-
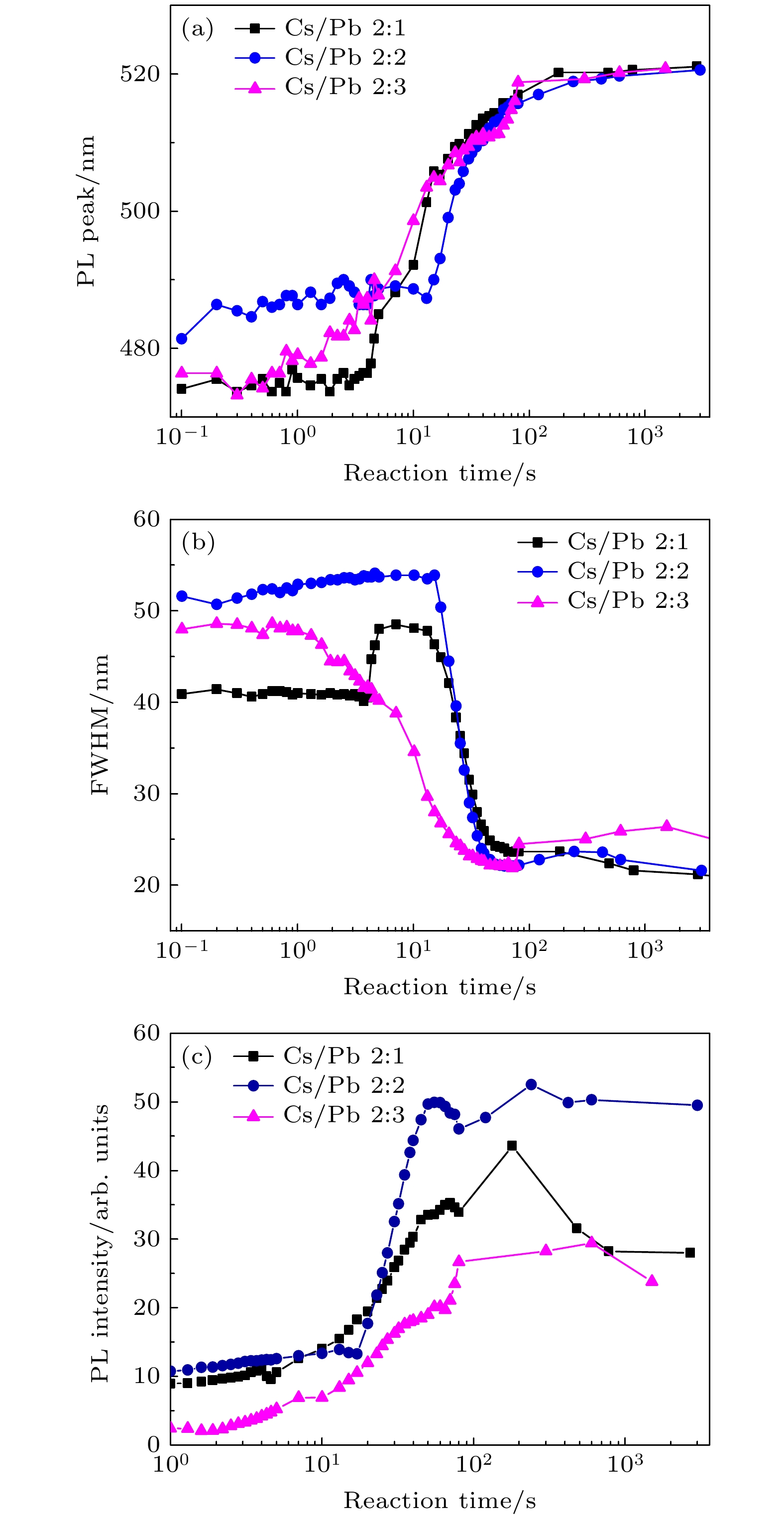
All-inorganic cesium lead halide perovskites have shown great potential applications in optoelectronic field due to their fascinating optical properties. Although perovskite materials have achieved great success in various fields, their inherent ionic properties and high dynamic surface properties have led to their poor stability, hindering their applications. The preparation of CsPbBr3-Cs4PbBr6 nanocrystals has proven to be an effective strategy to enhance their photoluminescence properties and stability. Herein, we report an easy synthesis of CsPbBr3-Cs4PbBr6 nanocrystals with a diphase structure at room temperature by using Cs-OA, Pb-OA and TOABr as precursors in toluene. It is found that the phase transformation and the relative composition between CsPbBr3 and Cs4PbBr6 are dependent on the concentration of TOABr and the ratio of Cs/Pb. The in-situ PL experiments reveal that the formation of ~12 nm CsPbBr3 nanocubes experiences the fast nucleation, the focusing growth of size-distribution in early growth stage and Ostwald ripening growth in the later stage at a TOABr concentration of 0.16 mmol. With the increase of concentration of TOABr or molar ratio of Cs/Pb > 1 (Cs/Pb < 1), [PbBr4]2– complex and [PbBr3]– complex can coexist and compete with each other in toluene, and the CsPbBr3 nucleations dominate in the early stage, then CsPbBr3-Cs4PbBr6 nanocomposites are gradually formed on CsPbBr3 nucleations as photoluminescence centers due to the continuous generation of [PbBr4]2– complex between TOABr and Pb2+. The relative composition of Cs4PbBr6 in CsPbBr3-Cs4PbBr6 nanocomposites can be improved from 4% to 85% with the concentration of TOABr increasing or Cs/Pb < 1. The optimized CsPbBr3-Cs4PbBr6 composite nanocrystals possess high PLQY and stability. Our work provides an understanding of the mechanism of phase transformation in cesium lead halide perovskite materials.
-
Keywords:
- CsPbBr3-Cs4PbBr6 composite nanocrystals /
- in-situ study /
- phase transformation /
- formation kinetic
[1] Peng C, Zhang R, Chen H, Liu Y, Zhang S L, Fang T, Guo R, Zhang J, Shan Q, Jin Y, Wang L, Hou L, Zeng H B 2023 Adv. Mater. 35 2206969
 Google Scholar
Google Scholar
[2] Kim Y H, Kim S, Kakekhani A, et al. 2021 Nat. Photonics 15 148
 Google Scholar
Google Scholar
[3] Liu X K, Xu W, Bai S, Jin Y, Wang J, Friend R H, Gao F 2021 Nat. Mater. 20 10
 Google Scholar
Google Scholar
[4] Protesescu L, Yakunin S, Bodnarchuk M I, Krieg F, Caputo R, Hendon C H, Yang R X, Walsh A, Kovalenko M V 2015 Nano Lett. 15 3692
 Google Scholar
Google Scholar
[5] Li X M, Wu Y, Zhang S L, Cai B, Gu Y, Song J Z, Zeng H B 2016 Adv. Funct. Mater. 26 2435
 Google Scholar
Google Scholar
[6] Ng C K, Wang C, Jasieniak J J 2019 Langmuir 35 11609
 Google Scholar
Google Scholar
[7] Ng C K, Yin W, Li H, Jasieniak J J 2020 Nanoscale 12 4859
 Google Scholar
Google Scholar
[8] Chen M, Zou Y T, Wu L Z, Pan Q, Yang D, Hu H C, Tan Y S, Zhong Q X, Xu Y, Liu H Y, Sun B Q, Zhang Q 2017 Adv. Funct. Mater. 27 1701121
 Google Scholar
Google Scholar
[9] Long Z, Ren H, Sun J H, Ouyang J, Na N 2017 Chem. Commun. 53 9914
 Google Scholar
Google Scholar
[10] Liu W, Zheng J, Cao S, Wang L, Gao F, Chou K C, Hou X, Yang W 2018 Inorg. Chem. 57 1598
 Google Scholar
Google Scholar
[11] Tong Y, Bladt E, Aygüler M F, Manzi A, Milowska K Z, Hintermayr V A, Docampo P, Bals S, Urban A S, Polavarapu L, Feldmann J 2016 Angew. Chem. Int. Ed. 55 13887
 Google Scholar
Google Scholar
[12] Tong Y, Yao E P, Manzi A, Bladt E, Wang K, Döblinger M, Bals S, Müller-Buschbaum P, Urban A S, Polavarapu L, Feldmann J 2018 Adv. Mater. 30 1801117
 Google Scholar
Google Scholar
[13] De Roo J, Ibáñez M, Geiregat P, Nedelcu G, Walravens W, Maes J, Martins J C, Van Driessche I, Kovalenko M V, Hens Z 2016 ACS Nano 10 2071
 Google Scholar
Google Scholar
[14] Wang Y, Yuan J Y, Zhang X L, Ling X F, Larson B W, Zhao Q, Yang Y G, Shi Y, Luther J M, Ma W L 2020 Adv. Mater. 32 2000449
 Google Scholar
Google Scholar
[15] Shankar H, Ghosh S, Kar P 2022 J. Mater. Chem. C 10 11532
 Google Scholar
Google Scholar
[16] 陈雪莲, 焦琥珀, 申岩冰, 潘喜强 2023 72 097801
 Google Scholar
Google Scholar
Chen X L, Jiao H P, Shen Y B, Pan X Q 2023 Acta Phys. Sin. 72 097801
 Google Scholar
Google Scholar
[17] Scharf E, Krieg F, Elimelech O, Oded M, Levi A, Dirin D N, Kovalenko M V, Banin U 2022 Nano Lett. 22 4340
 Google Scholar
Google Scholar
[18] Zhang C, Lian L Y, Zhang J B, Su X M, Liu S S, Gao Y L, Lian Z Y, Sun D Z, Luo W, Zheng H M, Zhang D L 2022 J. Phys. Chem. C 126 4172
 Google Scholar
Google Scholar
[19] Grisorio R, Fasulo F, Muñoz-García A B, Pavone M, Conelli D, Fanizza E, Striccoli M, Allegretta I, Terzano R, Margiotta N, Vivo P, Suranna G P 2022 Nano Lett. 22 4437
 Google Scholar
Google Scholar
[20] Song S, Lv Y C, Cao B Q, Wang W Z 2023 Adv. Funct. Mater. 33 2300493
 Google Scholar
Google Scholar
[21] 陈雪莲, 焦琥珀, 申岩冰, 潘喜强 2022 71 096802
 Google Scholar
Google Scholar
Chen X L, Jiao H P, Shen Y B, Pan X Q 2022 Acta Phys. Sin. 71 096802
 Google Scholar
Google Scholar
[22] Su Y C, Jing Q, Xu Y, Xing X, Lu Z D 2019 ACS Omega 4 22209
 Google Scholar
Google Scholar
[23] Li X W, Cai W S, Guan H L, Zhao S Y, Cao S L, Chen C, Liu M, Zang Z G 2021 Chem. Eng. J. 419 129551
 Google Scholar
Google Scholar
[24] Zhang J B, Jiang P F, Wang Y, Liu X F, Ma J M, Tu G L 2020 ACS Appl. Mater. Interfaces 12 3080
 Google Scholar
Google Scholar
[25] Cho H B, Min J W, Kim H J, Viswanath N S M, Samanta T, Han J H, Park Y M, Jang S W, Im W B 2023 ACS Appl. Electron. Mater. 5 66
 Google Scholar
Google Scholar
[26] Kim H, Park J H, Kim K, Lee D, Song M H, Park J 2022 Adv. Sci. 9 2104660
 Google Scholar
Google Scholar
[27] Bao Z, Chiu H D, Wang W G, Su Q, Yamada T, Chang Y C, Chen S M, Kanemitsu Y, Chung R J, Liu R S 2020 J. Phys. Chem. Lett. 11 10196
 Google Scholar
Google Scholar
[28] Xu L M, Li J H, Fang T, Zhao Y L, Yuan S C, Dong Y H, Song J Z 2019 Nanoscale Adv. 1 980
 Google Scholar
Google Scholar
[29] Zhao X, Shen S L, Gan L, Zhang J L, Zhou W L, Yu L P, Lian S X 2023 J. Lumin. 261 119909
 Google Scholar
Google Scholar
[30] Wang C F, Zhang C Y, Wang F C, Chen J, Ren E L, Kong J F, Li L, Xu J Y, Zhang Y 2022 Opt. Mater. 128 112444
 Google Scholar
Google Scholar
[31] Wang X J, Liu Y Q, Liu N Q, Sun R J, Zheng W, Liu H, Zhang Y H 2021 J. Mater. Chem. A 9 4658
 Google Scholar
Google Scholar
[32] Balakrishnan S K, Kamat P V 2018 Chem. Mater. 30 74
 Google Scholar
Google Scholar
[33] Qiao Z, Wang X, Zhai Y F, Yu R Z, Fang Z, Chen G 2023 Nano Lett. 23 10788
 Google Scholar
Google Scholar
[34] Yoon S J, Stamplecoskie K G, Kamat P V 2016 J. Phys. Chem. C 7 1368
 Google Scholar
Google Scholar
[35] Hui J, Jiang Y N, Gökçinar Ö Ö, Tang J B, Yu Q Y, Zhang M, Yu K 2020 Chem. Mater. 32 4574
 Google Scholar
Google Scholar
[36] Montanarella F, Akkerman Q A, Bonatz D, van der Sluijs M M, van der Bok J C, Prins P T, Aebli M, Mews A, Vanmaekelbergh D, Kovalenko M V 2023 Nano Lett. 23 667
 Google Scholar
Google Scholar
[37] Xu Z S, Yang Y J, Wang P, Liu X F, Qiu J R 2024 Ceram. Int. 50 8952
 Google Scholar
Google Scholar
[38] Kovalenko M V, Protesescu L, Bodnarchuk M I 2017 Science 358 745
 Google Scholar
Google Scholar
-
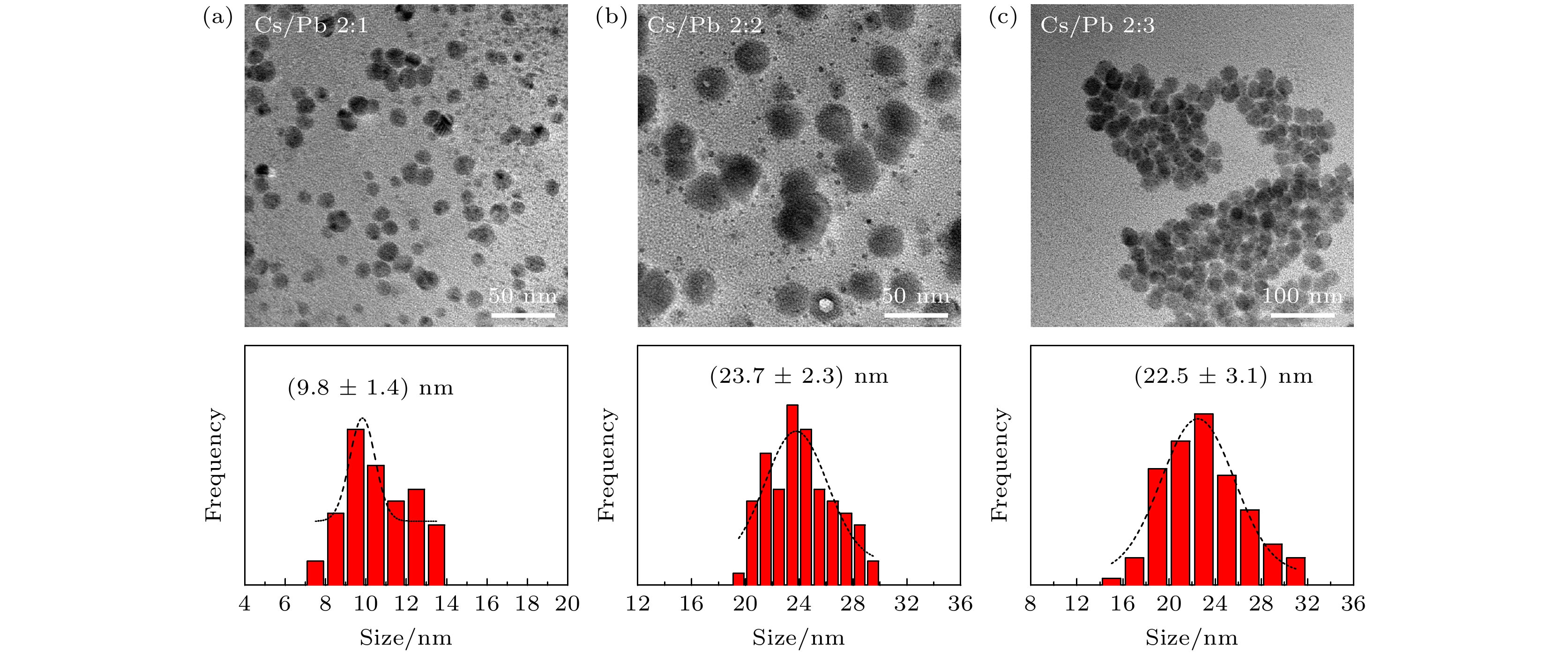
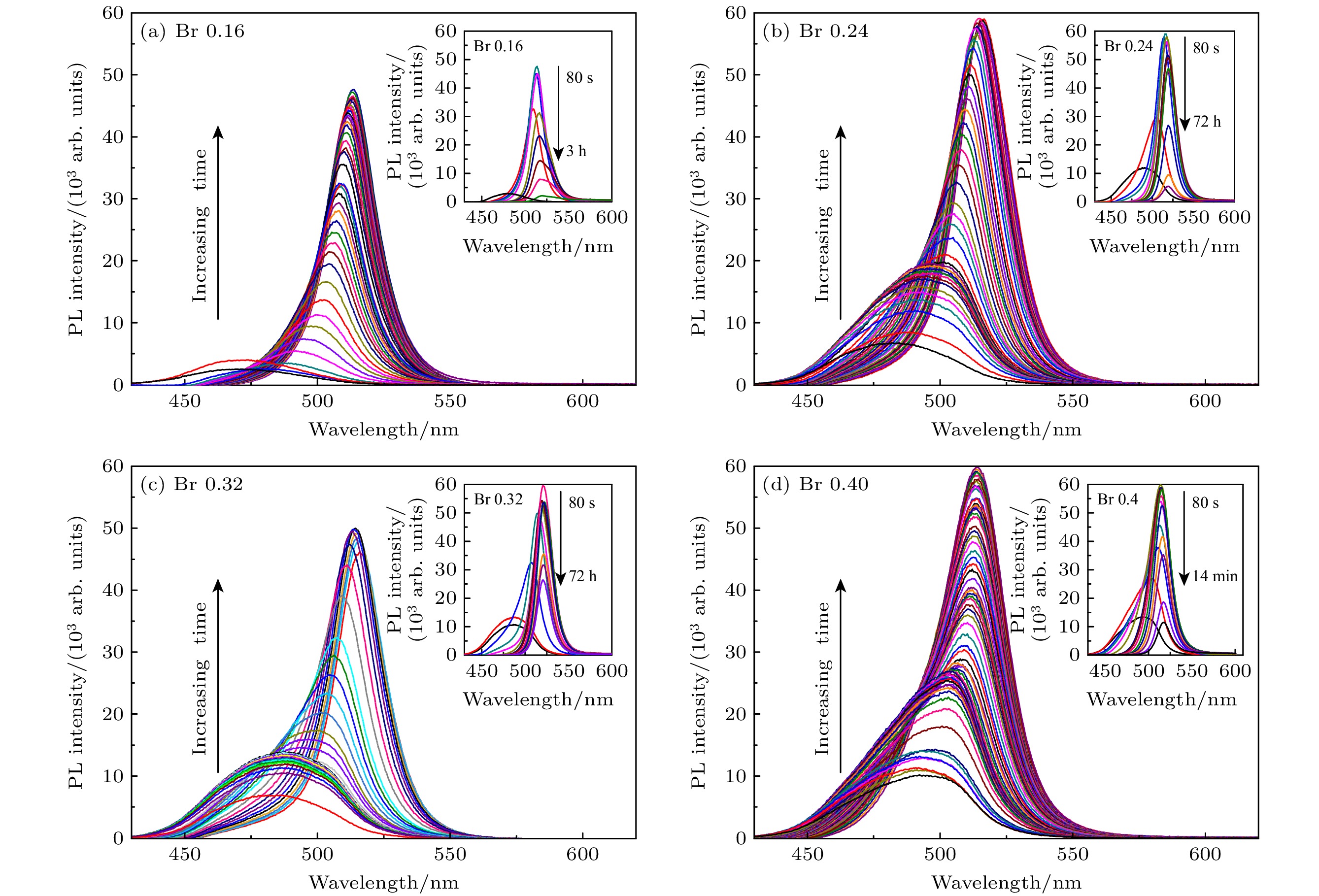
图 2 (a)—(d)不同TOABr用量下所得铯铅溴纳米晶的TEM表征和晶粒尺寸统计结果, 其中(a) Br 0.16 mmol; (b) Br 0.24 mmol; (c) Br 0.32 mmol; (d) Br 0.4 mmol; (e)为图(c)中选择的任意样品区域的HRTEM图(黄色线圈为出现小黑点区域), (f)为小黑点晶粒的尺寸分布图
Figure 2. TEM images and the corresponding histograms of cesium lead bromide nanocrystals synthesized at different dosages of TOABr: (a) Br 0.16 mmol, (b) Br 0.24 mmol, (c) Br 0.32 mmol, and (d) Br 0.4 mmol. (e) HRTEM image of sample in panel (c) (the yellow circles represent the small black dots); (f) size distribution of the small black dots from panel (e).
表 1 不同TOABr用量下所得纳米晶中单斜相CsPbBr3和六方相Cs4PbBr6的相占比
Table 1. Proportion of CsPbBr3 and Cs4PbBr6 in nanocrystals synthesized at different dosages of TOABr.
溴用量/mmol 0.16 0.24 0.32 0.4 CsPbBr3相占比/% 96 89 58 17 Cs4PbBr6相占比/% 4 11 42 83 表 2 不同Cs/Pb摩尔比下所得纳米晶中CsPbBr3相和Cs4PbBr6相的占比情况
Table 2. Proportion of CsPbBr3 and Cs4PbBr6 in nanocrystals synthesized at different molar ratio of Cs/Pb.
Cs/Pb摩尔比 2∶1 2∶2 2∶3 CsPbBr3相占比/% 15 58 28 Cs4PbBr6相占比/% 85 42 72 -
[1] Peng C, Zhang R, Chen H, Liu Y, Zhang S L, Fang T, Guo R, Zhang J, Shan Q, Jin Y, Wang L, Hou L, Zeng H B 2023 Adv. Mater. 35 2206969
 Google Scholar
Google Scholar
[2] Kim Y H, Kim S, Kakekhani A, et al. 2021 Nat. Photonics 15 148
 Google Scholar
Google Scholar
[3] Liu X K, Xu W, Bai S, Jin Y, Wang J, Friend R H, Gao F 2021 Nat. Mater. 20 10
 Google Scholar
Google Scholar
[4] Protesescu L, Yakunin S, Bodnarchuk M I, Krieg F, Caputo R, Hendon C H, Yang R X, Walsh A, Kovalenko M V 2015 Nano Lett. 15 3692
 Google Scholar
Google Scholar
[5] Li X M, Wu Y, Zhang S L, Cai B, Gu Y, Song J Z, Zeng H B 2016 Adv. Funct. Mater. 26 2435
 Google Scholar
Google Scholar
[6] Ng C K, Wang C, Jasieniak J J 2019 Langmuir 35 11609
 Google Scholar
Google Scholar
[7] Ng C K, Yin W, Li H, Jasieniak J J 2020 Nanoscale 12 4859
 Google Scholar
Google Scholar
[8] Chen M, Zou Y T, Wu L Z, Pan Q, Yang D, Hu H C, Tan Y S, Zhong Q X, Xu Y, Liu H Y, Sun B Q, Zhang Q 2017 Adv. Funct. Mater. 27 1701121
 Google Scholar
Google Scholar
[9] Long Z, Ren H, Sun J H, Ouyang J, Na N 2017 Chem. Commun. 53 9914
 Google Scholar
Google Scholar
[10] Liu W, Zheng J, Cao S, Wang L, Gao F, Chou K C, Hou X, Yang W 2018 Inorg. Chem. 57 1598
 Google Scholar
Google Scholar
[11] Tong Y, Bladt E, Aygüler M F, Manzi A, Milowska K Z, Hintermayr V A, Docampo P, Bals S, Urban A S, Polavarapu L, Feldmann J 2016 Angew. Chem. Int. Ed. 55 13887
 Google Scholar
Google Scholar
[12] Tong Y, Yao E P, Manzi A, Bladt E, Wang K, Döblinger M, Bals S, Müller-Buschbaum P, Urban A S, Polavarapu L, Feldmann J 2018 Adv. Mater. 30 1801117
 Google Scholar
Google Scholar
[13] De Roo J, Ibáñez M, Geiregat P, Nedelcu G, Walravens W, Maes J, Martins J C, Van Driessche I, Kovalenko M V, Hens Z 2016 ACS Nano 10 2071
 Google Scholar
Google Scholar
[14] Wang Y, Yuan J Y, Zhang X L, Ling X F, Larson B W, Zhao Q, Yang Y G, Shi Y, Luther J M, Ma W L 2020 Adv. Mater. 32 2000449
 Google Scholar
Google Scholar
[15] Shankar H, Ghosh S, Kar P 2022 J. Mater. Chem. C 10 11532
 Google Scholar
Google Scholar
[16] 陈雪莲, 焦琥珀, 申岩冰, 潘喜强 2023 72 097801
 Google Scholar
Google Scholar
Chen X L, Jiao H P, Shen Y B, Pan X Q 2023 Acta Phys. Sin. 72 097801
 Google Scholar
Google Scholar
[17] Scharf E, Krieg F, Elimelech O, Oded M, Levi A, Dirin D N, Kovalenko M V, Banin U 2022 Nano Lett. 22 4340
 Google Scholar
Google Scholar
[18] Zhang C, Lian L Y, Zhang J B, Su X M, Liu S S, Gao Y L, Lian Z Y, Sun D Z, Luo W, Zheng H M, Zhang D L 2022 J. Phys. Chem. C 126 4172
 Google Scholar
Google Scholar
[19] Grisorio R, Fasulo F, Muñoz-García A B, Pavone M, Conelli D, Fanizza E, Striccoli M, Allegretta I, Terzano R, Margiotta N, Vivo P, Suranna G P 2022 Nano Lett. 22 4437
 Google Scholar
Google Scholar
[20] Song S, Lv Y C, Cao B Q, Wang W Z 2023 Adv. Funct. Mater. 33 2300493
 Google Scholar
Google Scholar
[21] 陈雪莲, 焦琥珀, 申岩冰, 潘喜强 2022 71 096802
 Google Scholar
Google Scholar
Chen X L, Jiao H P, Shen Y B, Pan X Q 2022 Acta Phys. Sin. 71 096802
 Google Scholar
Google Scholar
[22] Su Y C, Jing Q, Xu Y, Xing X, Lu Z D 2019 ACS Omega 4 22209
 Google Scholar
Google Scholar
[23] Li X W, Cai W S, Guan H L, Zhao S Y, Cao S L, Chen C, Liu M, Zang Z G 2021 Chem. Eng. J. 419 129551
 Google Scholar
Google Scholar
[24] Zhang J B, Jiang P F, Wang Y, Liu X F, Ma J M, Tu G L 2020 ACS Appl. Mater. Interfaces 12 3080
 Google Scholar
Google Scholar
[25] Cho H B, Min J W, Kim H J, Viswanath N S M, Samanta T, Han J H, Park Y M, Jang S W, Im W B 2023 ACS Appl. Electron. Mater. 5 66
 Google Scholar
Google Scholar
[26] Kim H, Park J H, Kim K, Lee D, Song M H, Park J 2022 Adv. Sci. 9 2104660
 Google Scholar
Google Scholar
[27] Bao Z, Chiu H D, Wang W G, Su Q, Yamada T, Chang Y C, Chen S M, Kanemitsu Y, Chung R J, Liu R S 2020 J. Phys. Chem. Lett. 11 10196
 Google Scholar
Google Scholar
[28] Xu L M, Li J H, Fang T, Zhao Y L, Yuan S C, Dong Y H, Song J Z 2019 Nanoscale Adv. 1 980
 Google Scholar
Google Scholar
[29] Zhao X, Shen S L, Gan L, Zhang J L, Zhou W L, Yu L P, Lian S X 2023 J. Lumin. 261 119909
 Google Scholar
Google Scholar
[30] Wang C F, Zhang C Y, Wang F C, Chen J, Ren E L, Kong J F, Li L, Xu J Y, Zhang Y 2022 Opt. Mater. 128 112444
 Google Scholar
Google Scholar
[31] Wang X J, Liu Y Q, Liu N Q, Sun R J, Zheng W, Liu H, Zhang Y H 2021 J. Mater. Chem. A 9 4658
 Google Scholar
Google Scholar
[32] Balakrishnan S K, Kamat P V 2018 Chem. Mater. 30 74
 Google Scholar
Google Scholar
[33] Qiao Z, Wang X, Zhai Y F, Yu R Z, Fang Z, Chen G 2023 Nano Lett. 23 10788
 Google Scholar
Google Scholar
[34] Yoon S J, Stamplecoskie K G, Kamat P V 2016 J. Phys. Chem. C 7 1368
 Google Scholar
Google Scholar
[35] Hui J, Jiang Y N, Gökçinar Ö Ö, Tang J B, Yu Q Y, Zhang M, Yu K 2020 Chem. Mater. 32 4574
 Google Scholar
Google Scholar
[36] Montanarella F, Akkerman Q A, Bonatz D, van der Sluijs M M, van der Bok J C, Prins P T, Aebli M, Mews A, Vanmaekelbergh D, Kovalenko M V 2023 Nano Lett. 23 667
 Google Scholar
Google Scholar
[37] Xu Z S, Yang Y J, Wang P, Liu X F, Qiu J R 2024 Ceram. Int. 50 8952
 Google Scholar
Google Scholar
[38] Kovalenko M V, Protesescu L, Bodnarchuk M I 2017 Science 358 745
 Google Scholar
Google Scholar
-
 2024年第73卷096801补充材料.pdf
2024年第73卷096801补充材料.pdf

Catalog
Metrics
- Abstract views: 5532
- PDF Downloads: 94
- Cited By: 0

















 DownLoad:
DownLoad: