-
The intermolecular interactions involving the water molecule play important roles in many fields of physics, chemistry, and biology. High-resolution spectroscopy of Van der Waals complexes formed by a rare gas atom and a water molecule can provide a wealth of information about these intermolecular interactions. The precise experimental data can be used to test the accuracies and efficiencies of various theoretical methods of constructing the intermolecular potential energy surfaces and calculating the bound states. In this work, the high-resolution infrared absorption spectrum of the Ar-D2O complex in the v2 bending region of D2O is measured by using an external cavity quantum cascade laser. A segmented rapid-scan data acquisition method is employed. The Ar-D2O complex is generated in a slit supersonic jet expansion by passing Ar gas through a vessel containing liquid D2O. Four new rovibrational subbands are assigned in the spectral range of 1150–1190 cm–1, namely
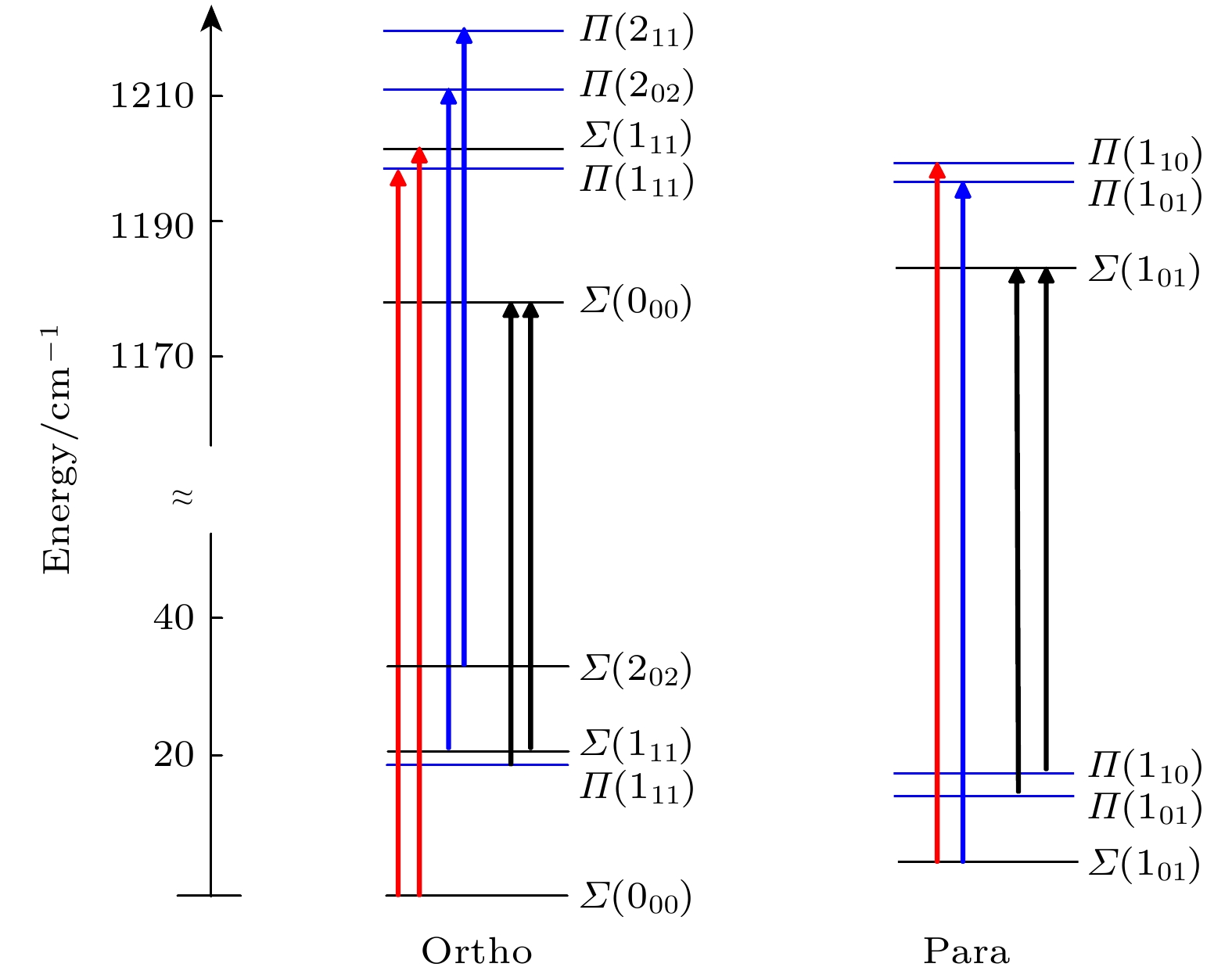
$\Sigma \left( {{0_{00}}, {v_2} = 1} \right) \leftarrow \Sigma \left( {{1_{11}}} \right)$ ,$\Sigma \left( {{0_{00}}, {v_2} = 1} \right) \leftarrow \Pi \left( {{1_{11}}} \right)$ ,$\Sigma \left( {{1_{01}}, {v_2} = 1} \right) \leftarrow \Pi \left( {{1_{10}}} \right)$ and$\Sigma \left( {{1_{01}}, {v_2} = 1} \right) $ $\leftarrow \Pi \left( {{1_{01}}} \right) $ . The first two subbands belong to the otho- species of Ar-D2O, while the latter two belong to the para- species. The observed rovibrational transitions together with the previously reported pure rotational spectra having the common lower vibrational sub-states are analyzed by a weighted least-squares fitting using a pseudo-diatomic effective Hamiltonian. An experimental error of 10 kHz for the far-infrared transitions and 0.001 cm–1 for the infrared transitions are set in the global fitting when using Pickett’s program SPFIT, respectively. The molecular constants including vibrational substate energy, rotational and centrifugal distortion constants, and Coriolis coupling constant, are determined accurately. The previous results for the$\Pi \left( {{1_{11}}, {v_2} = 0} \right)$ substate are found to be likely incorrect. The energy of the$\Sigma \left( {{0_{00}}, {v_2} = 1} \right)$ and$\Sigma \left( {{1_{01}}, {v_2} = 1} \right)$ substates are determined experimentally for the first time. The band origin of Ar-D2O in the D2O v2 bending mode region is determined to be 1177.92144(13) cm–1, which is a red shift about 0.458 cm–1 compared with the head of D2O monomer. The experimental vibrational substate energy is compared with its theoretical value based on a four-dimensional intermolecular potential energy surface which includes the normal coordinate of the D2O v2 bending mode. The experimental and theoretical results are in good agreement with each other. But the calculated energy levels are generally higher than the experimental values, so, there is still much room for improving the theoretical calculations.[1] Fraser G T, Lovas F J, Suenram R D, Matsumura K 1990 J. Mol. Spectrosc. 144 97
 Google Scholar
Google Scholar
[2] Zwart E, Meerts W L 1991 Chem. Phys. 151 407
 Google Scholar
Google Scholar
[3] Germann T C, Gutowsky H S 1993 J. Chem. Phys. 98 5235
 Google Scholar
Google Scholar
[4] Cohen R C, Busarow K L, Laughlin K B, Blake G A, Havenith M, Lee Y T, Saykally R J 1988 J. Chem. Phys. 89 4494
 Google Scholar
Google Scholar
[5] Cohen R C, Busarow K L, Lee Y T, Saykally R J 1990 J. Chem. Phys. 92 169
 Google Scholar
Google Scholar
[6] Cohen R C, Saykally R J 1991 J. Chem. Phys. 95 7891
 Google Scholar
Google Scholar
[7] Suzuki S, Bumgarner R E, Stockman P A, Green P G, Blake G A 1991 J. Chem. Phys. 94 824
 Google Scholar
Google Scholar
[8] Zou L Y, Widicus Weaver S L 2016 J. Mol. Spectrosc. 324 12
 Google Scholar
Google Scholar
[9] Weida M J, Nesbitt D J 1997 J. Chem. Phys. 106 3078
 Google Scholar
Google Scholar
[10] Verdes D, Linnartz H 2002 Chem. Phys. Lett. 355 538
 Google Scholar
Google Scholar
[11] Li S, Zheng R, Zhu Y, Duan C X 2012 J. Mol. Spectrosc. 272 27
 Google Scholar
Google Scholar
[12] Stewart J T, McCall B J 2012 J. Mol. Spectrosc. 282 34
 Google Scholar
Google Scholar
[13] Liu X, Xu Y 2014 J. Mol. Spectrosc. 301 1
 Google Scholar
Google Scholar
[14] Lascola R, Nesbitt D J 1991 J. Chem. Phys. 95 7917
 Google Scholar
Google Scholar
[15] Nesbitt D J, Lascola R 1992 J. Chem. Phys. 97 8096
 Google Scholar
Google Scholar
[16] Kuma S, Slipchenko M N, Momose T, Vilesov A F 2010 J. Phys. Chem. A 114 9022
 Google Scholar
Google Scholar
[17] Didriche K, Földes T 2013 J. Chem. Phys. 138 104307
 Google Scholar
Google Scholar
[18] Vanfleteren T, Földes T, Herman M 2015 Chem. Phys. Lett. 627 36
 Google Scholar
Google Scholar
[19] Cohen R C, Saykally R J 1993 J. Chem. Phys. 98 6007
 Google Scholar
Google Scholar
[20] Hutson J M 1990 J. Chem. Phys. 92 157
 Google Scholar
Google Scholar
[21] Bulski M, Wormer P E S, Avoird A V D 1991 J. Chem. Phys. 94 8096
 Google Scholar
Google Scholar
[22] Chalasiński G, Szczȩśniak M M, Scheiner S 1991 J. Chem. Phys. 94 2807
 Google Scholar
Google Scholar
[23] Tao F M, Klemperer W 1994 J. Chem. Phys. 101 1129
 Google Scholar
Google Scholar
[24] Hodges M P, Wheatley R J, Harvey A H 2002 J. Chem. Phys. 117 7169
 Google Scholar
Google Scholar
[25] Makarewicz J 2008 J. Chem. Phys. 129 184310
 Google Scholar
Google Scholar
[26] Wang S H, He S S, Dai L C, Feng E Y, Huang W Y 2015 J. Chem. Phys. 142 224307
 Google Scholar
Google Scholar
[27] He S S, Chen D, Li Y, Feng E Y, Huang W Y 2016 Chem. Phys. Lett. 665 71
 Google Scholar
Google Scholar
[28] Li S, Zheng R, Duan C X 2014 Chin. Phys. B. 23 123301
 Google Scholar
Google Scholar
[29] Luo W, Duan C X 2016 Chin. Phys. Lett. 33 024207
 Google Scholar
Google Scholar
[30] Li X, Liu Z, Duan C X 2021 J. Mol. Spectrosc. 377 111424
 Google Scholar
Google Scholar
[31] Li X, Pu Y Y, Liu Z, Sun Y X, Duan C X 2022 J. Mol. Spectrosc. 383 111559
 Google Scholar
Google Scholar
[32] Drouin B J 2017 J. Mol. Spectrosc. 340 1
 Google Scholar
Google Scholar
[33] 王申浩 2015 硕士学位论文 (芜湖: 安徽师范大学)
Wang S H 2015 M. S. Dessertation (Wuhu: Anhui Normal University) (in Chinese)
-
图 3 Ar-D2O的
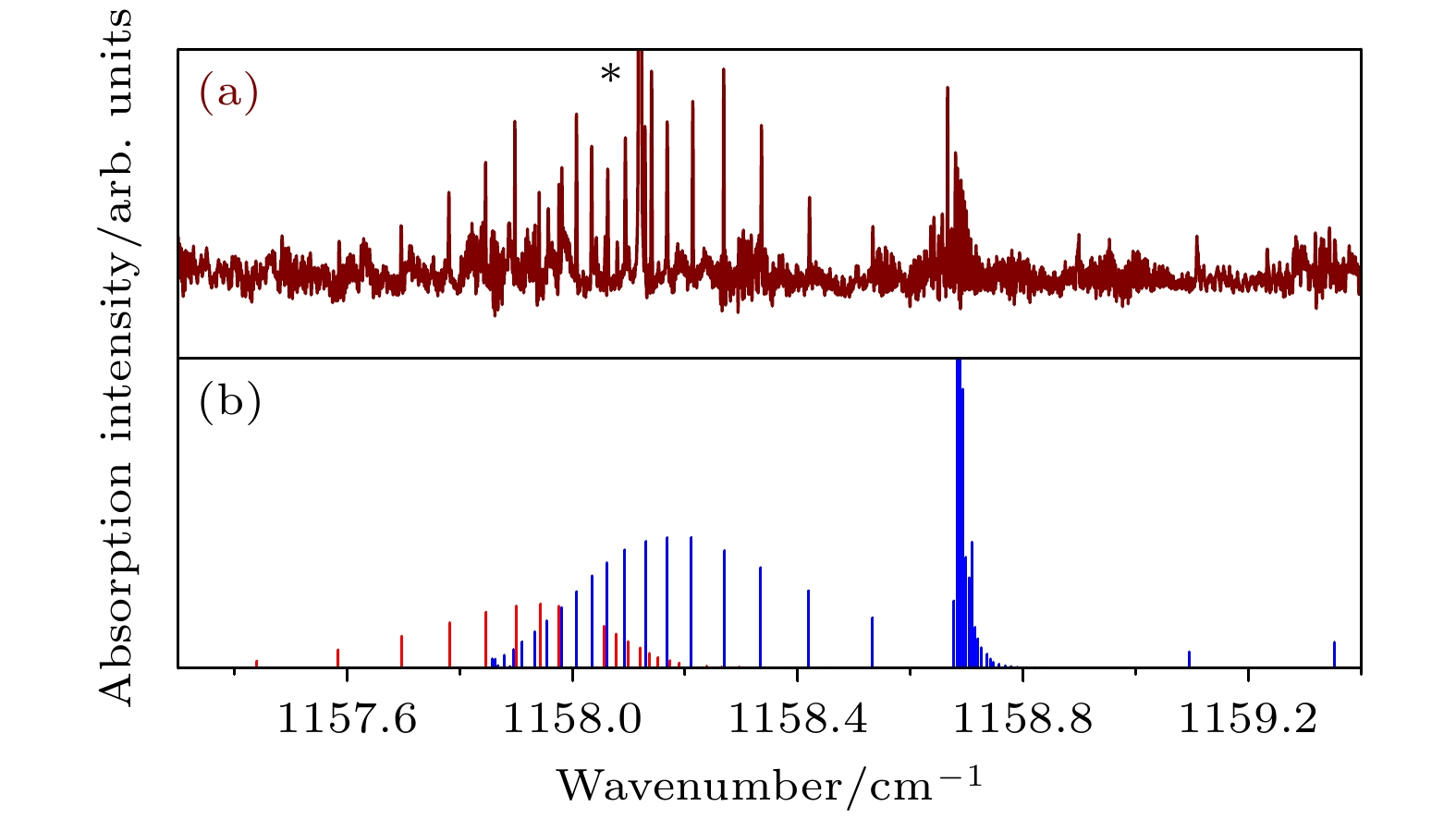
$\Sigma \left( { {1_{01}}, {v_2} = 1} \right) \leftarrow \Pi \left( {{1_{01}}} \right)$ 谱带 (a) 实验光谱; (b) 模拟光谱. 星号所示为D2O单体线Figure 3. The spectrum for
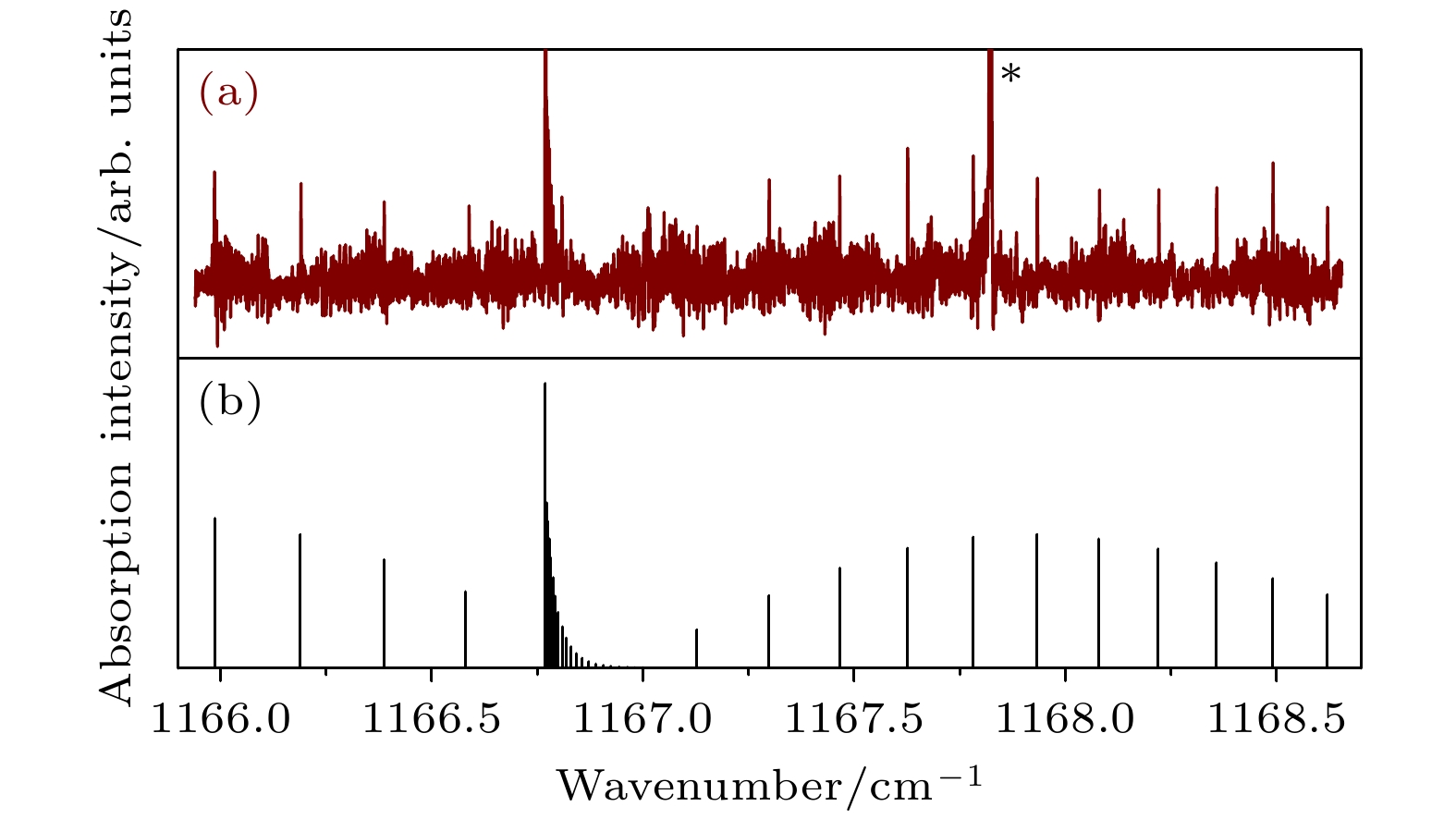
$\Sigma \left( { {1_{01}}, {v_2} = 1} \right) \leftarrow \Pi \left( {{1_{01}}} \right)$ band of Ar-D2O: (a) Observed spectrum; (b) Simulated spectrum. Line marked with an asterisk is from the D2O monomer.图 4 Ar-D2O的
$\Sigma \left( {{1_{01}}, {v_2} = 1} \right) \leftarrow \Pi \left( {{1_{10}}} \right)$ 谱带 (a) 实验光谱; (b) 模拟光谱. 星号所示为D2O单体线Figure 4. The spectrum for
$\Sigma \left( {{1_{01}}, {v_2} = 1} \right) \leftarrow \Pi \left( {{1_{10}}} \right)$ band of Ar-D2O: (a) Observed spectrum; (b) Simulated spectrum. Line marked with an asterisk is from the D2O monomer.图 5 Ar-D2O的
$\Sigma \left( {{0_{00}}, {v_2} = 1} \right) \leftarrow \Sigma \left( {{1_{11}}} \right)$ 和$\Sigma( {0_{00}}, $ $ {v_2} = 1 ) \leftarrow \Pi \left( {{1_{11}}} \right)$ 谱带 (a) 实验光谱; (b) 模拟光谱; 图中红色为$\Sigma \left( {{0_{00}}, {v_2} = 1} \right) \leftarrow \Sigma \left( {{1_{11}}} \right)$ 跃迁谱带, 蓝色为$\Sigma \left( {{0_{00}}, {v_2} = 1} \right) \leftarrow \Pi \left( {{1_{11}}} \right)$ 跃迁谱带. 星号所示为D2O单体线Figure 5. The spectra for
$\Sigma \left( {{0_{00}}, {v_2} = 1} \right) \leftarrow \Sigma \left( {{1_{11}}} \right)$ and$\Sigma \left( {{0_{00}}, {v_2} = 1} \right) \leftarrow \Pi \left( {{1_{11}}} \right)$ bands of Ar-D2O: (a) Observed spectrum; (b) Simulated spectrum. The red is$\Sigma ( {0_{00}}, {v_2} = $ $ 1 ) \leftarrow \Sigma \left( {{1_{11}}} \right)$ band and the blue is$\Sigma ( {0_{00}}, {v_2} = 1) \leftarrow$ $\Pi \left( {{1_{11}}} \right) $ band. Line marked with an asterisk is from the D2O monomer.表 1 Ar-D2O在远红外区域的跃迁谱线的重新拟合 (单位: MHz) a
Table 1. Refitting of transition frequencies of Ar-D2O in the far-infrared region (in MHz) a.
Assignment $\Pi \left( {{1_{01}}} \right) \leftarrow \Sigma \left( {{1_{01}}} \right)$b $\Pi \left( {{1_{10}}} \right) \leftarrow \Sigma \left( {{1_{01}}} \right)$b $\Sigma \left( {{1_{11}}} \right) \leftarrow \Sigma \left( {{0_{00}}} \right)$c $\Pi \left( {{1_{11}}} \right) \leftarrow \Sigma \left( {{0_{00}}} \right)$c P(15) 593671.56(–85) P(14) 594125.10(89) P(13) 594617.34(63) P(12) 595159.22(–50) P(11) 595761.68(–81) P(10) 596438.86(–22) P(9) 286151.60(1) 380426.47(0) 597208.18(5) P(8) 290294.16(–2) 383658.52(1) 598095.30(–5) P(7) 294584.78(0) 387168.84(0) 599137.41(19) P(6) 299029.93(–1) 390958.16(1) 600387.39(59) 529456.94(–55) P(5) 303635.47(1) 395026.72(2) 601924.22(95) 538944.20(–44) P(4) 308406.24(0) 399374.40(0) 603862.02(–15) 548068.80(41) P(3) 313346.09(–1) 404000.86(0) 606374.16(–75) P(2) 318457.69(–2) 408905.45(1) 609688.76(68) 564472.24(–33) P(1) 614050.94(–94) Q(1) 329209.16(0) 419686.25(0) 576854.64(14) Q(2) 329225.88(0) 419967.95(2) 576845.60(10) Q(3) 329249.43(0) 420389.80(–2) 576832.02(8) Q(4) 329278.03(0) 420951.17(1) 576813.78(2) Q(5) 329309.36(0) 421650.90(0) 576790.88(1) Q(6) 329340.61(–1) 422487.66(1) 576763.12(–4) Q(7) 329368.59(0) 423459.73(0) 576730.48(–3) Q(8) 329389.75(1) 424565.04(0) 576692.81(1) Q(9) 329400.26(0) 425801.07(0) 586649.90(–1) Q(10) 576601.72(3) Q(11) 576548.01(–4) Q(12) 576488.93(5) Q(13) 576424.08(–2) Q(14) R(0) 334830.48(0) 425278.22(1) 626461.13((64) 581244.48(–50) R(1) 340629.81(2) 431284.54(–2) 634322.63(–7) R(2) 346594.45(1) 437562.59(–1) 642975.68(–31) 587181.98(–24) R(3) 352719.13(1) 444110.37(1) 652188.92(–61) 589211.88(44) R(4) 358997.30(0) 450925.49(–2) 661789.14(–50) 590861.04(70) R(5) 365421.36(5) 458005.35(–1) 671655.74(25) 592246.68(33) R(6) 371982.39(–4) 681704.80(21) 593449.38(99) R(7) 378671.09(–2) 691879.92(–12) 594518.75(–43) R(8) 385477.11(4) 702141.48(–1) 595494.34(–90) R(9) 392389.53(–2) 712459.36(2) 596401.33(–39) R(10) 722811.14(14) 597256.58(–2) R(11) 733178.48(–15) 598073.14(10) R(12) 598861.05(8) R(13) 599628.49(43) R(14) 600380.64(31) R(15) 601122.42(7) R(16) 601856.02(–96) R(17) 602584.84(46) a括号中的数字为 (实验值-计算值)×102; b 实验观测谱线来自于文献[2]; c 实验观测谱线来自于文献[7]. 表 2 Ar-D2O在D2O单体v2弯曲振动模附近的新观测谱线及拟合偏差 (单位: cm–1)a
Table 2. Newly observed transition frequencies and fitting residuals of Ar-D2O in v2 bending region of D2O (in cm–1)a.
Assignment $\Sigma \left( {{0_{00}}} \right) \leftarrow \Sigma \left( {{1_{11}}} \right)$ $\Sigma \left( {{0_{00}}} \right) \leftarrow \Pi \left( {{1_{11}}} \right)$ $\Sigma \left( {{1_{01}}} \right) \leftarrow \Pi \left( {{1_{10}}} \right)$ $\Sigma \left( {{1_{01}}} \right) \leftarrow \Pi \left( {{1_{01}}} \right)$ P(13) 1157.9570(5) P(12) 1157.9810(0) P(11) 1158.0070(4) P(10) 1158.0340(3) 1164.6769(–2) P(9) 1158.0627(0) 1161.7230(–17) 1164.9049(6) P(8) 1158.0939(–3) 1161.9839(2) 1165.1281(–3) P(7) 1158.1287(–2) 1162.2345(–1) 1165.3492(3) P(6) 1158.1681(–1) 1162.4770(–2) 1165.5657(2) P(5) 1158.2135(–3) 1162.7108(–7) 1165.7774(–5) P(4) 1158.2685(2) 1162.9371(–2) 1165.9857(–1) P(3) 1158.3354(–2) 1163.1541(–5) 1166.1901(11) P(2) 1158.4209(–4) 1163.3632(–1) 1166.3873(1) P(1) 1158.5331(–1) 1166.5815(10) Q(1) 1163.7504(2) Q(2) 1163.7416(1) Q(3) 1163.7289(3) Q(4) 1158.6820(–3) 1163.7117(4) Q(5) 1158.6835(–3) 1163.6902(3) Q(6) 1158.6853(–2) 1163.6645(3) 1166.7712(0) Q(7) 1158.6874(–1) 1163.6348(4) 1166.7728(–1) Q(8) 1158.6899(0) 1163.6005(0) 1166.7752(0) Q(9) 1158.6927(1) 1163.5627(1) 1166.7782(0) Q(10) 1158.6957(1) 1163.5207(–1) 1166.7821(1) Q(11) 1158.6991(1) 1163.4753(1) 1166.7868(0) Q(12) 1158.7028(0) 1163.4260(0) 1166.7927(0) Q(13) 1158.7070(1) 1163.3730(–3) Q(14) 1163.3171(–1) R(1) 1157.5857(8) 1167.1275(0) R(2) 1157.6960(2) 1159.3539(–3) 1164.2752(0) 1167.2987(–4) R(3) 1157.7804(3) 1159.6412(0) 1164.4305(–5) 1167.4657(2) R(4) 1157.8455(0) 1159.9463(1) 1164.5782(2) 1167.6264(0) R(5) 1157.8977(2) 1164.7160(0) 1167.7819(–4) R(6) 1157.9401(0) 1164.8454(5) 1167.9332(0) R(7) 1157.9762(2) 1164.9655(5) 1168.0803(10) R(8) 1165.0767(6) 1168.2210(2) R(9) 1165.1766(–17) 1168.3581(1) R(10) 1158.0580(0) 1163.2720(2) 1168.4914(3) R(11) 1158.0795(–5) 1168.6204(1) R(12) 1158.0994(–6) 1168.7455(–4) R(13) 1168.8687(5) R(14) 1158.1360(–1) R(15) 1158.1528(2) a括号中的数字为 (实验值-计算值) ×104. 表 3 Ar-D2O各振动子能级的分子参数a
Table 3. Molecular constants of vibrational sub-states of Ar-D2Oa.
Parameter Ground state D2O (v2 = 1) excited $\Sigma \left( {{0_{00}}} \right)$ Ref. [7] This work This work v/cm–1 1177.92144 (32) $B$/MHz 2795.93 2795.86781(44) 2797.88(11) $D$/kHz 78.137 77.7551(54) 77.16(46) $H$/Hz –2.406 –2.930 (19) –2.930(19) b $\Sigma \left( {{1_{11}}} \right)$ Ref. [7] This work Ref. [11] v/cm–1) 20.669081(11) 20.6690759(17) 1199.84075(22) $B$/MHz) 2808.409(30) 2808.36099(61) 2835.137(51) $D$/kHz) 136.24(89) 136.328(14) 137.005(33) $H$/Hz) –23.3(69) –20.27(10) — $L$/Hz) –0.084(18) –0.09110(29) — $\Pi \left( {{1_{11}}} \right)$ Ref. [7] This work Ref. [11] v/cm–1) 19.335135(11) 19.2419471 (16) 1198.12738(22) $B$/ MHz 2793.526(22) 2793.46903(54) 2767.084(51) ${D^{\text{e}}}$/kHz 13.84(74) 13.308(12) 20.806(33) ${D^{\text{f}}}$/ kHz 79.06(33) 78.7624(73) — $ {H^{\text{e}}} $/Hz –1.49(58) –17.565(94) — $ {H^{\text{f}}} $/Hz –1.7(13) –1.902(27) — ${L^{\text{e}}}$/Hz 0.140(14) 0.14473(24) — $\beta $/MHz 5141.09(12) 3635.3021(12) 3509.22(19) $\Sigma \left( {{1_{01}}} \right)$ Ref. [2] This work This work v /cm–1 1177.74889(26) $B$/MHz 2729.114(10) 2729.11326(75) 2734.85(98) $D$/kHz 52.96(24) 52.965(19) 53.90(42) $H$/Hz –13.5(17) –13.40(13) –13.40(13) $\Pi \left( {{1_{01}}} \right)$ Ref. [2] This work Ref. [12] v/cm–1 10.9809467(18) 10.9809468(17) 1189.41215(11) ${B^{\text{e}}}$/MHz 2815.2130(92) 2815.21185(76) — ${B^{\text{f}}}$/MHz 2733.497(12) 2742.423 (66) ${D^{\text{e}}}$/kHz 110.24(18) 110.229(16) — ${D^{\text{f}}}$/kHz 78.66(31) 78.665(28) 75.65(25) $ {H^{\text{e}}} $/Hz 23.2(11) 23.228(96) — $ {H^{\text{f}}} $/Hz 5.0(23) 5.07(21) — $\Pi \left( {{1_{10}}} \right)$ Ref. [2] This work Ref. [11] v /cm–1 13.9945245(20) 13.9945245(19) 1192.86911(21) ${B^{\text{e}}}$/MHz 2866.584(19) 2866.5846(12) 2855.13(60) ${B^{\text{f}}}$/MHz 2799.615(18) 2799.6154(11) 2793.37(19) ${D^{\text{e}}}$/kHz 61.65(90) 61.646(40) 47.97(79) ${D^{\text{f}}}$/kHz 63.21(68) 63.211(30) 35.08(20) $ {H^{\text{e}}} $/Hz –32(13) –31.95(37) — $ {H^{\text{f}}} $/Hz –22.2(74) –22.22(22) — a 括号中的数字为拟合标准偏差;
b 固定在基态值上.表 4 Ar-D2O实验与理论计算的振动子能级间隔比较
Table 4. Comparison between observed and calculated vibrational sub-state energies of Ar-D2O.
v2=0 D2O v2=1 excited Exp. Theo. c Exp.-Theo. Exp. Theo. c Exp.-Theo. $\Pi \left( {{1_{11}}} \right)$a 19.2419 19.4189 –0.177 20.2996 20.4349 –0.1353 $\Sigma \left( {{1_{11}}} \right)$a 20.6691 20.9706 –0.3015 21.3633 22.0928 –0.6647 $\Pi \left( {{1_{01}}} \right)$b 10.9809 10.9785 0.0024 11.6633 11.6329 0.0304 $ \Pi \left( {{1_{10}}} \right) $ b 13.9945 14.4571 –0.4624 15.1202 15.4173 –0.2971 a $\Pi \left( {{1_{11}}} \right)$和$\Sigma \left( {{1_{11}}} \right)$相对于$\Sigma \left( {{0_{00}}} \right)$的能级间隔; b $\Pi \left( {{1_{01}}} \right)$和$\Pi \left( {{1_{10}}} \right)$相对于$\Sigma \left( {{1_{01}}} \right)$的能级间隔; c 理论计算值来自于文献[33] . -
[1] Fraser G T, Lovas F J, Suenram R D, Matsumura K 1990 J. Mol. Spectrosc. 144 97
 Google Scholar
Google Scholar
[2] Zwart E, Meerts W L 1991 Chem. Phys. 151 407
 Google Scholar
Google Scholar
[3] Germann T C, Gutowsky H S 1993 J. Chem. Phys. 98 5235
 Google Scholar
Google Scholar
[4] Cohen R C, Busarow K L, Laughlin K B, Blake G A, Havenith M, Lee Y T, Saykally R J 1988 J. Chem. Phys. 89 4494
 Google Scholar
Google Scholar
[5] Cohen R C, Busarow K L, Lee Y T, Saykally R J 1990 J. Chem. Phys. 92 169
 Google Scholar
Google Scholar
[6] Cohen R C, Saykally R J 1991 J. Chem. Phys. 95 7891
 Google Scholar
Google Scholar
[7] Suzuki S, Bumgarner R E, Stockman P A, Green P G, Blake G A 1991 J. Chem. Phys. 94 824
 Google Scholar
Google Scholar
[8] Zou L Y, Widicus Weaver S L 2016 J. Mol. Spectrosc. 324 12
 Google Scholar
Google Scholar
[9] Weida M J, Nesbitt D J 1997 J. Chem. Phys. 106 3078
 Google Scholar
Google Scholar
[10] Verdes D, Linnartz H 2002 Chem. Phys. Lett. 355 538
 Google Scholar
Google Scholar
[11] Li S, Zheng R, Zhu Y, Duan C X 2012 J. Mol. Spectrosc. 272 27
 Google Scholar
Google Scholar
[12] Stewart J T, McCall B J 2012 J. Mol. Spectrosc. 282 34
 Google Scholar
Google Scholar
[13] Liu X, Xu Y 2014 J. Mol. Spectrosc. 301 1
 Google Scholar
Google Scholar
[14] Lascola R, Nesbitt D J 1991 J. Chem. Phys. 95 7917
 Google Scholar
Google Scholar
[15] Nesbitt D J, Lascola R 1992 J. Chem. Phys. 97 8096
 Google Scholar
Google Scholar
[16] Kuma S, Slipchenko M N, Momose T, Vilesov A F 2010 J. Phys. Chem. A 114 9022
 Google Scholar
Google Scholar
[17] Didriche K, Földes T 2013 J. Chem. Phys. 138 104307
 Google Scholar
Google Scholar
[18] Vanfleteren T, Földes T, Herman M 2015 Chem. Phys. Lett. 627 36
 Google Scholar
Google Scholar
[19] Cohen R C, Saykally R J 1993 J. Chem. Phys. 98 6007
 Google Scholar
Google Scholar
[20] Hutson J M 1990 J. Chem. Phys. 92 157
 Google Scholar
Google Scholar
[21] Bulski M, Wormer P E S, Avoird A V D 1991 J. Chem. Phys. 94 8096
 Google Scholar
Google Scholar
[22] Chalasiński G, Szczȩśniak M M, Scheiner S 1991 J. Chem. Phys. 94 2807
 Google Scholar
Google Scholar
[23] Tao F M, Klemperer W 1994 J. Chem. Phys. 101 1129
 Google Scholar
Google Scholar
[24] Hodges M P, Wheatley R J, Harvey A H 2002 J. Chem. Phys. 117 7169
 Google Scholar
Google Scholar
[25] Makarewicz J 2008 J. Chem. Phys. 129 184310
 Google Scholar
Google Scholar
[26] Wang S H, He S S, Dai L C, Feng E Y, Huang W Y 2015 J. Chem. Phys. 142 224307
 Google Scholar
Google Scholar
[27] He S S, Chen D, Li Y, Feng E Y, Huang W Y 2016 Chem. Phys. Lett. 665 71
 Google Scholar
Google Scholar
[28] Li S, Zheng R, Duan C X 2014 Chin. Phys. B. 23 123301
 Google Scholar
Google Scholar
[29] Luo W, Duan C X 2016 Chin. Phys. Lett. 33 024207
 Google Scholar
Google Scholar
[30] Li X, Liu Z, Duan C X 2021 J. Mol. Spectrosc. 377 111424
 Google Scholar
Google Scholar
[31] Li X, Pu Y Y, Liu Z, Sun Y X, Duan C X 2022 J. Mol. Spectrosc. 383 111559
 Google Scholar
Google Scholar
[32] Drouin B J 2017 J. Mol. Spectrosc. 340 1
 Google Scholar
Google Scholar
[33] 王申浩 2015 硕士学位论文 (芜湖: 安徽师范大学)
Wang S H 2015 M. S. Dessertation (Wuhu: Anhui Normal University) (in Chinese)
Catalog
Metrics
- Abstract views: 4288
- PDF Downloads: 55
- Cited By: 0























 DownLoad:
DownLoad: