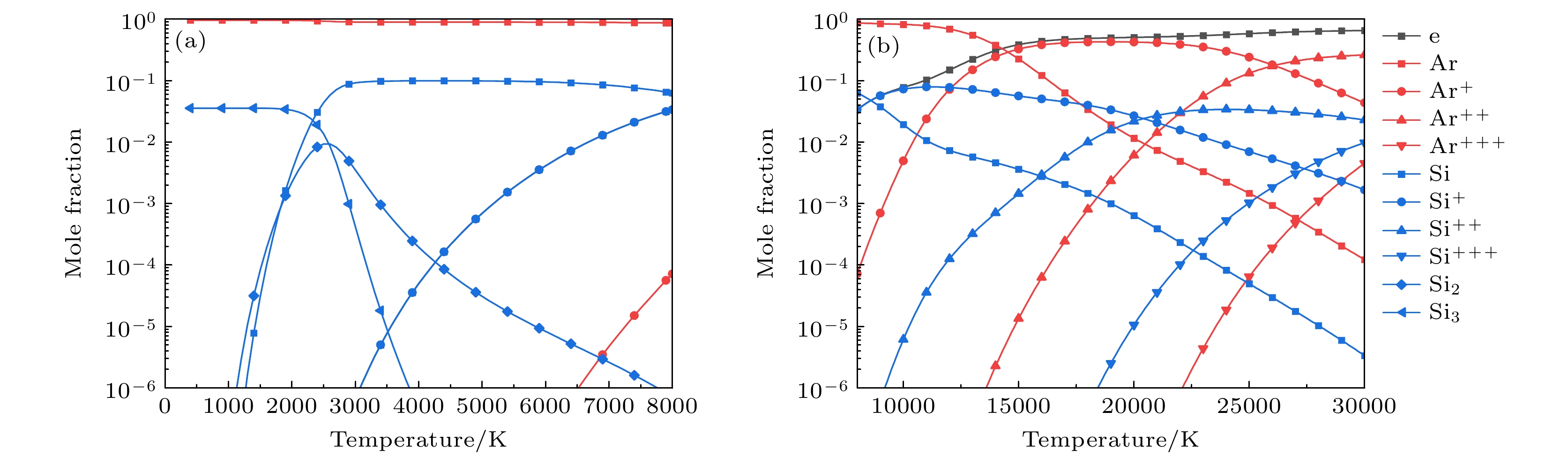
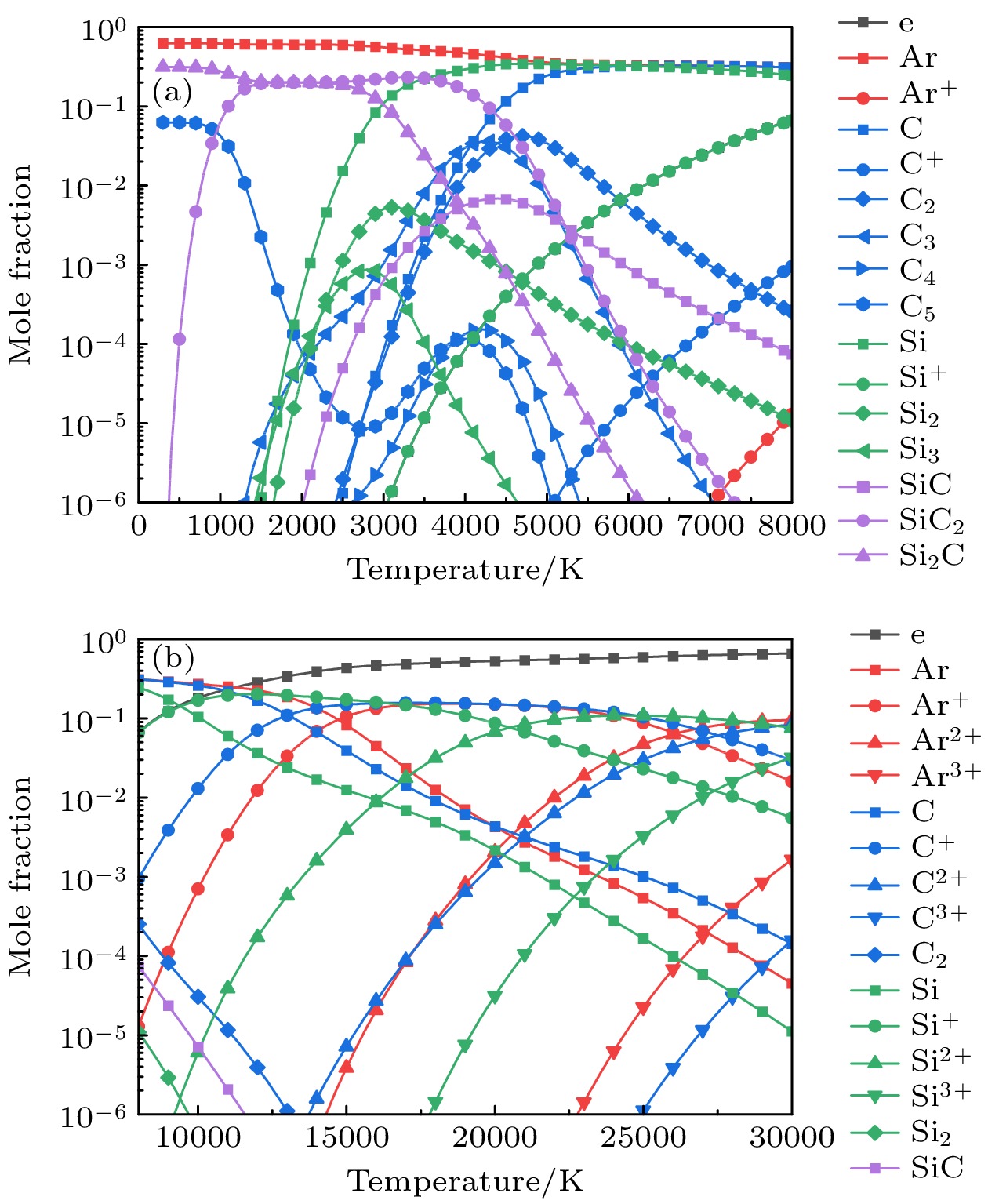
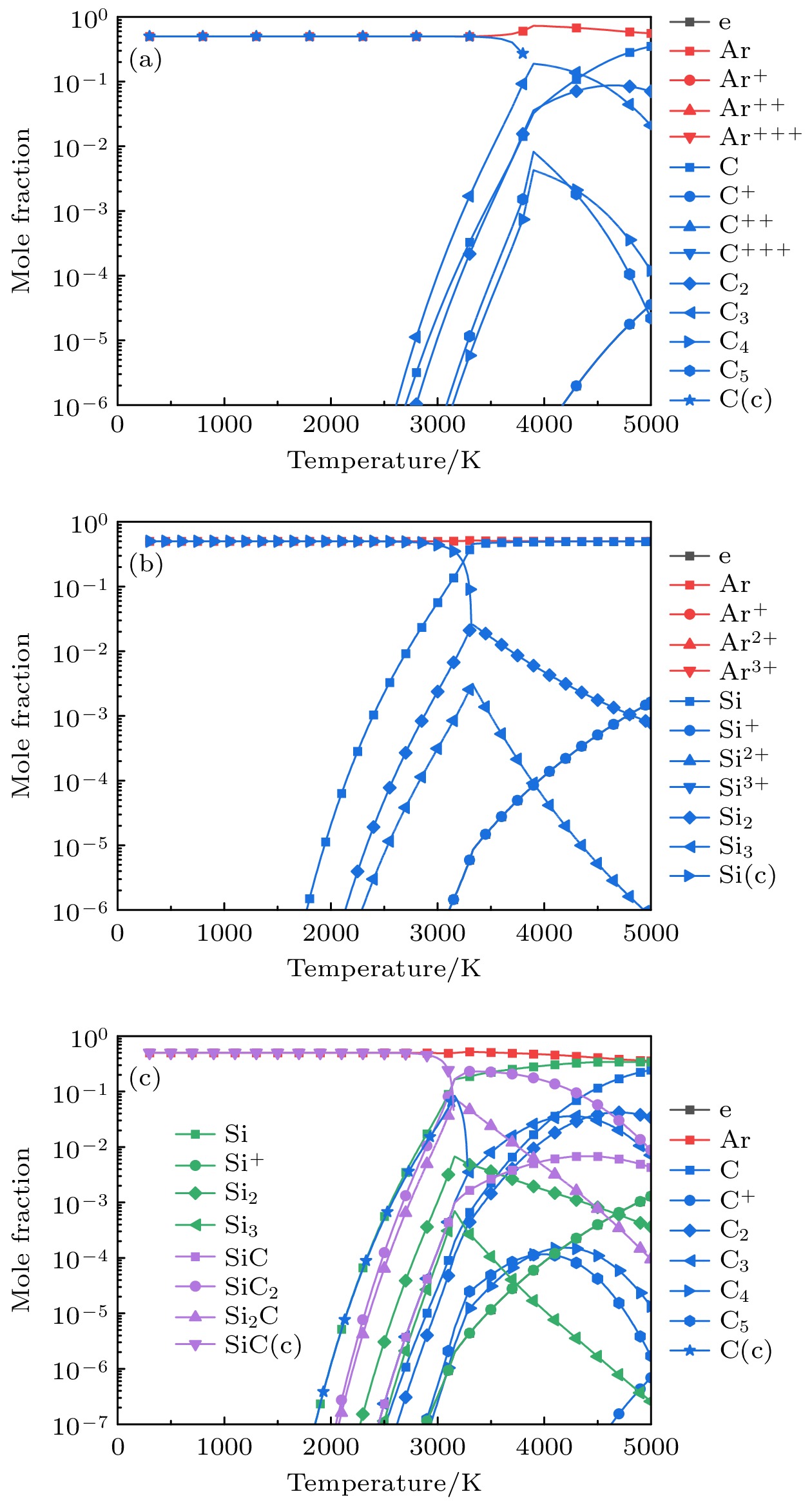
-
The compositions, thermodynamic properties and transport coefficients of the argon-carbon-silicon plasma at local thermodynamic equilibrium and local chemical equilibrium in temperatures range of 300-30000 K and pressure range of 0.1 to 10 atm and are different mixture ratios are investigated in this work. The condensed phases and Debye-Hückel corrections are both taken into account. The equilibrium component in gas phase is calculated by mass action law (Saha’s law and Gulberg-Waage’s law), Dalton’s partial pressure law, conservation of the elements and charge quasi-neutral equation, and at the same time the condensed species is calculated under the assumption of local phase equilibrium. Thermodynamic properties including density, enthalpy and specific heat are evaluated through a classical statistical mechanics approach. The transport coefficient calculations including viscosity, electrical conductivity, and thermal conductivity are carried out by using a third-order approximation (second-order for viscosity) of the Chapman-Enskog method. Collision integrals are obtained by using the relatively new data. The results show that the concentration and ratio of blend of C vapor and Si vapor can greatly affect the properties of the Ar plasma owing to the introduced C and Si vapor’ s own properties and their new reactions. While the pressure influences those properties through the shift of chemical equilibrium and the change of total number density. In addition, the introduction of condensed species leads the thermodynamic properties and transport coefficients of the lower temperature plasma to become almost the same as those of pure argon, and causes discontinuous points at phase-transition temperature. The final calculation results are in good agreement with those in the literature, and the slight difference in transport coefficient between them can be explained by the different selection of interaction potentials. The results are expected to provide reliable basic data for the numerical simulation of argon-carbon-silicon plasma.
-
Keywords:
- argon-carbon-silicon plasma /
- thermodynamic properties /
- transport coefficients /
- local thermodynamic equilibrium
[1] Kim T H, Oh J H, Kim M, Hong S H, Choi S 2020 Appl. Sci. Converg. Technol. 29 117
 Google Scholar
Google Scholar
[2] Wang C, Zhou J, Song M, Chen X, Zheng Y, Yang C, Xia W, Xia W 2022 Ceram. Int. 48 632
 Google Scholar
Google Scholar
[3] Zhou J W, Wang C, Song M, Chen X H, Xia W D 2021 Mater. Lett. 299 130072
 Google Scholar
Google Scholar
[4] Choi S, Lee H, Park D W 2016 J. Nanomater. 2016 5849041
 Google Scholar
Google Scholar
[5] Okuyama H, Saito S, Uda M, Nakata T, Sakka Y 2012 J. Alloys Compd. 520 127
 Google Scholar
Google Scholar
[6] Qadri S B, Imam M A, Feng C R, Rath B B, Yousuf M, Singh S K 2003 Appl. Phys. Lett. 83 548
 Google Scholar
Google Scholar
[7] Rai P, Kim Y S, Kang S K, Yu Y T 2012 Plasma Chem. Plasma Process. 32 211
 Google Scholar
Google Scholar
[8] Wan X H, Fan Y K, Ma W H, Li S Y, Huang X, Yu J 2018 Mater. Lett. 220 144
 Google Scholar
Google Scholar
[9] Zhang X Y, Hayashida R, Tanaka M, Watanabe T 2020 Powder Technol. 371 26
 Google Scholar
Google Scholar
[10] Zhang X Y, Liu Z S, Tanaka M, Watanabe T 2021 Chem. Eng. Sci. 230 116217
 Google Scholar
Google Scholar
[11] Mendoza Gonzalez N Y, El Morsli M, Proulx P 2008 J. Therm. Spray Technol. 17 533
 Google Scholar
Google Scholar
[12] Kim K S, Moradian A, Mostaghimi J, Soucy G 2009 Plasma Chem. Plasma Process. 30 91
 Google Scholar
Google Scholar
[13] Atsuchi N, Shigeta M, Watanabe T 2006 Int. J. Heat Mass Transf. 49 1073
 Google Scholar
Google Scholar
[14] Pan Z H, Chen X H, Yuan X, Wang C, Xia W D 2021 Plasma Chem. Plasma Process. 41 1183
 Google Scholar
Google Scholar
[15] Colombo V, Ghedini E, Gherardi M, Sanibondi P 2013 Plasma Sources Sci. Technol. 22 035010
 Google Scholar
Google Scholar
[16] Wang W Z, Rong M Z, Murphy A B, Wu Y, Spencer J W, Yan J D, Fang M T C 2011 J. Phys. D Appl. Phys. 44 355207
 Google Scholar
Google Scholar
[17] Wang W Z, Yan J D, Rong M Z, Murphy A B, Spencer J W 2012 Plasma Chem. Plasma Process. 32 495
 Google Scholar
Google Scholar
[18] Wu Y, Chen Z X, Rong M Z, Cressault Y, Yang F, Niu C P, Sun H 2016 J. Phys. D Appl. Phys. 49 405203
 Google Scholar
Google Scholar
[19] 王海兴, 孙素蓉, 陈士强 2012 61 195203
 Google Scholar
Google Scholar
Wang H X, Sun S R, Chen S Q 2012 Acta Phys. Sin. 61 195203
 Google Scholar
Google Scholar
[20] Akashi K, Tanaka Y, Nakano Y, Furukawa R, Ishijima T, Sueyasu S, Watanabe S, Nakamura K 2021 Plasma Chem. Plasma Process. 41 1121
 Google Scholar
Google Scholar
[21] Colonna G 2019 Rend. Lincei Sci. Fis. Nat. 30 537
 Google Scholar
Google Scholar
[22] Colonna G, D'Angola A, Pietanza L D, Capitelli M, Pirani F, Stevanato E, Laricchiuta A 2018 Plasma Sources Sci. Technol. 27 015007
 Google Scholar
Google Scholar
[23] Devoto R S 1966 Phys. Fluids 9 1230
 Google Scholar
Google Scholar
[24] Devoto R S 1967 Phys. Fluids 10 2105
 Google Scholar
Google Scholar
[25] Godin D, Trepanier J Y 2004 Plasma Chem. Plasma Process. 24 447
 Google Scholar
Google Scholar
[26] Kramida A, Ralchenko Y, Reader J, NIST ASD Team https://www.nist.gov/pml/atomic-spectra-database [2022-12-1]
[27] Chase M W, Davies C A, Downey J R, Frurip D J, McDonald R A, Syverud A N https://janaf.nist.gov/ [2022-12-1]
[28] André P, Bussière W, Rochette D 2007 Plasma Chem. Plasma Process. 27 381
 Google Scholar
Google Scholar
[29] André P, Brunet L, Bussière W, Caillard J, Lombard J M, Picard J P 2004 Eur. Phys. J. Appl. Phys. 25 169
 Google Scholar
Google Scholar
[30] Capitelli M, Cappelletti D, Colonna G, Gorse C, Laricchiuta A, Liuti G, Longo S, Pirani F 2007 Chem. Phys. 338 62
 Google Scholar
Google Scholar
[31] Colonna G, Laricchiuta A 2008 Comput. Phys. Commun. 178 809
 Google Scholar
Google Scholar
[32] Stallcop J R, Partridge H, Pradhan A, Levin E 2000 J. Thermophys Heat Transfer 14 480
 Google Scholar
Google Scholar
[33] Aubreton J, Bonnefoi C, Mexmain J M 1986 Phys. Appl. Rev. 21 365
 Google Scholar
Google Scholar
[34] Laricchiuta A, Bruno D, Capitelli M, Catalfamo C, Celiberto R, Colonna G, Diomede P, Giordano D, Gorse C, Longo S, Pagano D, Pirani F 2009 Eur. Phys. J. D 54 607
 Google Scholar
Google Scholar
[35] Sourd B, Aubreton J, Elchinger M F, Labrot M, Michon U 2006 J. Phys. D Appl. Phys. 39 1105
 Google Scholar
Google Scholar
-
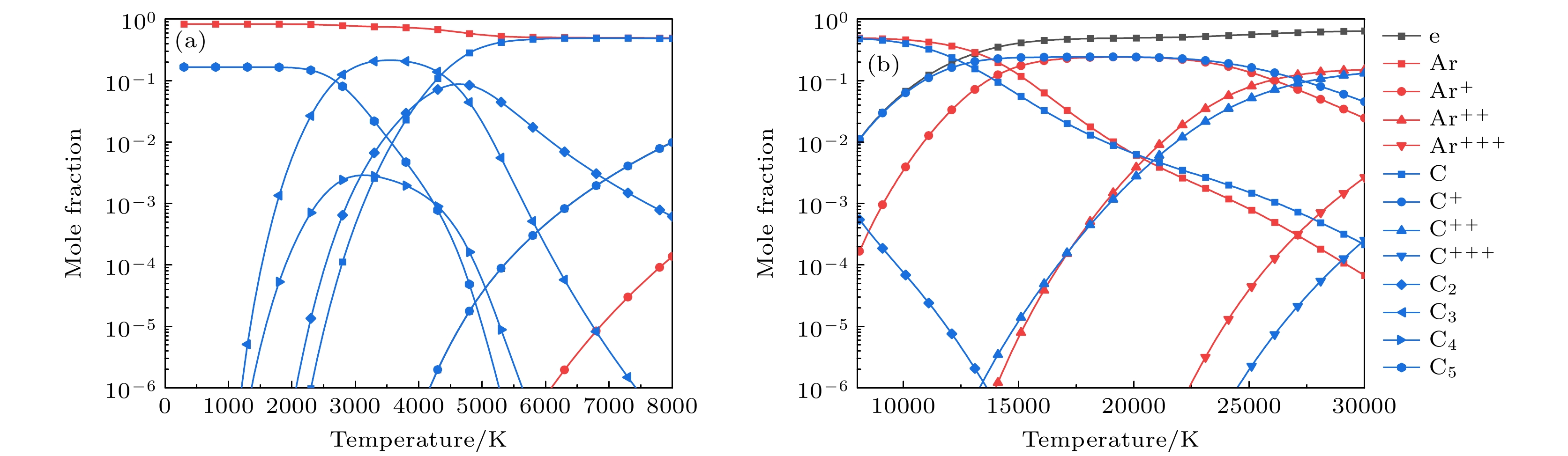
图 5 1 atm下, 带有不同SiC浓度的Ar-SiC等离子体的焓值 (a) 300—5000 K的焓值, 考虑凝聚态物种(折线图), 忽略凝聚态物种(散点图); (b) 300—30000 K下的焓值(不考虑凝聚态物种)
Figure 5. Enthalpy of Ar-SiC plasma with different SiC concentrations at 1 atm: (a) Enthalpy at 300–30000 K, (solid lines) considering condensed species, (dotted lines) neglecting condensed species; (b) results at 300–30000 K (neglecting condensed species).
图 10 1 atm下, 50%Ar和50%不同碳硅比混合物的等离子体的定压比热 (a) 300—5000 K的焓值, 考虑凝聚态物种(折线图), 忽略凝聚态物种(散点图); (b) 300—30000 K下的结果(不考虑凝聚态物种)
Figure 10. Specific heat at constant pressure of 50%Ar and 50% mixture with different C/Si ratios at 1 atm: (a) Results at 300–5000 K, (solid lines) considering condensed species, (dotted lines) neglecting condensed species; (b) results at 300–30000 K (neglecting condensed species).
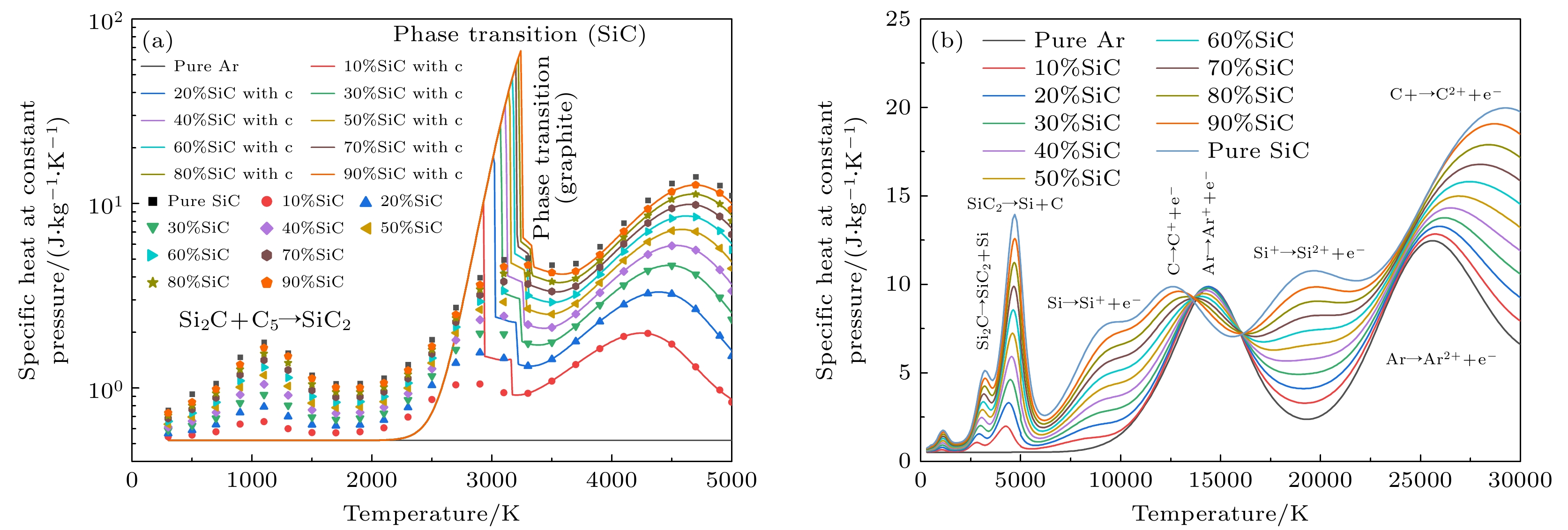
图 6 1 atm下, 带有不同SiC浓度的Ar-SiC等离子体的定压比热 (a) 300—5000 K的定压比热, 考虑凝聚态物种(折线图), 忽略凝聚态物种(散点图); (b) 300—30000 K下的结果(不考虑凝聚态物种)
Figure 6. Specific heat at constant pressure of Ar-SiC plasma with different SiC concentrations at 1 atm: (a) Results at 300–5000 K, (solid lines) considering condensed species, (dotted lines) neglecting condensed species; (b) results at 300–30000 K (neglecting condensed species).
图 7 不同气压下50%Ar-50%SiC等离子体的焓值变化曲线 (a) 300—5000 K的焓值, 考虑凝聚态物种(折线图), 忽略凝聚态物种(散点图); (b) 300—30000 K下的焓值(不考虑凝聚态物种)
Figure 7. Enthalpy of 50%Ar-50%SiC plasma at different pressures: (a) Results at 300–5000 K, (solid lines) considering condensed species, (dotted lines) neglecting condensed species; (b) results at 300–30000 K (neglecting condensed species).
图 8 在不同气压下50%Ar-50%SiC等离子体的定压比热Cp (a) 300—5000 K的定压比热, 考虑凝聚态物种(折线图), 忽略凝聚态物种(散点图); (b) 300—30000 K下的结果(不考虑凝聚态物种)
Figure 8. Specific heat of 50%Ar-50%SiC plasma at different constant pressure: (a) Results at 300–000 K, (solid lines) considering condensed species, (dotted lines) neglecting condensed species; (b) results at 300–30000 K (neglecting condensed species).
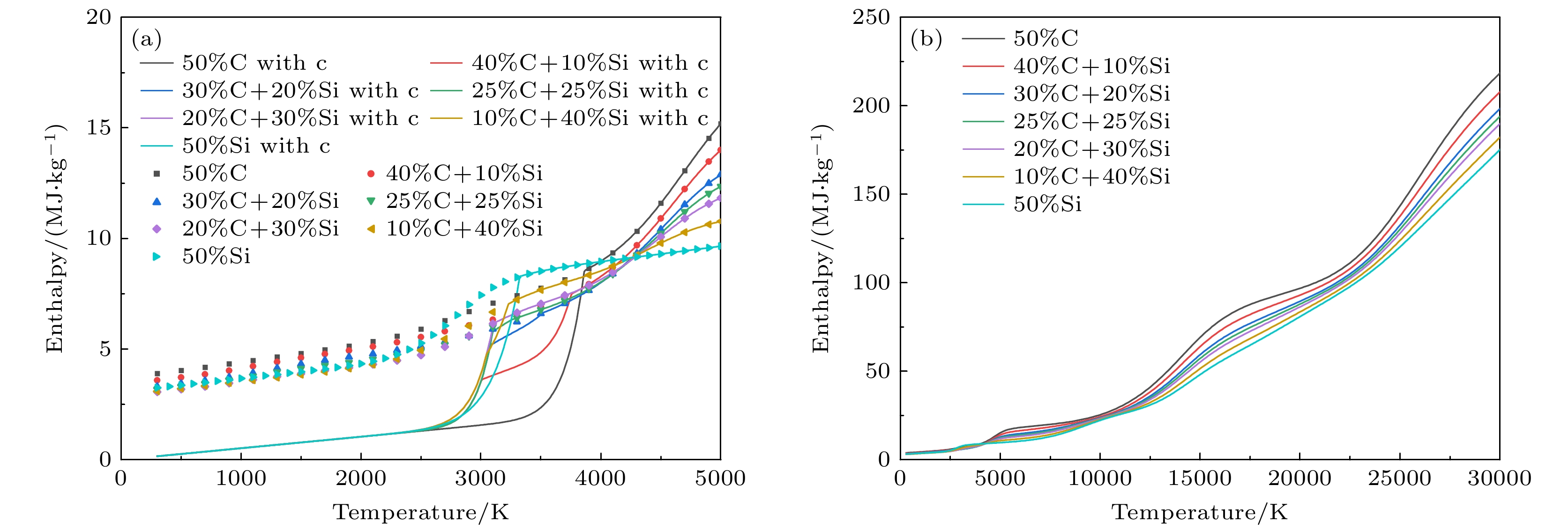
图 9 1 atm下, 50%Ar和50%不同碳硅比混合物的等离子体的焓值 (a)300—5000 K的焓值, 考虑凝聚态物种(折线图), 忽略凝聚态物种(散点图); (b) 300—30000 K下的结果(不考虑凝聚态物种)
Figure 9. Enthalpy of 50%Ar and 50% mixture with different C/Si ratios at 1 atm: (a) Results at 300–5000 K, (solid lines) considering condensed species, (dotted lines) neglecting condensed species; (b) results at 300–30000 K (neglecting condensed species).
图 11 电导率随温度变化曲线 (a)不同压力下50%Ar+50%SiC的曲线; (b) 1 atm下不同SiC浓度的曲线; (c) 50%Ar和50%不同碳硅比混合物的曲线
Figure 11. Temperature dependence of electrical conductivity: (a) Curves of 50%Ar and 50%SiC under different pressures; (b) curves of different SiC concentrations at 1 atm; (c) curves of 50%Ar and 50% mixtures with different C/Si ratios
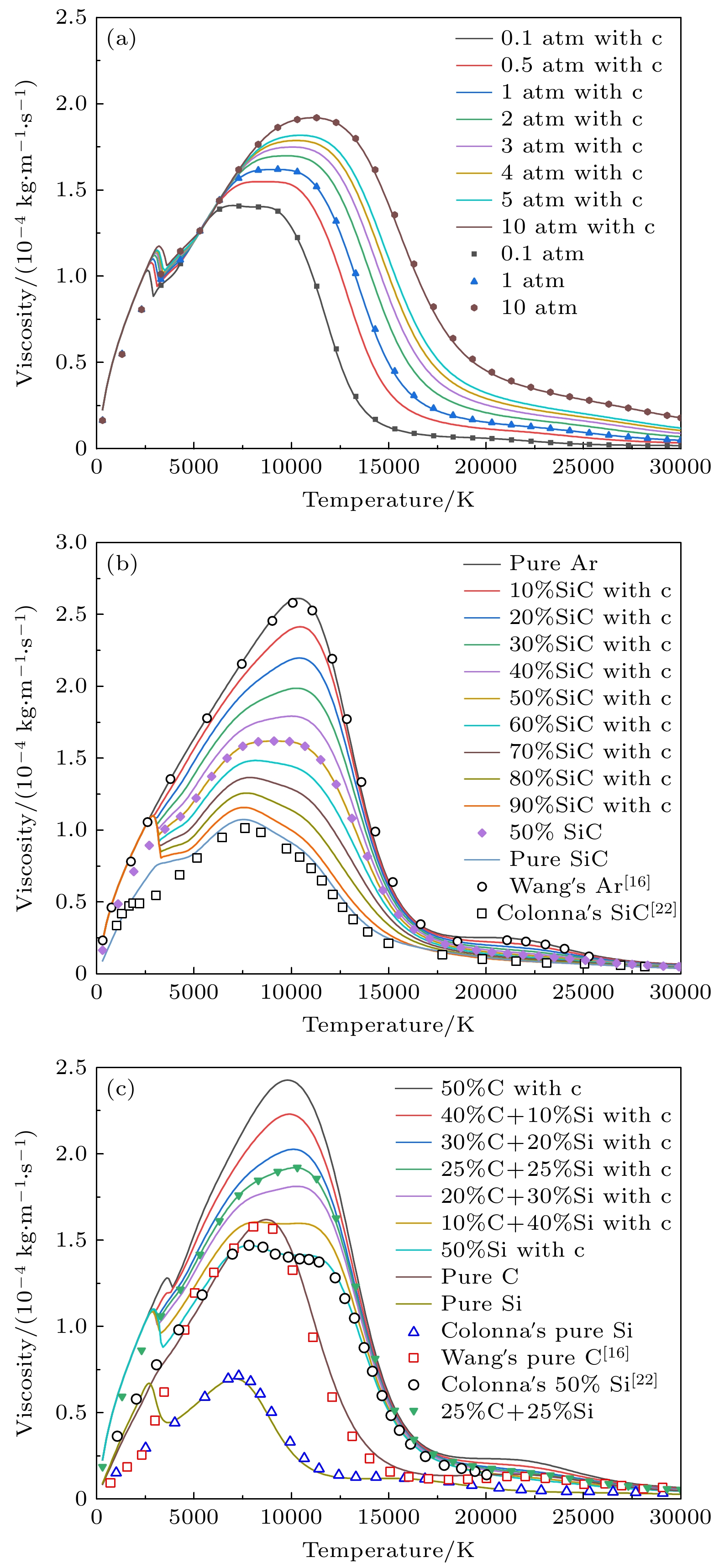
图 12 黏度随温度变化曲线 (a)不同压力下50%Ar+50%SiC的曲线; (b) 1 atm下不同SiC浓度的曲线; (c) 50%Ar和50%不同碳硅比混合物的曲线
Figure 12. Temperature dependence of viscosity: (a) curves of 50%Ar and 50%SiC under different pressures; (b) curves of different SiC concentrations at 1 atm; (c) curves of 50%Ar and 50% mixtures with different C/Si ratios.
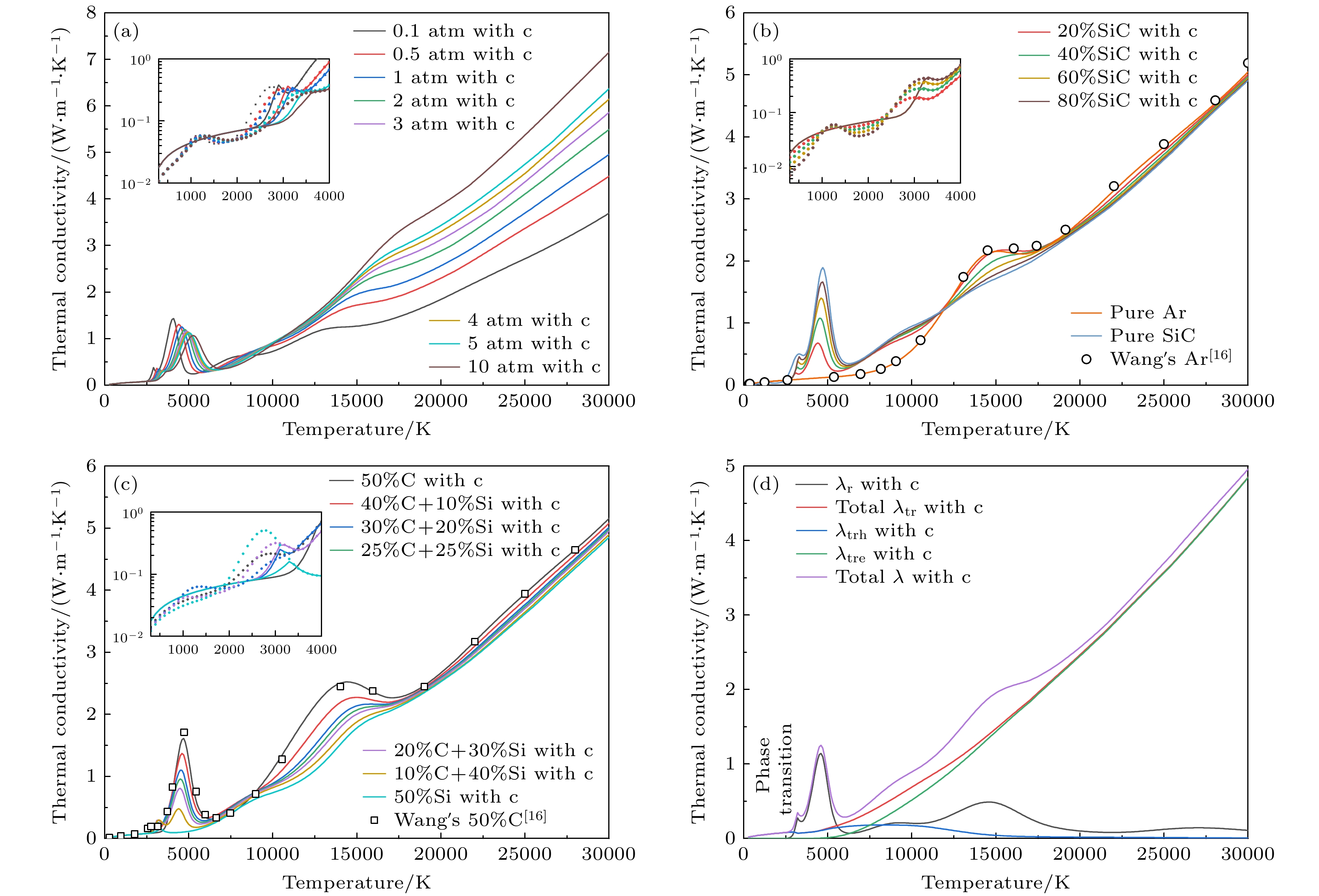
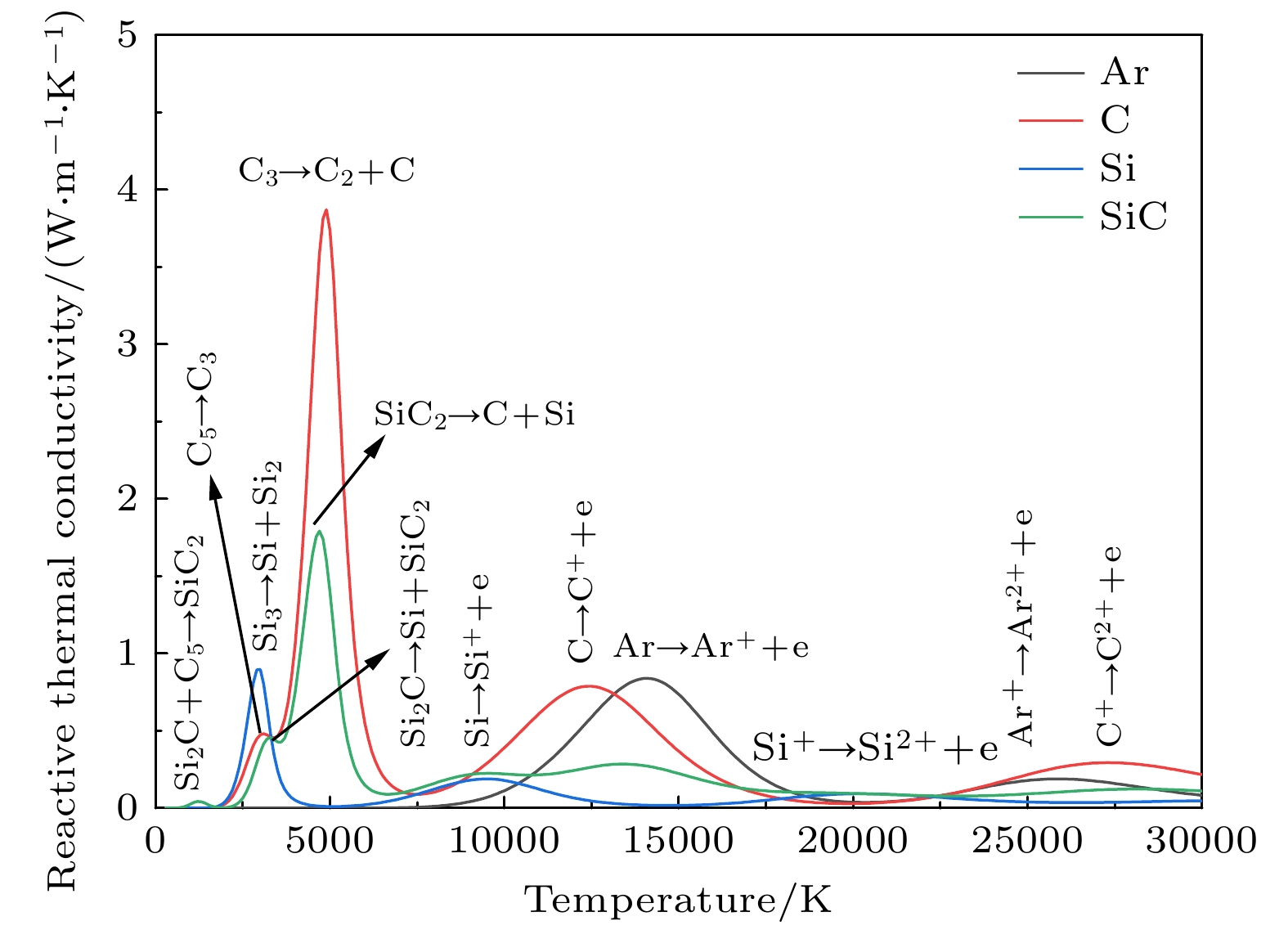
图 13 热导率随温度变化曲线 (a)不同压力下50%Ar+50%SiC的曲线; (b) 1 atm下不同SiC浓度的曲线; (c) 50%Ar和50%不同碳硅比混合物的曲线; (d)总热导率(total λ)、电子平动热导率(λtre)、重粒子平动热导率(λtrh)、总平动热导率(total λtr)、反应热导率(λr)随温度变化曲线
Figure 13. Temperature dependence of thermal conductivity: (a) Curves of 50%Ar and 50%SiC under different pressures; (b) curves of different SiC concentrations at 1 atm; (c) curves of 50%Ar and 50% mixtures with different C/Si ratios; (d) curves of Temperature dependence of total thermal conductivity (total λ), electron translational thermal conductivity (λtre), heavy species translational thermal conductivity (λtrh), total translational thermal conductivity (total λtr) and reactive thermal conductivity (λr).
表 1 计算考虑的物种
Table 1. Species considered in calculations.
元素 物种 Ar Ar, Ar+, Ar2+, Ar3+ C C(g), C+, C2+, C3+, C2, C3, C4, C5, C(c) Si Si(g), Si+, Si2+, Si3+, Si2, Si3, Si(c) C, Si SiC(g), SiC2, Si2C, SiC(c) 表 2 部分二体相互作用势选取
Table 2. Partial selection of interaction.
-
[1] Kim T H, Oh J H, Kim M, Hong S H, Choi S 2020 Appl. Sci. Converg. Technol. 29 117
 Google Scholar
Google Scholar
[2] Wang C, Zhou J, Song M, Chen X, Zheng Y, Yang C, Xia W, Xia W 2022 Ceram. Int. 48 632
 Google Scholar
Google Scholar
[3] Zhou J W, Wang C, Song M, Chen X H, Xia W D 2021 Mater. Lett. 299 130072
 Google Scholar
Google Scholar
[4] Choi S, Lee H, Park D W 2016 J. Nanomater. 2016 5849041
 Google Scholar
Google Scholar
[5] Okuyama H, Saito S, Uda M, Nakata T, Sakka Y 2012 J. Alloys Compd. 520 127
 Google Scholar
Google Scholar
[6] Qadri S B, Imam M A, Feng C R, Rath B B, Yousuf M, Singh S K 2003 Appl. Phys. Lett. 83 548
 Google Scholar
Google Scholar
[7] Rai P, Kim Y S, Kang S K, Yu Y T 2012 Plasma Chem. Plasma Process. 32 211
 Google Scholar
Google Scholar
[8] Wan X H, Fan Y K, Ma W H, Li S Y, Huang X, Yu J 2018 Mater. Lett. 220 144
 Google Scholar
Google Scholar
[9] Zhang X Y, Hayashida R, Tanaka M, Watanabe T 2020 Powder Technol. 371 26
 Google Scholar
Google Scholar
[10] Zhang X Y, Liu Z S, Tanaka M, Watanabe T 2021 Chem. Eng. Sci. 230 116217
 Google Scholar
Google Scholar
[11] Mendoza Gonzalez N Y, El Morsli M, Proulx P 2008 J. Therm. Spray Technol. 17 533
 Google Scholar
Google Scholar
[12] Kim K S, Moradian A, Mostaghimi J, Soucy G 2009 Plasma Chem. Plasma Process. 30 91
 Google Scholar
Google Scholar
[13] Atsuchi N, Shigeta M, Watanabe T 2006 Int. J. Heat Mass Transf. 49 1073
 Google Scholar
Google Scholar
[14] Pan Z H, Chen X H, Yuan X, Wang C, Xia W D 2021 Plasma Chem. Plasma Process. 41 1183
 Google Scholar
Google Scholar
[15] Colombo V, Ghedini E, Gherardi M, Sanibondi P 2013 Plasma Sources Sci. Technol. 22 035010
 Google Scholar
Google Scholar
[16] Wang W Z, Rong M Z, Murphy A B, Wu Y, Spencer J W, Yan J D, Fang M T C 2011 J. Phys. D Appl. Phys. 44 355207
 Google Scholar
Google Scholar
[17] Wang W Z, Yan J D, Rong M Z, Murphy A B, Spencer J W 2012 Plasma Chem. Plasma Process. 32 495
 Google Scholar
Google Scholar
[18] Wu Y, Chen Z X, Rong M Z, Cressault Y, Yang F, Niu C P, Sun H 2016 J. Phys. D Appl. Phys. 49 405203
 Google Scholar
Google Scholar
[19] 王海兴, 孙素蓉, 陈士强 2012 61 195203
 Google Scholar
Google Scholar
Wang H X, Sun S R, Chen S Q 2012 Acta Phys. Sin. 61 195203
 Google Scholar
Google Scholar
[20] Akashi K, Tanaka Y, Nakano Y, Furukawa R, Ishijima T, Sueyasu S, Watanabe S, Nakamura K 2021 Plasma Chem. Plasma Process. 41 1121
 Google Scholar
Google Scholar
[21] Colonna G 2019 Rend. Lincei Sci. Fis. Nat. 30 537
 Google Scholar
Google Scholar
[22] Colonna G, D'Angola A, Pietanza L D, Capitelli M, Pirani F, Stevanato E, Laricchiuta A 2018 Plasma Sources Sci. Technol. 27 015007
 Google Scholar
Google Scholar
[23] Devoto R S 1966 Phys. Fluids 9 1230
 Google Scholar
Google Scholar
[24] Devoto R S 1967 Phys. Fluids 10 2105
 Google Scholar
Google Scholar
[25] Godin D, Trepanier J Y 2004 Plasma Chem. Plasma Process. 24 447
 Google Scholar
Google Scholar
[26] Kramida A, Ralchenko Y, Reader J, NIST ASD Team https://www.nist.gov/pml/atomic-spectra-database [2022-12-1]
[27] Chase M W, Davies C A, Downey J R, Frurip D J, McDonald R A, Syverud A N https://janaf.nist.gov/ [2022-12-1]
[28] André P, Bussière W, Rochette D 2007 Plasma Chem. Plasma Process. 27 381
 Google Scholar
Google Scholar
[29] André P, Brunet L, Bussière W, Caillard J, Lombard J M, Picard J P 2004 Eur. Phys. J. Appl. Phys. 25 169
 Google Scholar
Google Scholar
[30] Capitelli M, Cappelletti D, Colonna G, Gorse C, Laricchiuta A, Liuti G, Longo S, Pirani F 2007 Chem. Phys. 338 62
 Google Scholar
Google Scholar
[31] Colonna G, Laricchiuta A 2008 Comput. Phys. Commun. 178 809
 Google Scholar
Google Scholar
[32] Stallcop J R, Partridge H, Pradhan A, Levin E 2000 J. Thermophys Heat Transfer 14 480
 Google Scholar
Google Scholar
[33] Aubreton J, Bonnefoi C, Mexmain J M 1986 Phys. Appl. Rev. 21 365
 Google Scholar
Google Scholar
[34] Laricchiuta A, Bruno D, Capitelli M, Catalfamo C, Celiberto R, Colonna G, Diomede P, Giordano D, Gorse C, Longo S, Pagano D, Pirani F 2009 Eur. Phys. J. D 54 607
 Google Scholar
Google Scholar
[35] Sourd B, Aubreton J, Elchinger M F, Labrot M, Michon U 2006 J. Phys. D Appl. Phys. 39 1105
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 7049
- PDF Downloads: 122
- Cited By: 0














 DownLoad:
DownLoad: