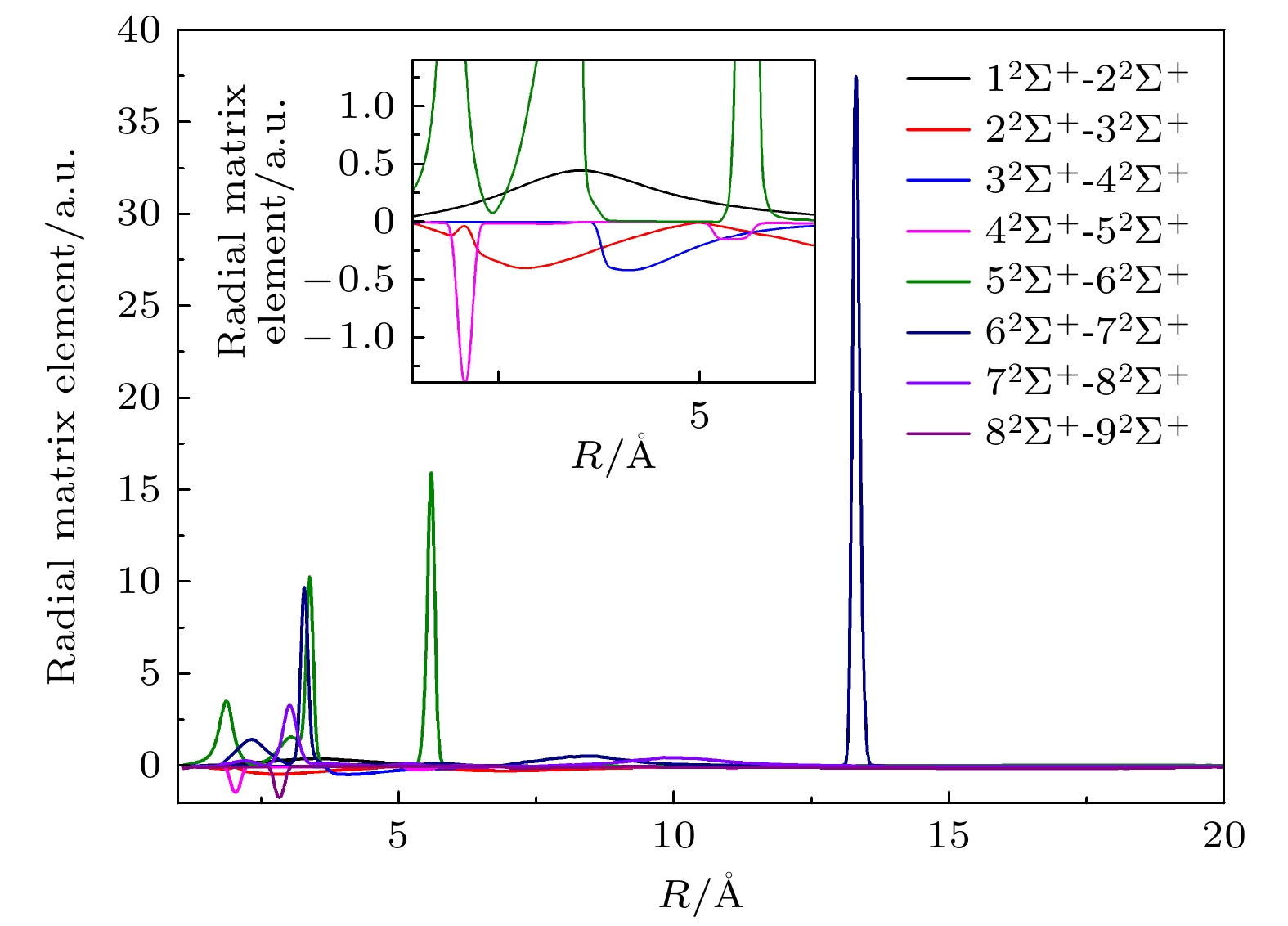
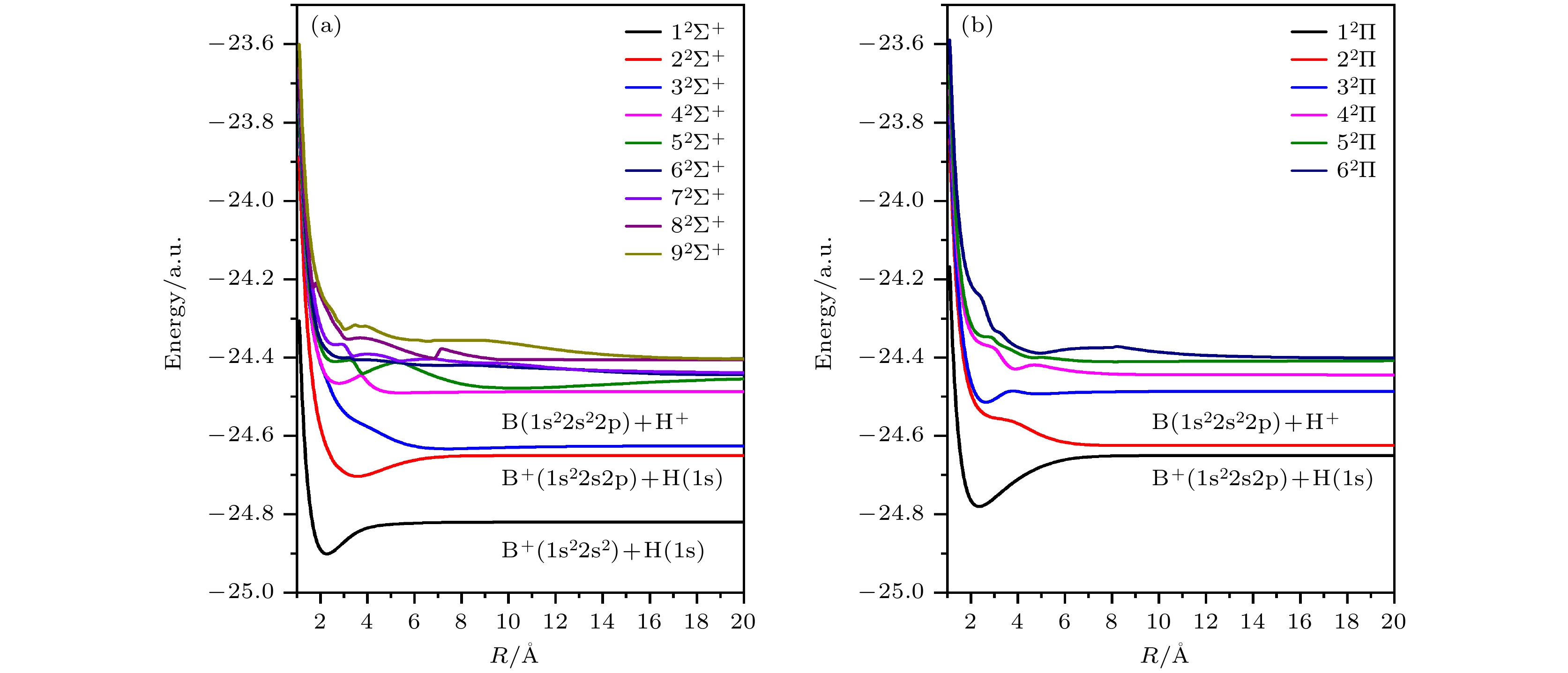
-
Electron transfer in heavy particle collisions, involving complicated electron correlation mechanisms, greatly affects the charge state balance in plasma, and is also one of important sources for X-ray radiation. Electron transfer cross section and rate coefficient are important atomic parameters required for the development of nuclear fusion plasma in the national defense industry. We systematically investigate the electron transfer process of proton impacting boron atom in an energy range of
${10}^{-3}-{10}^{3}\;{\rm{e}}{\rm{V}}/{\rm{u}}$ based on a fully quantum non-radiative molecular orbital close-coupling method. A total of 15 channels of electron transfer, excitation, and elastic scattering are obtained by using the multi-reference configuration interaction method, and the corresponding molecular energy of each channel is in good agreement with the experimental results. The phenomenon of avoiding crossing between the adiabatic potential energy curves of molecular states is obvious, which constituents the main pathway of electron transfer. The calculation shows that the electron transfer of the 2s orbital is dominated in the process of proton impacting boron atom, while the electron transfer of the 2p orbital contributes a little. In the low energy region, there are obvious quantum resonances in the electron transfer cross section, which originate from the coupling between high energy channel and low energy channel. In addition, we calculate the electron transfer rates of proton-impact boron atom at different temperatures, which can provide important atomic parameter which support for simulating and diagnosing complex plasma environments.-
Keywords:
- electron transfer /
- heavy ion collision /
- potential curve
[1] Janev R K, Presnyakov L P, Shevelko V P 1985 Physics of Highly Charged Ions (Berlin-Heidelberg: Springer) pp1–6
[2] Loarte A, Lipschultz B, Kukushkin A S 2007 Nucl. Fusion 47 S203
 Google Scholar
Google Scholar
[3] Kallman T R, Palmeri P 2007 Rev. Mod. Phys. 79 79
 Google Scholar
Google Scholar
[4] 刘春华 2009 博士学位论文 (北京: 中国科学院大学)
Liu C H 2009 Ph. D. Dissertation (Beijing: University of the Chinese Academy of Sciences) (in Chinese)
[5] 林晓贺 2019 博士学位论文 (北京: 北京理工大学)
Lin X H 2019 Ph. D. Dissertation (Beijing: Beijing Institute of Technology) (in Chinese)
[6] Liu L, Liu C H, Wang J G, Janev R K 2011 Phys. Rev. A 84 032710
 Google Scholar
Google Scholar
[7] Gao J W, Wu Y, Sisourat N, Wang J G, Dubois A 2017 Phys. Rev. A 96 052703
 Google Scholar
Google Scholar
[8] Gao J W, Wu Y, Wang J G, Sisourat N, Dubois A 2018 Phys. Rev. A 97 052709
 Google Scholar
Google Scholar
[9] Zhang Y W, Gao J G, Wu Y, Zhou F Y, Wang J G, Sisourat N, Dubois A 2020 Phys. Rev. A 102 022814
 Google Scholar
Google Scholar
[10] Liu C H, Wang J G, Janev R K 2012 Phys. Rev. A 85 042719
 Google Scholar
Google Scholar
[11] Yan L L, Wu Y, Qu Y Z, Wang J G, Buenker R J 2013 Phys. Rev. A 88 022706
 Google Scholar
Google Scholar
[12] Liu C H, Liu L, Wang J G 2014 Phys. Rev. A 90 012708
 Google Scholar
Google Scholar
[13] Liu C H, Wang J G 2013 Phys. Rev. A 87 042709
 Google Scholar
Google Scholar
[14] Liu C H, Wang J G, Janev R K 2014 Phys. Rev. A 89 062719
 Google Scholar
Google Scholar
[15] Bruhns H, Kreckel H, Savin D W, seely D G, Havener C C 2008 Phys. Rev. A 77 064702
 Google Scholar
Google Scholar
[16] Kim H J, Phaneuf R A, Meyer F W, Stelson P H 1987 Phys. Rev. A 17 854
[17] Gieler M, Ziegelwanger P, Aumayr F, Winter H, Fritsch W 1991 J. Phys. B 24 647
 Google Scholar
Google Scholar
[18] Ebel F, Salzborn E 1987 J. Phys. B 20 4531
 Google Scholar
Google Scholar
[19] Hayakawa S, Kadomura K, Kimura M, Dutta C M 2004 Phys. Rev. A 70 022708
 Google Scholar
Google Scholar
[20] Hayakawa H, Helm H, Briggs J S, Salzborn E 2007 Phys. Rev. Lett 99 173202
 Google Scholar
Google Scholar
[21] Kim H K, Schoffler M S, Houamer S, Chuluunbaatar O, Titze J N, Schmidt L P H, Jahnke T, Schmidt-Bocking H, Galstyan A, Popov Y V, Dorner R 2012 Phys. Rev. A 85 022707
 Google Scholar
Google Scholar
[22] Bayfield J E, Khayrallah G A 1975 Phys. Rev. A 11 920
 Google Scholar
Google Scholar
[23] Buenker R J, Liebermann H P, Whitten J L 2001 Chem. Phys. 265 1
 Google Scholar
Google Scholar
[24] Buenker R J, Liebermann H P, Izgorodina E I 2003 Chem. Phys. 291 115
 Google Scholar
Google Scholar
[25] Alcheev P G, Buenker R J, Chernov V, Zon B A 2003 J. Mol. Spectrosc. 218 190
 Google Scholar
Google Scholar
-
表 1 不同分子态对应的解离极限下的原子态以及态能量的理论结果和实验结果
Table 1. Atomic states corresponding to different molecular states under dissociation limit, and the theoretical and experimental results of state energy.
原子态 分子态 能量/eV 理论 实验 误差 $ {{\rm{B}}}^{+}\left(1{{\rm{s}}}^{2}2{{\rm{s}}}^{2}\right)+{\rm{H}}\left(1{\rm{s}}\right) $ $ 1{}_{}{}^{2}{{{\Sigma }}}^{+} $ 0 0 0 $ {{\rm{B}}}^{+}\left(1{{\rm{s}}}^{2}2{{\rm{s}}}^{}2{\rm{p}}\right)+{\rm{H}}\left(1{\rm{s}}\right) $ $ 2{}_{}{}^{2}{{{\Sigma }}}^{+}1{}_{}{}^{2}{{\Pi }} $ 4.6226 4.6230 0.0004 $ {{\rm{B}}}^{}\left(1{{\rm{s}}}^{2}2{{\rm{s}}}^{2}2{\rm{p}}\right)+{{\rm{H}}}^{+} $ $ 3{}_{}{}^{2}{{{\Sigma }}}^{+}2{}_{}{}^{2}{{\Pi }} $ 5.3042 5.3003 0.0039 $ {{\rm{B}}}^{+}\left(1{{\rm{s}}}^{2}2{{\rm{s}}}^{}2{\rm{p}}\right)+{\rm{H}}\left(1{\rm{s}}\right) $ $ 4{}_{}{}^{2}{{{\Sigma }}}^{+}3{}_{}{}^{2}{{\Pi }} $ 9.0663 9.1001 0.0338 $ {{\rm{B}}}^{+}\left(1{{\rm{s}}}^{2}2{{\rm{s}}}^{2}\right)+{\rm{H}}\left(2{\rm{p}}\right) $ $ 5{}_{}{}^{2}{{{\Sigma }}}^{+}4{}_{}{}^{2}{{\Pi }} $ 10.1769 10.1987 0.0218 $ {{\rm{B}}}^{+}\left(1{{\rm{s}}}^{2}2{{\rm{s}}}^{2}\right)+{\rm{H}}\left(2{\rm{s}}\right) $ $ 6{}_{}{}^{2}{{{\Sigma }}}^{+} $ 10.1929 10.1987 0.0058 $ {{\rm{B}}}^{}\left(1{{\rm{s}}}^{2}2{{\rm{s}}}^{2}3{\rm{s}}\right)+{{\rm{H}}}^{+} $ $ 7{}_{}{}^{2}{{{\Sigma }}}^{+} $ 10.2615 10.2597 0.0018 $ {{\rm{B}}}^{}\left(1{{\rm{s}}}^{2}2{\rm{s}}2{{\rm{p}}}^{2}\right)+{{\rm{H}}}^{+} $ $ 8{}_{}{}^{2}{{{\Sigma }}}^{+}5{}_{}{}^{2}{{\Pi }} $ 11.2491 11.2337 0.0154 $ {{\rm{B}}}^{}\left(1{{\rm{s}}}^{2}2{{\rm{s}}}^{2}3{\rm{s}}\right)+{{\rm{H}}}^{+} $ $ 9{}_{}{}^{2}{{{\Sigma }}}^{+}6{}_{}{}^{2}{{\Pi }} $ 11.3623 11.3273 0.0350 表 2 电子转移总的速率系数, 其中包含2s和2p轨道的电子转移
Table 2. Total rate coefficient of electron transfer, including electron transfer of 2s and 2p orbitals.
$ T/{\rm{K}} $ $ 2 $ $ 5 $ $ 10 $ $ 40 $ $\alpha /\left({10}^{-12}~{ {\rm{c} }{\rm{m} } }^{3}{\cdot{\rm{s} } }^{-1}\right)$ $ 1.72\times {10}^{-8} $ $ 5.09\times {10}^{-4} $ $ 1.40\times {10}^{-2} $ $ 1.24\times {10}^{-1} $ $ T/{\rm{K}} $ $ 100 $ $ 500 $ $ 1000 $ $ 6000 $ $\alpha /\left({10}^{-14}~{ {\rm{c} }{\rm{m} } }^{3}{\cdot{\rm{s} } }^{-1}\right)$ $ 14.7 $ $ 10.3 $ $ 8.62 $ $ 46.9 $ $T/{(10}^{5}~{\rm{K} })$ $ 0.1 $ $ 0.5 $ $ 1.0 $ $ 5.0 $ $\alpha /\left({10}^{-12}~{ {\rm{c} }{\rm{m} } }^{3}{\cdot{\rm{s} } }^{-1}\right)$ $ 1.72 $ $ 8.91\times {10}^{1} $ $ 3.86\times {10}^{2} $ $ 4.74\times {10}^{3} $ $T/{(10}^{6}~{\rm{K} })$ $ 1.0 $ $ 5.0 $ $ 10 $ $ 50 $ $\alpha /\left({10}^{-9}~{ {\rm{c} }{\rm{m} } }^{3}{\cdot{\rm{s} } }^{-1}\right)$ $ 9.38 $ $ 25.8 $ $ 26.8 $ $ 7.19 $ -
[1] Janev R K, Presnyakov L P, Shevelko V P 1985 Physics of Highly Charged Ions (Berlin-Heidelberg: Springer) pp1–6
[2] Loarte A, Lipschultz B, Kukushkin A S 2007 Nucl. Fusion 47 S203
 Google Scholar
Google Scholar
[3] Kallman T R, Palmeri P 2007 Rev. Mod. Phys. 79 79
 Google Scholar
Google Scholar
[4] 刘春华 2009 博士学位论文 (北京: 中国科学院大学)
Liu C H 2009 Ph. D. Dissertation (Beijing: University of the Chinese Academy of Sciences) (in Chinese)
[5] 林晓贺 2019 博士学位论文 (北京: 北京理工大学)
Lin X H 2019 Ph. D. Dissertation (Beijing: Beijing Institute of Technology) (in Chinese)
[6] Liu L, Liu C H, Wang J G, Janev R K 2011 Phys. Rev. A 84 032710
 Google Scholar
Google Scholar
[7] Gao J W, Wu Y, Sisourat N, Wang J G, Dubois A 2017 Phys. Rev. A 96 052703
 Google Scholar
Google Scholar
[8] Gao J W, Wu Y, Wang J G, Sisourat N, Dubois A 2018 Phys. Rev. A 97 052709
 Google Scholar
Google Scholar
[9] Zhang Y W, Gao J G, Wu Y, Zhou F Y, Wang J G, Sisourat N, Dubois A 2020 Phys. Rev. A 102 022814
 Google Scholar
Google Scholar
[10] Liu C H, Wang J G, Janev R K 2012 Phys. Rev. A 85 042719
 Google Scholar
Google Scholar
[11] Yan L L, Wu Y, Qu Y Z, Wang J G, Buenker R J 2013 Phys. Rev. A 88 022706
 Google Scholar
Google Scholar
[12] Liu C H, Liu L, Wang J G 2014 Phys. Rev. A 90 012708
 Google Scholar
Google Scholar
[13] Liu C H, Wang J G 2013 Phys. Rev. A 87 042709
 Google Scholar
Google Scholar
[14] Liu C H, Wang J G, Janev R K 2014 Phys. Rev. A 89 062719
 Google Scholar
Google Scholar
[15] Bruhns H, Kreckel H, Savin D W, seely D G, Havener C C 2008 Phys. Rev. A 77 064702
 Google Scholar
Google Scholar
[16] Kim H J, Phaneuf R A, Meyer F W, Stelson P H 1987 Phys. Rev. A 17 854
[17] Gieler M, Ziegelwanger P, Aumayr F, Winter H, Fritsch W 1991 J. Phys. B 24 647
 Google Scholar
Google Scholar
[18] Ebel F, Salzborn E 1987 J. Phys. B 20 4531
 Google Scholar
Google Scholar
[19] Hayakawa S, Kadomura K, Kimura M, Dutta C M 2004 Phys. Rev. A 70 022708
 Google Scholar
Google Scholar
[20] Hayakawa H, Helm H, Briggs J S, Salzborn E 2007 Phys. Rev. Lett 99 173202
 Google Scholar
Google Scholar
[21] Kim H K, Schoffler M S, Houamer S, Chuluunbaatar O, Titze J N, Schmidt L P H, Jahnke T, Schmidt-Bocking H, Galstyan A, Popov Y V, Dorner R 2012 Phys. Rev. A 85 022707
 Google Scholar
Google Scholar
[22] Bayfield J E, Khayrallah G A 1975 Phys. Rev. A 11 920
 Google Scholar
Google Scholar
[23] Buenker R J, Liebermann H P, Whitten J L 2001 Chem. Phys. 265 1
 Google Scholar
Google Scholar
[24] Buenker R J, Liebermann H P, Izgorodina E I 2003 Chem. Phys. 291 115
 Google Scholar
Google Scholar
[25] Alcheev P G, Buenker R J, Chernov V, Zon B A 2003 J. Mol. Spectrosc. 218 190
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 4330
- PDF Downloads: 87
- Cited By: 0



















 DownLoad:
DownLoad: