-
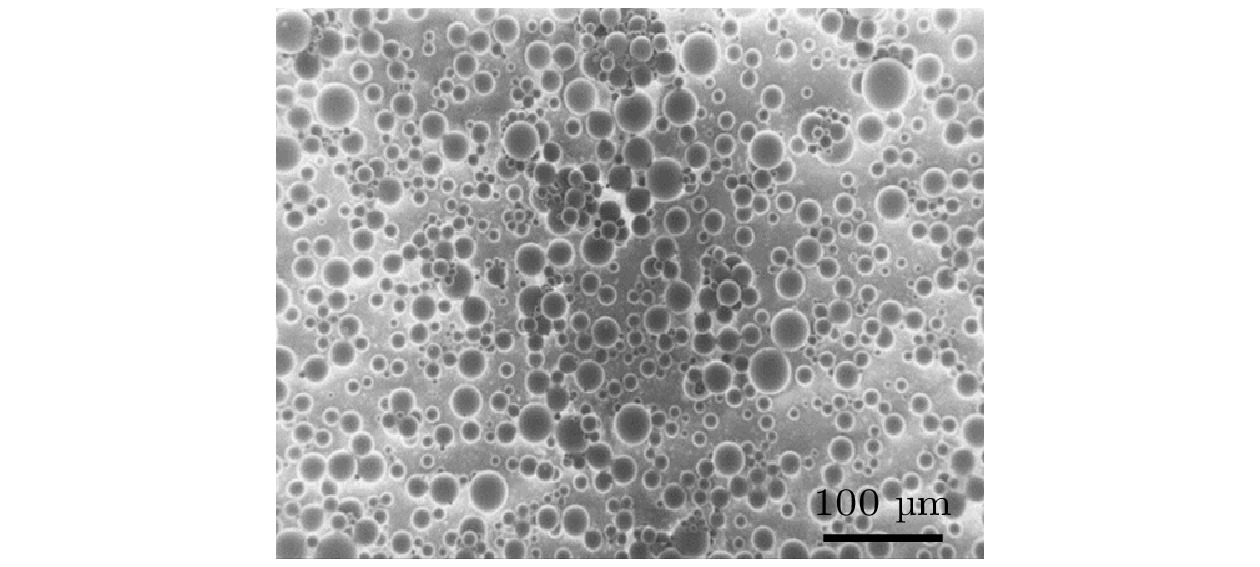
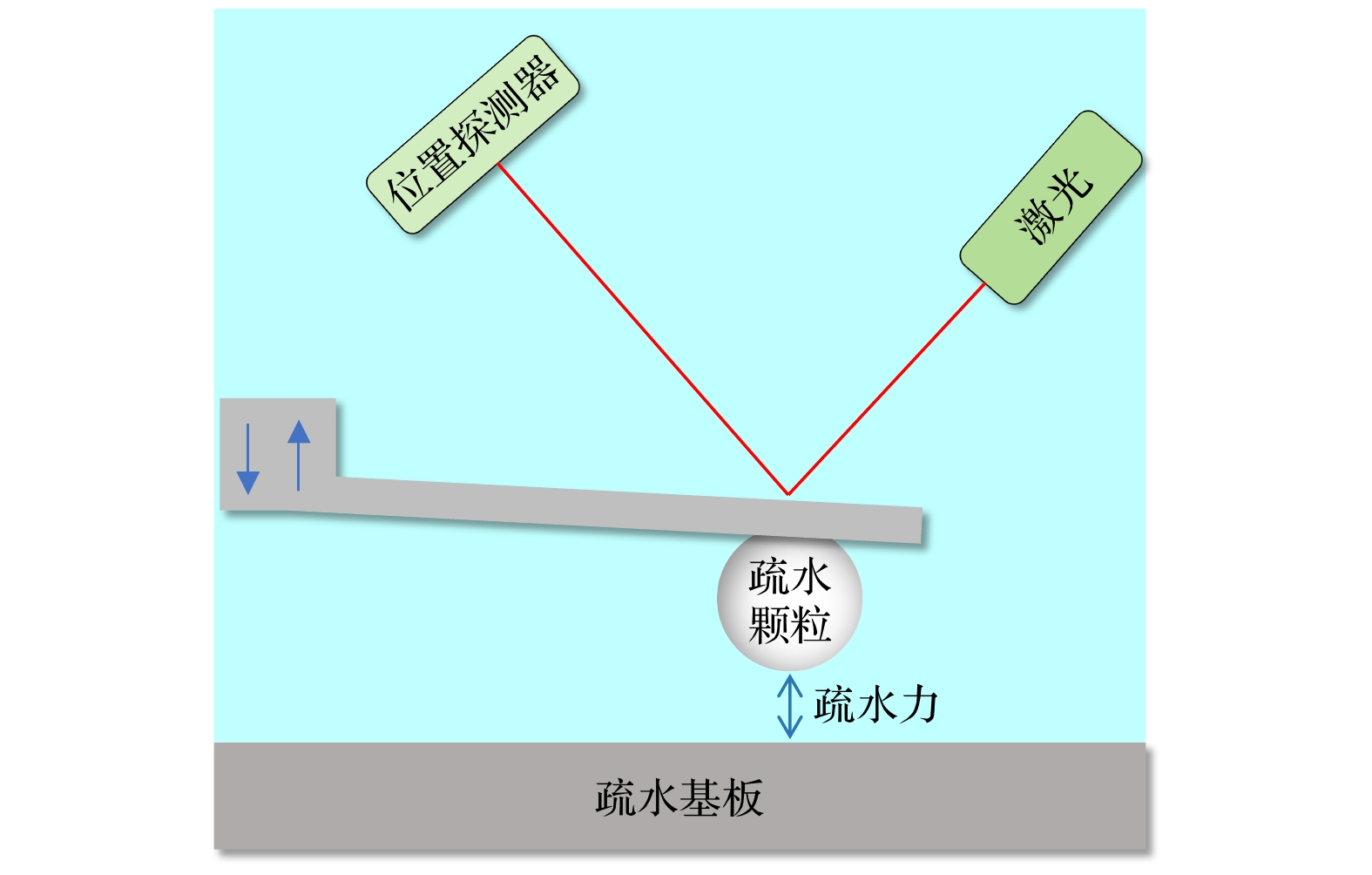
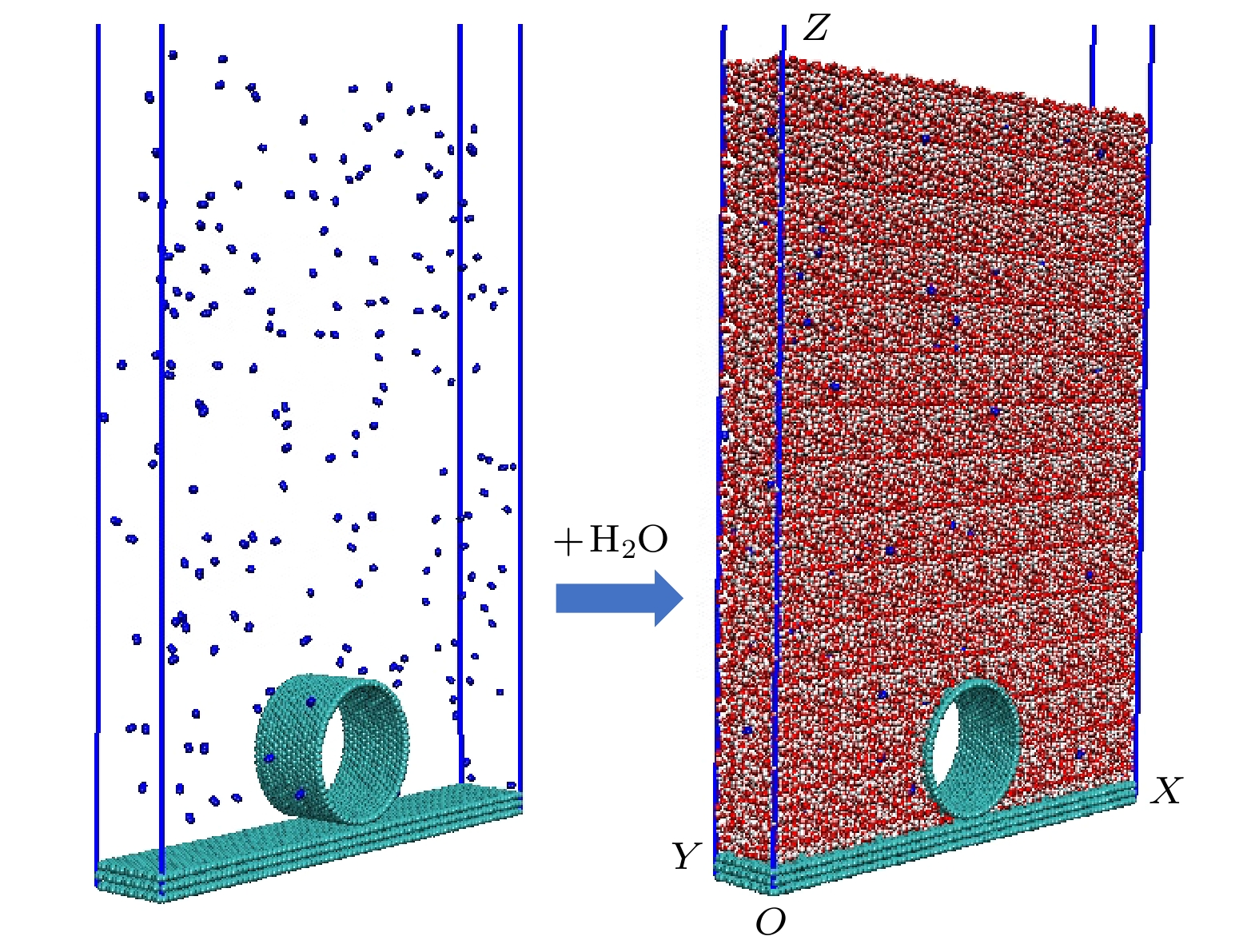
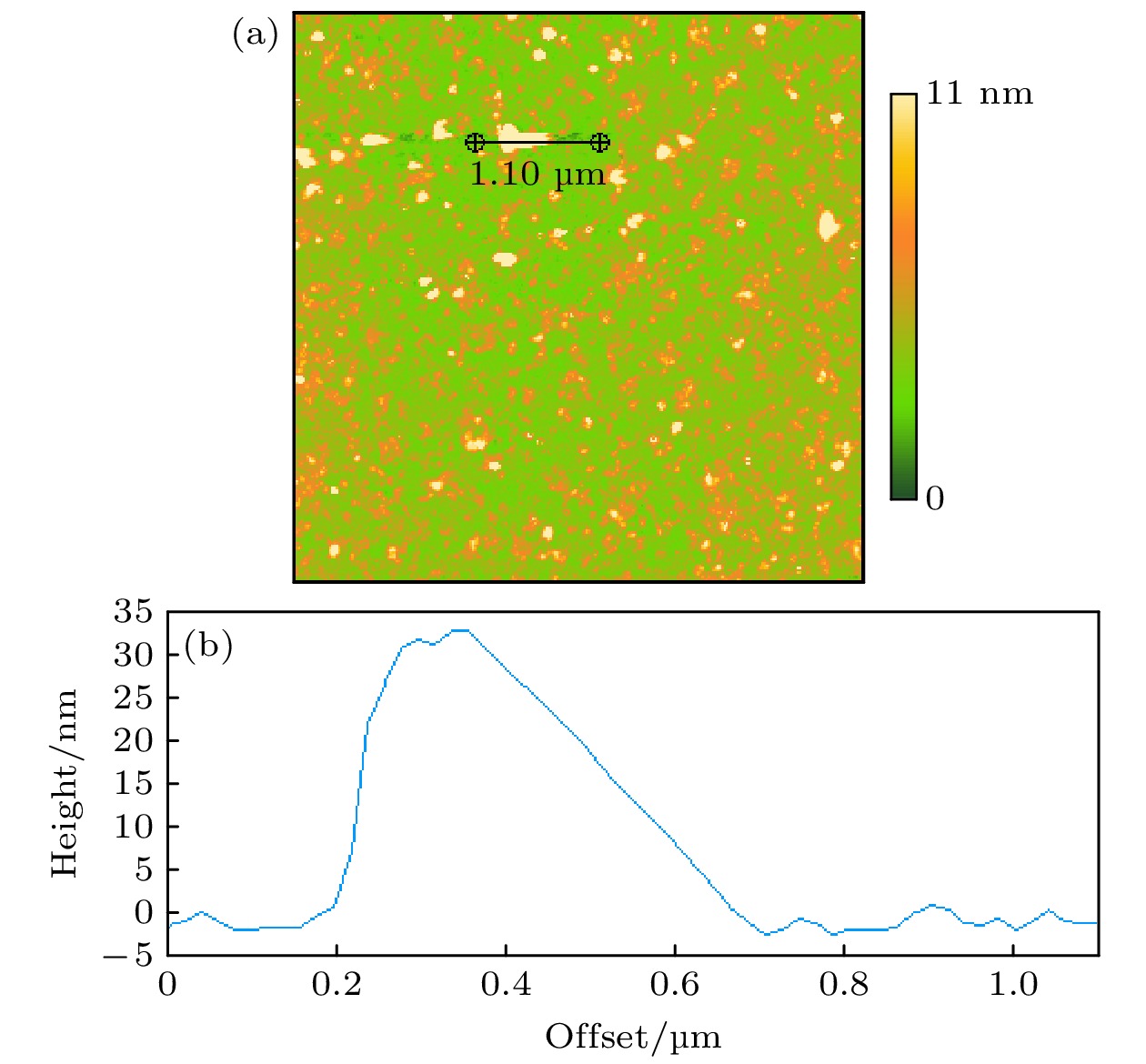
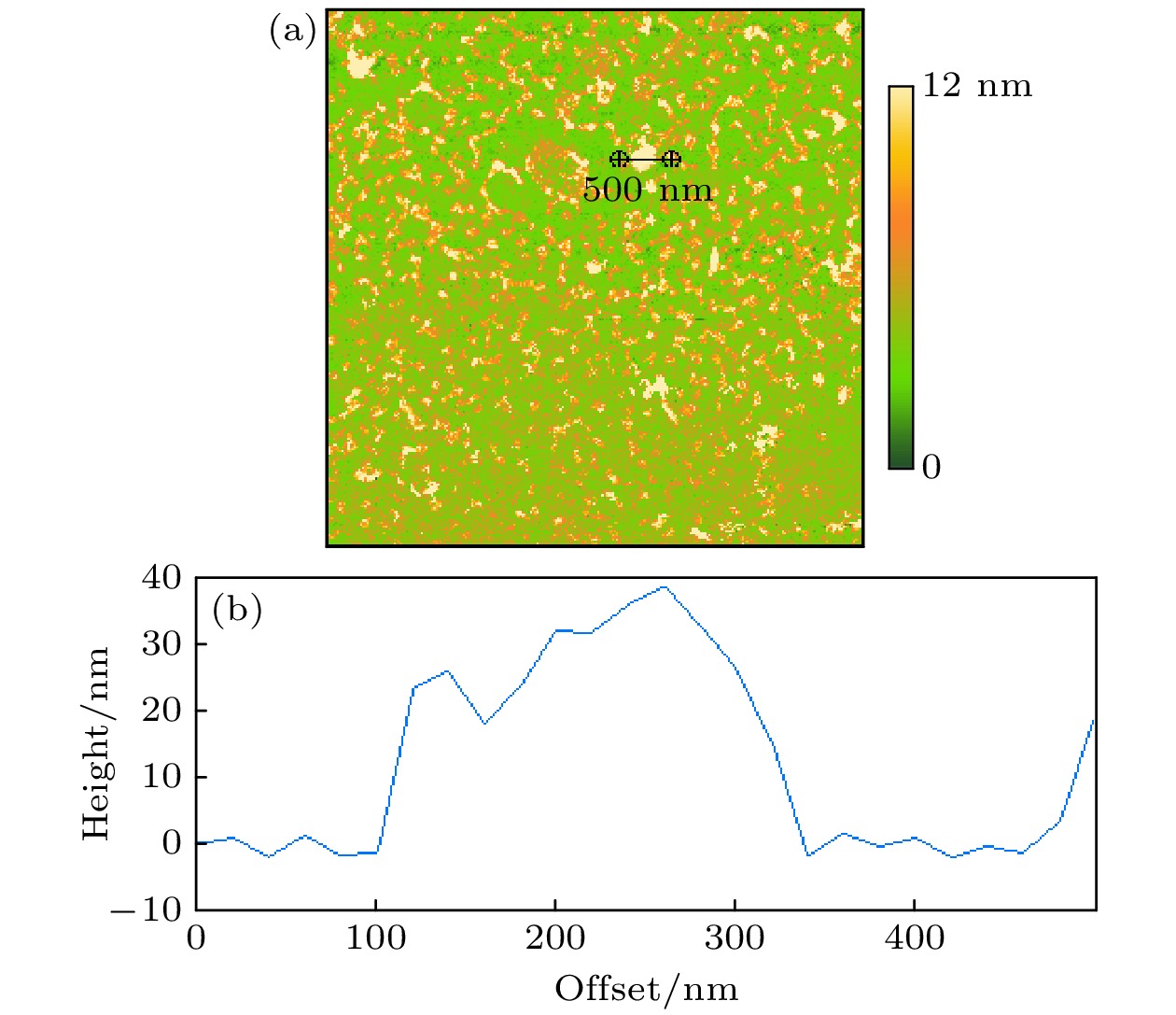
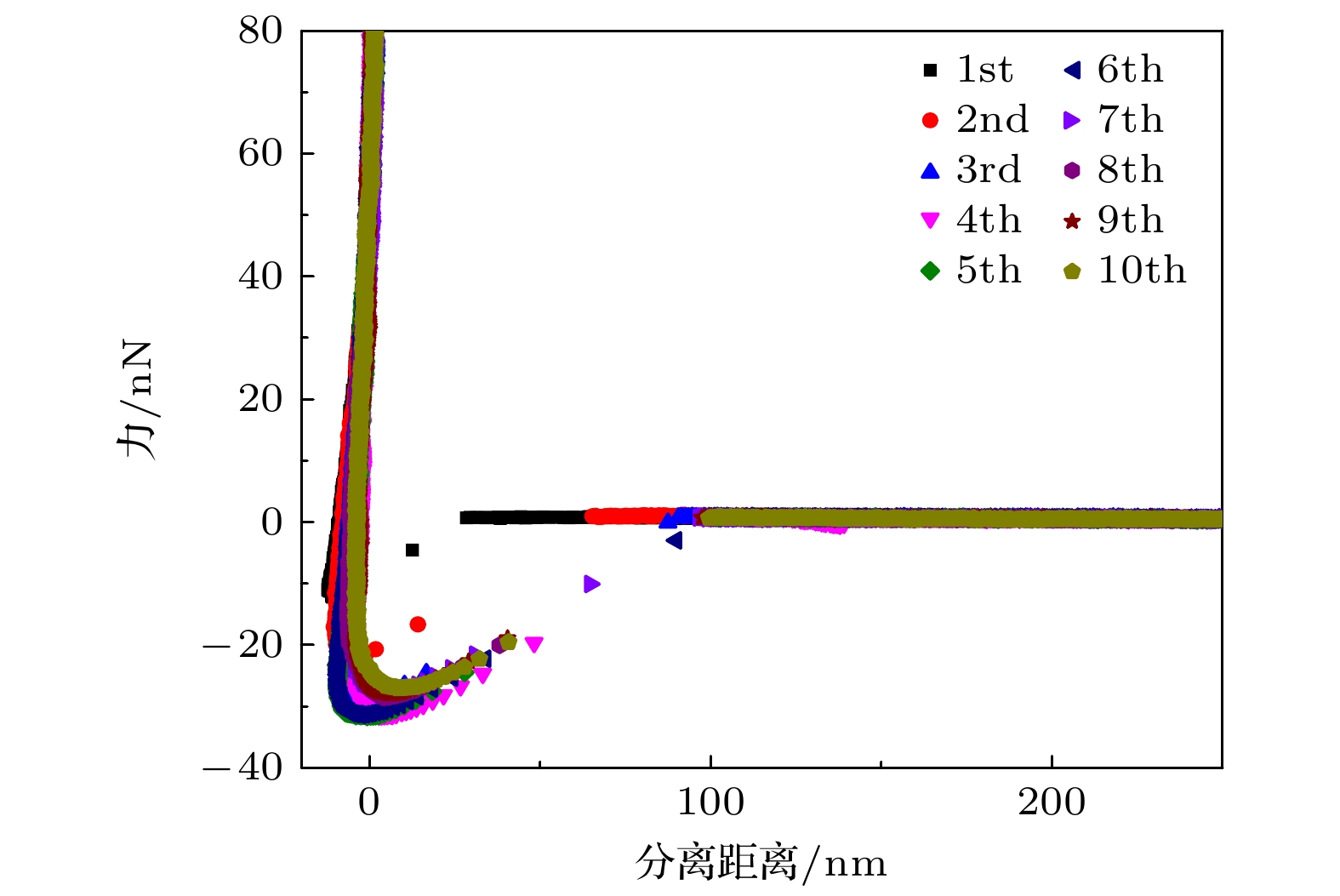
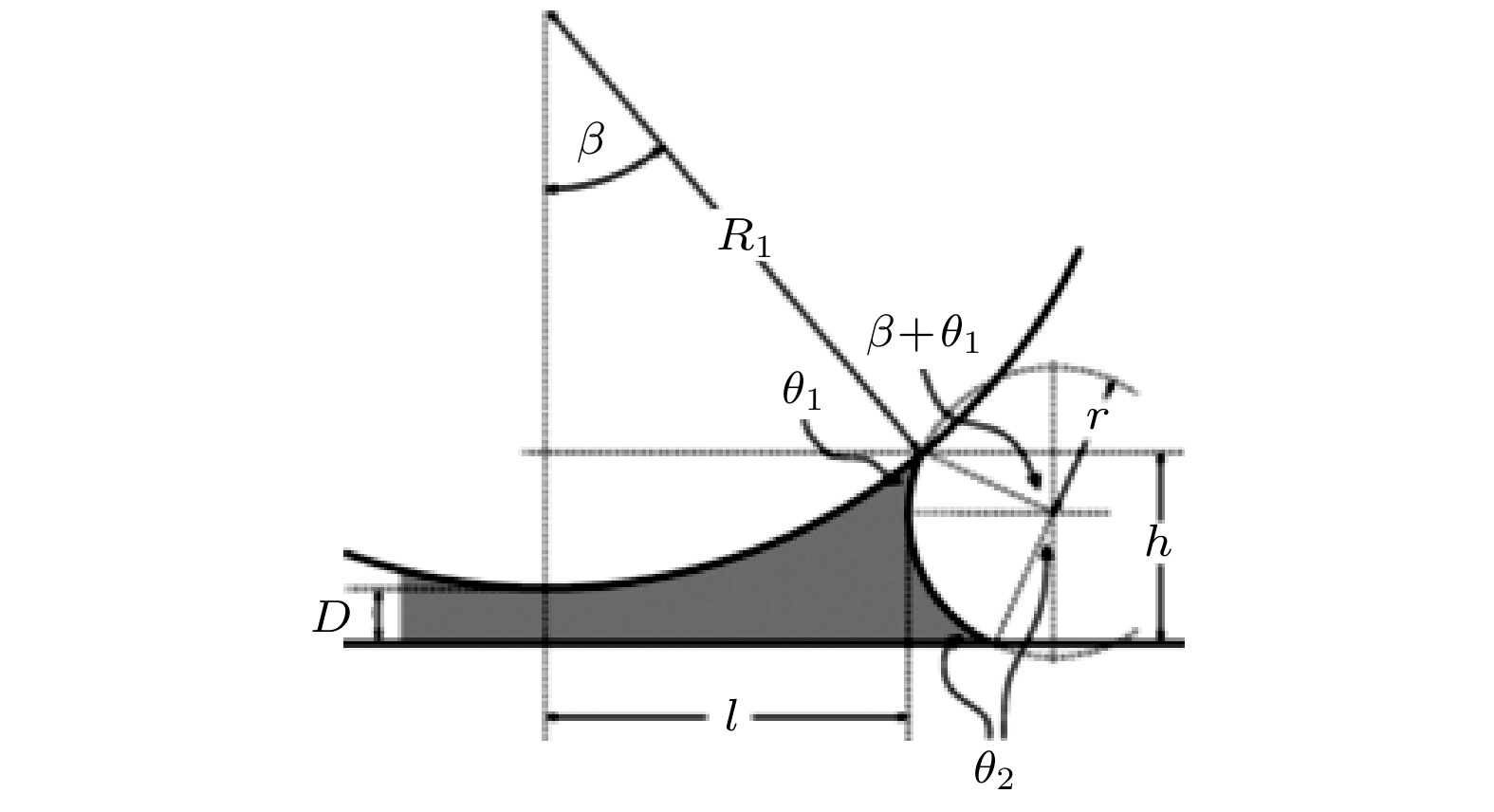
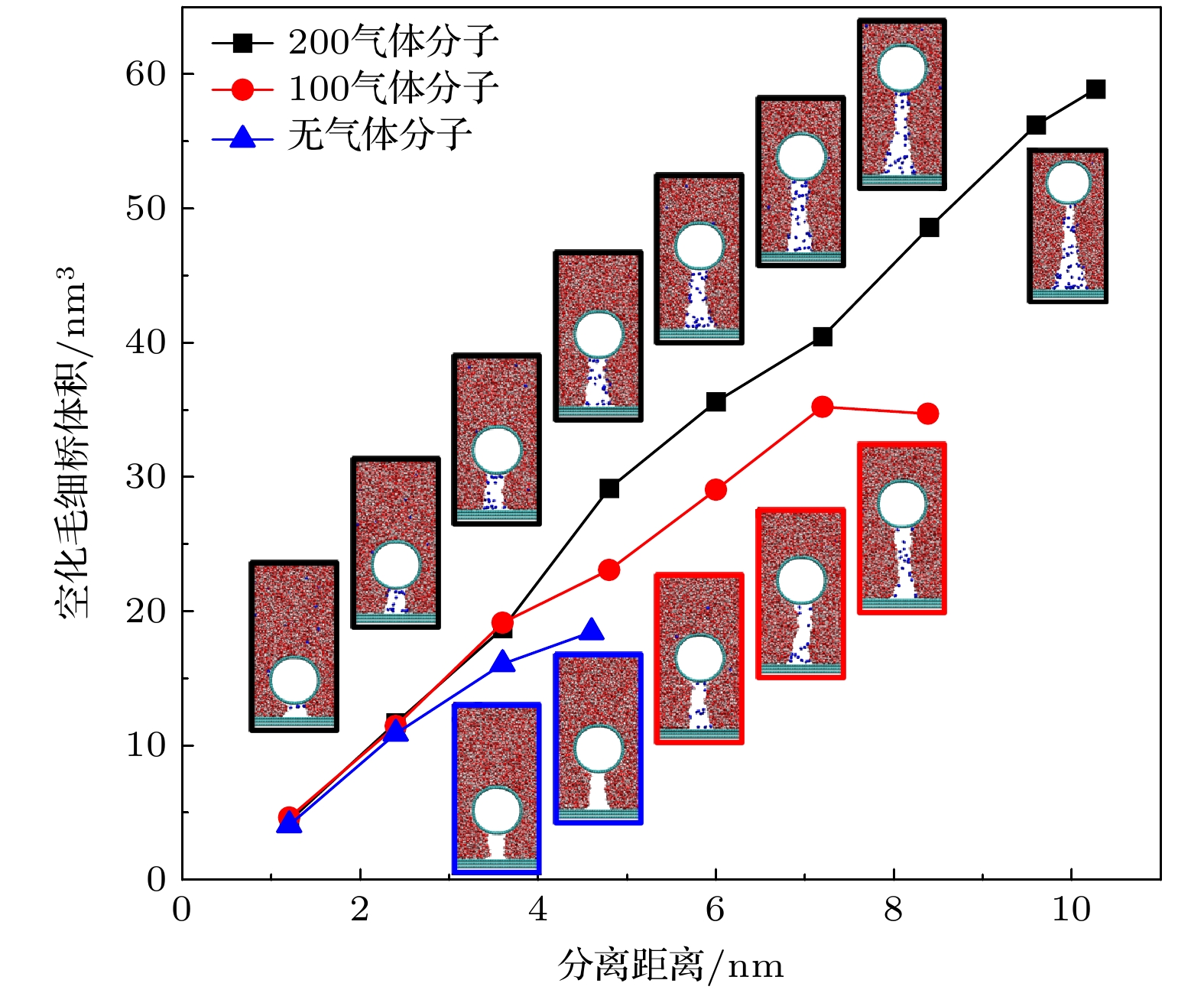
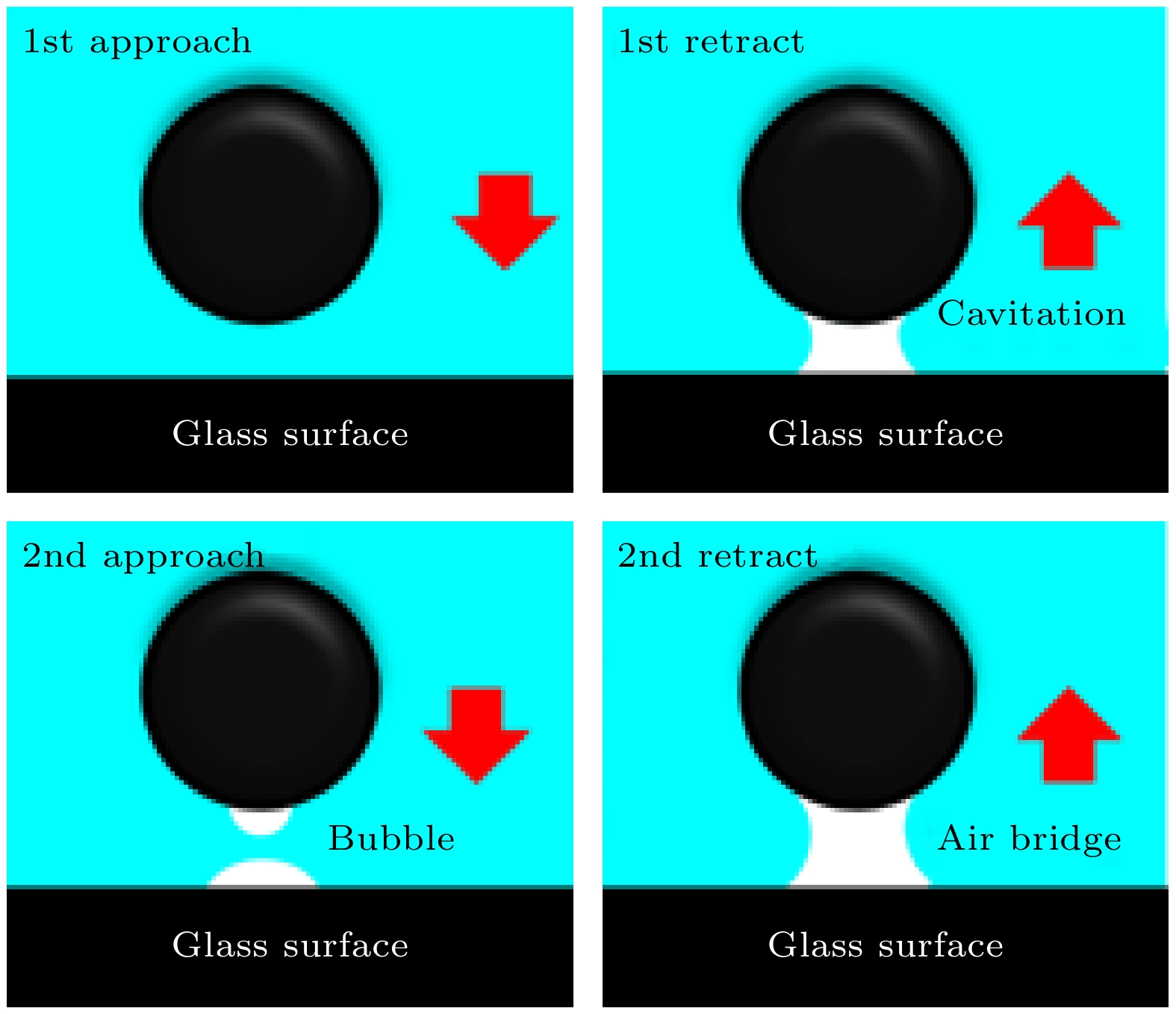
Hydrophobic force, a key driving force in colloid physicochemical system and biological macromolecular system, exhibits a distinct multi-scale effect. The prevailing scholarly consensus attributes long-range hydrophobic force to bubble bridging, facilitated by unstable liquid film cavitation, while short-range force is thought to arise from the reorganization of water molecules at the solid-liquid interface. However, a comprehensive theoretical study remains elusive. To further elucidate the mechanism of the long-range hydrophobic force based on unstable liquid film cavitation, we carry out systematic research on the long-range hydrophobic force between perfluorooctyl trichlorosilane hydrophobic particles and the surface, by utilizing atomic force microscopy (AFM) and molecular dynamics simulations. According to AFM force tests, the long-range hydrophobic force escalates incrementally with subsequent close contacts before reaching a plateau. On the tenth contact, the penetration curve exhibits a sudden jump to an adhesion distance of 502.01 nm. A distinct step in the retraction curve suggests the rupture of the cavitation bubble capillary bridge. Importantly, the classical capillary force mathematical model provides an effective fit for the penetration curve. Our calculations estimate the volume of the capillary bridge at 0.30 μm³, offering direct theoretical evidence of the unstable liquid film cavitation bubble capillary bridge. Further insights are gained from large-scale tensile molecular dynamics simulations by using GROningen MAchine for Chemical Simulations (GROMACS). The inherent correlation mechanism of the formation, evolution, and mechanical behavior of the cavitation bubble capillary bridge in the separation process of hydrophobic particles are further explored from a molecular perspective. The results demonstrate that the local pressure drop occurring at the moment of “jump-out” separation of the hydrophobic particles attracts nitrogen molecules to diffuse into the liquid film, thereby forming a cavitation bubble capillary bridge. Simultaneously, a jumping behavior is observed in the calculated spring potential curve at the moment of capillary bridge rupture. Finally, the influence of solution gas content on long-range hydrophobic force is investigated. There is a positive correlation between gas molecule content and both the growth rate of cavitation bubble capillary bridge volume and the length of capillary bridge stretch-rupture, further demonstrating the gas concentration dependence of long-range hydrophobic forces. In a word, revealing the long-range hydrophobic force mechanism based on the cavitation of unstable liquid film can enhance our understanding of the interaction between colloid physical chemistry and biological macromolecules. Meanwhile, hydrophobic force is the fundamental driving force of particle-bubble adhesion in mineral flotation system, and the revelation of its action mechanism has important guiding significance for regulating the actual mineral flotation process.
-
Keywords:
- long-range hydrophobic force /
- atomic force microscope /
- cavitation /
- capillary force /
- molecular dynamics simulation
[1] 马红孺 2016 65 184701
 Google Scholar
Google Scholar
Ma H R 2016 Acta Phys. Sin. 65 184701
 Google Scholar
Google Scholar
[2] Nikolov A, Lee J, Wasan D 2023 Adv. Colloid Interface Sci. 313 102847
 Google Scholar
Google Scholar
[3] 彭家略, 郭浩, 尤天涯, 纪献兵, 徐进良 2021 70 044701
 Google Scholar
Google Scholar
Peng J L, Guo H, You T Y, Ji X B, Xu J L 2021 Acta Phys. Sin. 70 044701
 Google Scholar
Google Scholar
[4] 赵昶, 纪献兵, 杨聿昊, 孟宇航, 徐进良, 彭家略 2022 71 214701
 Google Scholar
Google Scholar
Zhao C, Ji X B, Yang Y H, Meng Y H, Xu J L, Peng J L 2022 Acta Phys. Sin. 71 214701
 Google Scholar
Google Scholar
[5] Li Q, Liao C, Hou J, Wang W, Zhang J 2022 Fuel 316 123271
 Google Scholar
Google Scholar
[6] 顾帼华, 锁军, 柳建设, 钟素姣 2006 中国有色金属学报 16 1462
 Google Scholar
Google Scholar
Gu G H, Suo J, Liu J S, Zhong S J 2006 Chin. J. of Nonferrous Met. 16 1462
 Google Scholar
Google Scholar
[7] 张天辉, 曹镜声, 梁颖, 刘向阳 2016 65 176401
 Google Scholar
Google Scholar
Zhang T H, Cao J S, Liang Y, Liu X Y 2016 Acta Phys. Sin. 65 176401
 Google Scholar
Google Scholar
[8] 邢耀文, 桂夏辉, 曹亦俊, 刘炯天 2019 煤炭学报 44 3185
 Google Scholar
Google Scholar
Xing Y W, Gui X H, Cao Y J, Liu J T 2019 J. China Coal Soc. 44 3185
 Google Scholar
Google Scholar
[9] 蒋昊, 罗汇丰, 谢佳辉, 韩文平 2022 中国有色金属学报 32 545
 Google Scholar
Google Scholar
Jiang H, Liu H F, Xie J H, Han W P 2022 Chin. J. of Nonferrous Met. 32 545
 Google Scholar
Google Scholar
[10] Christenson H K, Claesson P M 2001 Adv. Colloid Interface Sci. 91 391
 Google Scholar
Google Scholar
[11] Meyer E E, Rosenberg K J, Israelachvili J 2006 Proc. Natl. Acad. Sci. U. S. A. 103 15739
 Google Scholar
Google Scholar
[12] Kékicheff P 2019 Adv. Colloid Interface Sci. 270 191
 Google Scholar
Google Scholar
[13] 王市委, 陈松降, 陶秀祥, 石开仪, 陈鹏, 陈文辉 2019 煤炭学报 44 2236
 Google Scholar
Google Scholar
Wang S W, Chen S J, Tao X X, Shi K Y, Chen P, Chen W H 2019 J. China Coal Soc. 44 2236
 Google Scholar
Google Scholar
[14] Christensen H, Claesson P 1988 Science 239 390
 Google Scholar
Google Scholar
[15] Yakubov G E, Butt H J, Vinogradova O I 2000 J. Phys. Chem. B 104 3407
 Google Scholar
Google Scholar
[16] Azadi M, Nguyen A V, Yakubov G E 2015 Langmuir 31 1941
 Google Scholar
Google Scholar
[17] Faghihnejad A, Zeng H 2012 Soft Matter 8 2746
 Google Scholar
Google Scholar
[18] Abraham M J, Murtola T, Schulz R, Páll S, Smith J C, Hess B, Lindahl E 2015 SoftwareX 1 19
 Google Scholar
Google Scholar
[19] Humphrey W, Dalke A, Schulten K 1996 J. Mol. Graph. 14 33
 Google Scholar
Google Scholar
[20] Yang H, Zhang F, Xing Y, Gui X, Cao Y 2022 Front. Mater. 8 644
 Google Scholar
Google Scholar
[21] Pandit S, Maroli N, Naskar S, Khatri B, Maiti P K, De M 2022 Nanoscale 14 7881
 Google Scholar
Google Scholar
[22] Wang X, Ramírez-Hinestrosa S, Dobnikar J, Frenkel D 2020 Phys. Chem. Chem. Phys. 22 10624
 Google Scholar
Google Scholar
[23] Vassetti D, Pagliai M, Procacci P 2019 J. Chem. Theory Comput. 15 1983
 Google Scholar
Google Scholar
[24] Potoff J J, Ilja Siepmann J 2001 AIChE J. 47 1676
 Google Scholar
Google Scholar
[25] Sedighi M, Talaie M R, Sabzyan H, Aghamiri S, Chen P 2022 Fuel 308 121965
 Google Scholar
Google Scholar
[26] Xia Y C, Zhang R, Cao Y J, Xing Y W, Gui X H 2020 Fuel 262 116535
 Google Scholar
Google Scholar
[27] Zimmermann K 1991 J. Comput. Chem. 12 310
 Google Scholar
Google Scholar
[28] Bussi G, Donadio D, Parrinello M 2007 J. Chem. Phys. 126 14101
 Google Scholar
Google Scholar
[29] Essmann U, Perera L, Berkowitz M L, Darden T, Lee H, Pedersen L G 1995 J. Chem. Phys. 103 8577
 Google Scholar
Google Scholar
[30] Hammer M U, Anderson T H, Chaimovich A, Shell M S, Israelachvili J 2010 Faraday Discuss. 146 299
 Google Scholar
Google Scholar
[31] Mezger M, Reichert H, Schöder S, Okasinski J, Schröder H, Dosch H, Palms D, Ralston J, Honkimäki V 2006 Proc. Natl. Acad. Sci. U. S. A. 103 18401
 Google Scholar
Google Scholar
[32] Butt H J, Kappl M 2009 Adv. Colloid Interface Sci. 146 48
 Google Scholar
Google Scholar
[33] Brown D, Neyertz S 1995 Molecular Physics 84 577
 Google Scholar
Google Scholar
-
-
[1] 马红孺 2016 65 184701
 Google Scholar
Google Scholar
Ma H R 2016 Acta Phys. Sin. 65 184701
 Google Scholar
Google Scholar
[2] Nikolov A, Lee J, Wasan D 2023 Adv. Colloid Interface Sci. 313 102847
 Google Scholar
Google Scholar
[3] 彭家略, 郭浩, 尤天涯, 纪献兵, 徐进良 2021 70 044701
 Google Scholar
Google Scholar
Peng J L, Guo H, You T Y, Ji X B, Xu J L 2021 Acta Phys. Sin. 70 044701
 Google Scholar
Google Scholar
[4] 赵昶, 纪献兵, 杨聿昊, 孟宇航, 徐进良, 彭家略 2022 71 214701
 Google Scholar
Google Scholar
Zhao C, Ji X B, Yang Y H, Meng Y H, Xu J L, Peng J L 2022 Acta Phys. Sin. 71 214701
 Google Scholar
Google Scholar
[5] Li Q, Liao C, Hou J, Wang W, Zhang J 2022 Fuel 316 123271
 Google Scholar
Google Scholar
[6] 顾帼华, 锁军, 柳建设, 钟素姣 2006 中国有色金属学报 16 1462
 Google Scholar
Google Scholar
Gu G H, Suo J, Liu J S, Zhong S J 2006 Chin. J. of Nonferrous Met. 16 1462
 Google Scholar
Google Scholar
[7] 张天辉, 曹镜声, 梁颖, 刘向阳 2016 65 176401
 Google Scholar
Google Scholar
Zhang T H, Cao J S, Liang Y, Liu X Y 2016 Acta Phys. Sin. 65 176401
 Google Scholar
Google Scholar
[8] 邢耀文, 桂夏辉, 曹亦俊, 刘炯天 2019 煤炭学报 44 3185
 Google Scholar
Google Scholar
Xing Y W, Gui X H, Cao Y J, Liu J T 2019 J. China Coal Soc. 44 3185
 Google Scholar
Google Scholar
[9] 蒋昊, 罗汇丰, 谢佳辉, 韩文平 2022 中国有色金属学报 32 545
 Google Scholar
Google Scholar
Jiang H, Liu H F, Xie J H, Han W P 2022 Chin. J. of Nonferrous Met. 32 545
 Google Scholar
Google Scholar
[10] Christenson H K, Claesson P M 2001 Adv. Colloid Interface Sci. 91 391
 Google Scholar
Google Scholar
[11] Meyer E E, Rosenberg K J, Israelachvili J 2006 Proc. Natl. Acad. Sci. U. S. A. 103 15739
 Google Scholar
Google Scholar
[12] Kékicheff P 2019 Adv. Colloid Interface Sci. 270 191
 Google Scholar
Google Scholar
[13] 王市委, 陈松降, 陶秀祥, 石开仪, 陈鹏, 陈文辉 2019 煤炭学报 44 2236
 Google Scholar
Google Scholar
Wang S W, Chen S J, Tao X X, Shi K Y, Chen P, Chen W H 2019 J. China Coal Soc. 44 2236
 Google Scholar
Google Scholar
[14] Christensen H, Claesson P 1988 Science 239 390
 Google Scholar
Google Scholar
[15] Yakubov G E, Butt H J, Vinogradova O I 2000 J. Phys. Chem. B 104 3407
 Google Scholar
Google Scholar
[16] Azadi M, Nguyen A V, Yakubov G E 2015 Langmuir 31 1941
 Google Scholar
Google Scholar
[17] Faghihnejad A, Zeng H 2012 Soft Matter 8 2746
 Google Scholar
Google Scholar
[18] Abraham M J, Murtola T, Schulz R, Páll S, Smith J C, Hess B, Lindahl E 2015 SoftwareX 1 19
 Google Scholar
Google Scholar
[19] Humphrey W, Dalke A, Schulten K 1996 J. Mol. Graph. 14 33
 Google Scholar
Google Scholar
[20] Yang H, Zhang F, Xing Y, Gui X, Cao Y 2022 Front. Mater. 8 644
 Google Scholar
Google Scholar
[21] Pandit S, Maroli N, Naskar S, Khatri B, Maiti P K, De M 2022 Nanoscale 14 7881
 Google Scholar
Google Scholar
[22] Wang X, Ramírez-Hinestrosa S, Dobnikar J, Frenkel D 2020 Phys. Chem. Chem. Phys. 22 10624
 Google Scholar
Google Scholar
[23] Vassetti D, Pagliai M, Procacci P 2019 J. Chem. Theory Comput. 15 1983
 Google Scholar
Google Scholar
[24] Potoff J J, Ilja Siepmann J 2001 AIChE J. 47 1676
 Google Scholar
Google Scholar
[25] Sedighi M, Talaie M R, Sabzyan H, Aghamiri S, Chen P 2022 Fuel 308 121965
 Google Scholar
Google Scholar
[26] Xia Y C, Zhang R, Cao Y J, Xing Y W, Gui X H 2020 Fuel 262 116535
 Google Scholar
Google Scholar
[27] Zimmermann K 1991 J. Comput. Chem. 12 310
 Google Scholar
Google Scholar
[28] Bussi G, Donadio D, Parrinello M 2007 J. Chem. Phys. 126 14101
 Google Scholar
Google Scholar
[29] Essmann U, Perera L, Berkowitz M L, Darden T, Lee H, Pedersen L G 1995 J. Chem. Phys. 103 8577
 Google Scholar
Google Scholar
[30] Hammer M U, Anderson T H, Chaimovich A, Shell M S, Israelachvili J 2010 Faraday Discuss. 146 299
 Google Scholar
Google Scholar
[31] Mezger M, Reichert H, Schöder S, Okasinski J, Schröder H, Dosch H, Palms D, Ralston J, Honkimäki V 2006 Proc. Natl. Acad. Sci. U. S. A. 103 18401
 Google Scholar
Google Scholar
[32] Butt H J, Kappl M 2009 Adv. Colloid Interface Sci. 146 48
 Google Scholar
Google Scholar
[33] Brown D, Neyertz S 1995 Molecular Physics 84 577
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 4752
- PDF Downloads: 276
- Cited By: 0















 DownLoad:
DownLoad: