen H G, Huo H, Liang F, Wu R, Gong P F, Lin Z S 2024 Inorg. Chem. Front. 11 5528
 Google Scholar
Google Scholar
[24] Cheng J L, Xu D, Lu J, Zhang F F, Hou X L 2023 Inorg. Chem. 62 20340
 Google Scholar
Google Scholar
[25] Chen Z X, Xu F, Cao S N, Li Z F, Yang H X, Ai X P, Cao Y L 2017 Small 13 1603148
 Google Scholar
Google Scholar
[26] Cao X Y, Yang Q, Zhu L M, Xie L L 2018 Ionics 24 943
 Google Scholar
Google Scholar
[27] Yang H, Li J, Zhang X G, Jin Y L 2008 J. Mater. Process. Technol. 207 265
 Google Scholar
Google Scholar
[28] Zhu L M, Li W X, Xie L L, Yang Q, Cao X Y 2019 Chem. Eng. J. 372 1056
 Google Scholar
Google Scholar
[29] Feng L L, Zhang W, Xu L N, Li D Z, Zhang Y Y 2020 Solid State Sci. 103 106187
 Google Scholar
Google Scholar
[30] Kim H J, Jo J H, Choi J U, Voronina N, Myung S T 2020 J. Power Sources 478 229072
 Google Scholar
Google Scholar
[31] Shchelkanova M, Shekhtman G, Pershina S, Vovkotrub E 2021 Materials 14 6976
 Google Scholar
Google Scholar
[32] Wu W Z, Ding J, Peng H R, Li G C 2011 Mater. Lett. 65 2155
 Google Scholar
Google Scholar
[33] Zhu J Z, Li X L, Chen S Y, Huang C M, Feng J J, Kuang Q, Fan Q H, Dong Y Z, Zhao Y M 2020 Electrochim. Acta 355 136799
 Google Scholar
Google Scholar
[34] Wadsley A D 1957 Acta Crystallogr. 10 261
 Google Scholar
Google Scholar
[35] Bachmann H G, Barnes W H 1962 Can Mineral 7 219
[36] Baddour-Hadjean R, Boudaoud A, Bach S, Emery N, Pereira-Ramos J P 2014 Inorg. Chem. 53 1764
 Google Scholar
Google Scholar
[37] Oka Y, Yao T, Yamamoto N 1997 Mater. Res. Bull. 32 1201
 Google Scholar
Google Scholar
[38] Segall M D, Lindan P J D, Probert M J 2002 J. Phys. : Condens. Matter 14 2717
 Google Scholar
Google Scholar
[39] Payne M C, Teter M P, Allan D C, Arias T A, Joannopoulos A J 1992 Rev. Mod. Phys. 64 1045
 Google Scholar
Google Scholar
[40] Srivastava G P, Weaire D 1987 Adv. Phys. 36 463
 Google Scholar
Google Scholar
[41] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[42] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[43] Hamann D R, Schlüter M, Chiang C 1979 Phys. Rev. Lett. 43 1494
 Google Scholar
Google Scholar
[44] Kong Q R, Yang Y, Liu L L, Bian Q, Lei B H, Li L P, Yang Z H, Su Z, Pan S L 2016 J. Mater. Res. 31 488
 Google Scholar
Google Scholar
[45] 阎守胜 2011 固体物理基础(北京: 北京大学出版社)
Yan S S 2011 Fundamentals of Solid-state Physics (Beijing: Peking University Press
[46] Hiscocks J, Frisch M J 2009 Gaussian 09: IOps Reference 9 (USA: Gaussian
[47] Riffet V, Contreras-Garcıa J, Carrasco J, Calatayud M 2016 J. Phys. Chem. C 120 4259
 Google Scholar
Google Scholar
[48] Mulliken R S 1931 Chem. Rev. 9 347
 Google Scholar
Google Scholar
[49] 张博, 王云杰, 齐亚杰, 和志豪, 丁家福, 苏欣 2024 人工晶体学报 53 999
 Google Scholar
Google Scholar
Zhang B, Wang Y J, Qi Y J, He Z H, Ding J F, Su X 2024 J. Synth. Cryst. 53 999
 Google Scholar
Google Scholar
[50] Su X, Wang Y J, Yang Z H, Huang X C, Pan S L, Li F, Lee M H 2013 J. Phys. Chem. C 117 14149
 Google Scholar
Google Scholar
[51] Tudi A, Han S, Yang Z H, Pan S L 2022 Coord. Chem. Rev. 459 214380
 Google Scholar
Google Scholar
[52] Wang X Y, Zhang B B, Yang D Q, Wang Y 2022 Dalton Trans. 51 14059
 Google Scholar
Google Scholar
[53] Bai S, Yang D Q, Zhang B B, Li L, Wang Y 2022 Dalton Trans. 51 3421
 Google Scholar
Google Scholar
[54] Chu Y, Wang H S, Chen Q, Su X, Chen Z X, Yang Z H, Li J J, Pan S L 2024 Adv. Funct. Mater. 34 2314933
 Google Scholar
Google Scholar
[55] Ding Y Y, Zhu M M, Wang J B, Li B, Qi H X, Liu L L, Chu Y Q 2024 Inorg. Chem. 63 20003
 Google Scholar
Google Scholar
[56] Lin L, Jiang X X, Wu C, Lin Z S, Huang Z P, Humphrey M G, Zhang C 2021 Dalton Trans. 50 7238
 Google Scholar
Google Scholar
[57] Su X, Chu Y, Yang Z H, Lei B H, Cao C, Wang Y, Pan S L 2020 J. Phys. Chem. C 124 24949
 Google Scholar
Google Scholar
[58] Bai S, Zhang X, Zhang B B, Li L, Wang Y J 2021 Inorg. Chem. 60 10006
 Google Scholar
Google Scholar
[59] Li S, Dou D, Chen C, Shi Q, Zhang B B, Wang Y J 2024 Inorg. Chem. 63 24076
 Google Scholar
Google Scholar
[60] Chu Y, Wang H S, Abutukadi T, Li Z, Mutailipu M, Su X, Yang Z H, Li J J, Pan S L 2023 Small 19 2305074
 Google Scholar
Google Scholar
[61] Liu H J, Liang C W, Liang W I, Chen H J, Yang J C, Peng C Y, Chu Y H 2012 Phys. Rev. B Condens. Matter Mater. Phys. 85 014104
 Google Scholar
Google Scholar
[62] 王云杰, 和志豪, 丁家福, 苏欣 2025 人工晶体学报 54 85
 Google Scholar
Google Scholar
Wang Y J, He Z H, Ding J F, Su X 2025 J. Synth. Cryst. 54 85
 Google Scholar
Google Scholar
[63] 丁家福, 和志豪, 王云杰, 苏欣 2025 人工晶体学报 54 95
 Google Scholar
Google Scholar
Ding J F, He Z H, Wang Y J, Su X 2025 J. Synth. Cryst. 54 95
 Google Scholar
Google Scholar
[64] 储冬冬, 杨志华, 潘世烈 2024 人工晶体学报 53 1475
 Google Scholar
Google Scholar
Chu D D, Yang Z H, Pan S L 2024 J. Synth. Cryst. 53 1475
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 1844
- PDF Downloads: 31
- Cited By: 0














 DownLoad:
DownLoad: