-
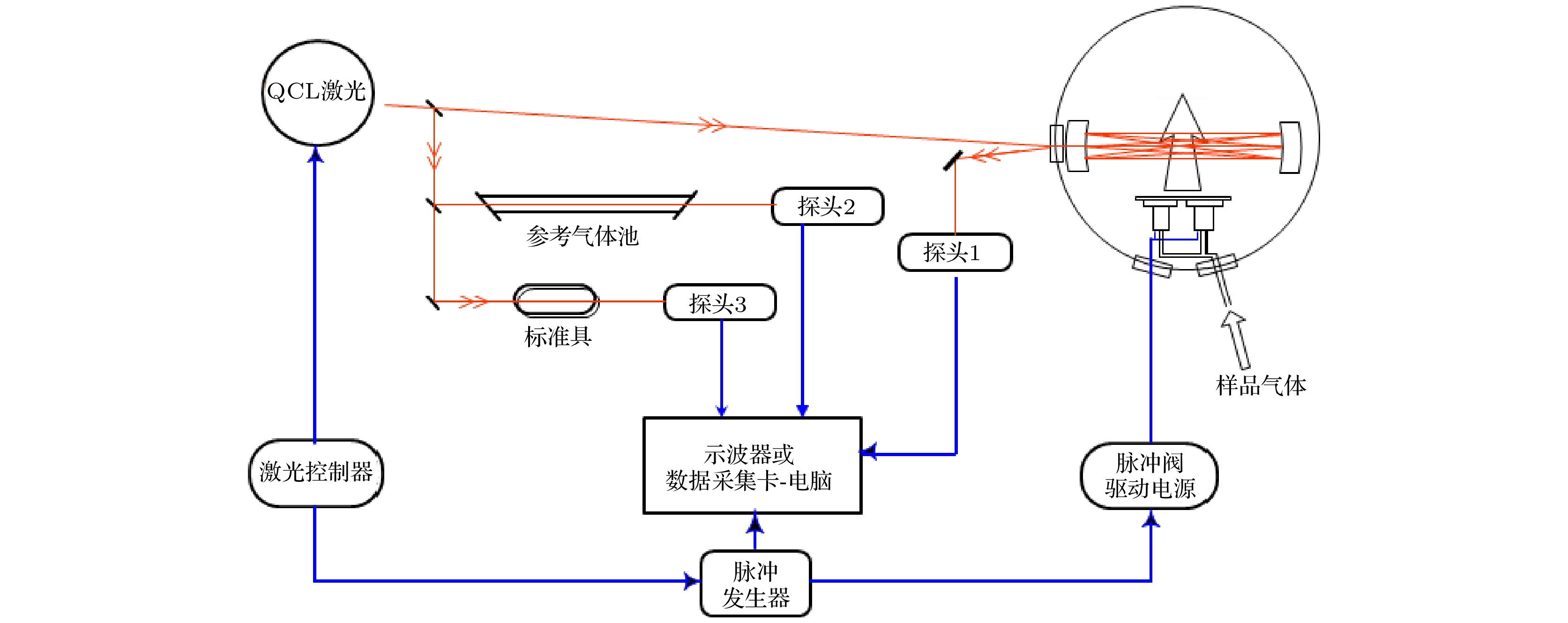
六氟化硫(SF6)是一种长寿命的温室气体, 其红外吸收光谱对模拟大气辐射平衡非常重要. SF6也是研究激光分离同位素原理和技术的典型体系之一. 由于SF6分子较重, 其室温下的红外光谱非常密集, 给利用吸收光谱技术监测不同SF6同位素分子的相对浓度带来很大困难. 本文利用超声射流冷却和像散型多程吸收池技术, 测量了32SF6和33SF6同位素分子在10.6 μm波段的高分辨红外激光吸收光谱. 处于振动基态的32SF6和33SF6分子在狭缝型超声射流中的转动温度约为10 K, 谱线线宽约为0.0008 cm–1. 在此条件下观测到了SF6一个新的热带, 其Q支的位置在941.0 cm–1附近. 将其初步归属为32SF6的(v1+v2+v3)–(v1+v2) 带, 对该热带进行简化的转动分析, 并讨论利用该热带和33SF6的v3基频带进行33SF6/32SF6的相对浓度监测的可行性.Sulfur hexafluoride (SF6) is a greenhouse gas of very long lifetime. Its infrared absorption spectrum is very important in modeling the atmospheric radiation balances. The SF6 is also a prototypical system for studying the principles and techniques of laser isotope separation using powerful infrared lasers. As a very heavy molecule, the infrared spectrum of SF6 at room temperature is very dense, which poses a great challenge to monitoring the relative abundances of different SF6 isotopomers by direct absorption spectroscopy. Supersonic jet expansions have been widely used to simplify the gas phase molecular spectra. In this work, astigmatic multi-pass absorption cell and distributed feed-back quantum cascade lasers (QCLs) are used to measure jet-cooled rovibrational absorption spectra of 32SF6 and 33SF6 at 10.6 μm. The spectrometer works in a segmented rapid-scan mode. The gas mixtures (SF6∶Ar∶He = 0.12∶1∶100) are expanded through an 80 mm
$ \times $ 300 μm pulsed slit nozzle. Two QCLs running at room temperature are used and each one covers a spectral range of about 3.0 cm–1. The v3 fundamental bands of both 32SF6 and 33SF6 are observed. The rotational temperature of 32SF6 and 33SF6 in the ground state in the supersonic jet are both estimated at 10 K and the linewidth is about 0.0008 cm–1 by comparing the simulated spectrum with the observed spectrum with the PGOPHER program. A new weak vibrational band centered around 941.0 cm–1 is observed and tentatively assigned to the (v1+v2+v3)–(v1+v2) hot band of 32SF6. The effective Hamiltonian used to analyze the rovibrational spectrum of SF6 is briefly introduced. A simplified rotational analysis for this hot band is performed with the XTDS program developed by the Dijon group. The band-origin of this hot band is determined to be 941.1785(21) cm–1. The rotational temperature of this hot band is estimated at 50 K. A new scheme by measuring the jet-cooled absorption spectrum of this hot band of 32SF6 and the v3 fundamental band of 33SF6 is proposed for measuring the relative abundance of 33SF6/32SF6.-
Keywords:
- vibration-rotational spectrum /
- greenhouse gas /
- isotope separation /
- supersonic jet
[1] Geller L, Elkins J, Lobert J, Clarke A, Hurst D, Butler J, Myers R 1997 Geophys. Res. Lett. 24 675
 Google Scholar
Google Scholar
[2] Makarov G N 2005 Phys. Usp. 48 37
 Google Scholar
Google Scholar
[3] Zellweger J M, Philippoz J M, Melinon P, Monot, van den Bergh H 1984 Phys. Rev. Lett. 52 522
 Google Scholar
Google Scholar
[4] Eerkens J W 1998 Laser Part. Beams 16 295
 Google Scholar
Google Scholar
[5] Makarov G N 2015 Phys. Usp. 58 670
 Google Scholar
Google Scholar
[6] Sai Prasad M B, Padma Nilaya J, Ghosh A, Biswas D J 2020 Chem. Phys. 538 110831
 Google Scholar
Google Scholar
[7] Faye M, Boudon V, Loët M, Roy P, Manceron L 2017 J. Quant. Spectrosc. Radiat. Transfer 190 38
 Google Scholar
Google Scholar
[8] Faye M, Manceron L, Roy P, Boudon V, Loët M 2018 J. Mol. Spectrosc. 348 37
 Google Scholar
Google Scholar
[9] Ke H, Boudon V, Richard C, Madhur, Faye M, Manceron L 2020 J. Mol. Spectrosc. 368 111251
 Google Scholar
Google Scholar
[10] Boudon V, Hepp M, Herman M, Pak I, Pierre G 1998 J. Mol. Spectrosc. 192 359
 Google Scholar
Google Scholar
[11] Boudon V, Doménech J L, Bermejo D, Willner H 2004 J. Mol. Spectrosc. 228 392
 Google Scholar
Google Scholar
[12] Boudon V, Doménech J L, Ramos A, Bermejo D, Willner H 2006 Mol. Phys. 104 2653
 Google Scholar
Google Scholar
[13] Faye M, Manceron L, Roy P, Boudon V, Loëte M 2018 J. Mol. Spectrosc. 346 23
 Google Scholar
Google Scholar
[14] Luo W, Zhang Y L, Li W G, Duan C X 2017 J. Mol. Spectrosc. 334 22
 Google Scholar
Google Scholar
[15] Liu Z, Luo W, Duan C X 2019 J. Chem. Phys. 150 064302
 Google Scholar
Google Scholar
[16] Li X, Liu Z, Duan C X 2021 J. Mol. Spectrosc. 377 111424
 Google Scholar
Google Scholar
[17] Gordon I E, Rothman L S, Hargreaves R J, et al. 2022 J. Quant. Spectrosc. Radiat. Transfer 277 107949
 Google Scholar
Google Scholar
[18] Western C M 2017 J. Quant. Spectrosc. Radiat. Transfer. 186 221
 Google Scholar
Google Scholar
[19] Asselin P, Turner A C, Bruel L, Brenner V, Gaveau M A, Mons M 2018 Phys. Chem. Chem. Phys. 20 28105
 Google Scholar
Google Scholar
[20] Rey M M, Chizhmakova I S, Nikitin A V, Tyuterev V G 2021 Phys. Chem. Chem. Phys. 23 12115
 Google Scholar
Google Scholar
[21] Champion J P, Loëte M, Pierre G 1992 Spectroscopy of the Earth's Atmosphere and Interstellar Medium (San Diego: Academic Press) pp339–422
[22] Boudon V, Champion J P, Gabard T, et al. 2004 J. Mol. Spectrosc. 228 620
 Google Scholar
Google Scholar
[23] Wenger C, Boudon V, Rotger M, Sanzharov J P, Champion J P 2008 J. Mol. Spectrosc. 251 102
 Google Scholar
Google Scholar
-
图 2 32SF6单体v3带附近的吸收光谱 (a) 32SF6单体v3振动带的模拟光谱, 线宽0.0008 cm–1 (~24 MHz), 转动温度10 K; (b)实验测量光谱
Fig. 2. Absorption spectrum at the v3 band region of 32SF6: (a) The simulated spectrum of the v3 band of 32SF6 with a linewidth of 0.0008 cm–1 (~24 MHz) and a rotational temperature of 10 K; (b) the experimental spectrum.
图 3 33SF6单体v3带附近的吸收光谱 (a) 33SF6单体v3振动带的模拟光谱, 线宽0.0008 cm–1 (~24 MHz), 转动温度10 K; (b)实验测量光谱
Fig. 3. Absorption spectrum at the v3 band region of 33SF6: (a) The simulated spectrum of the v3 band of 33SF6 with a linewidth of 0.0008 cm–1 (~24 MHz) and a rotational temperature of 10 K; (b) the experimental spectrum.
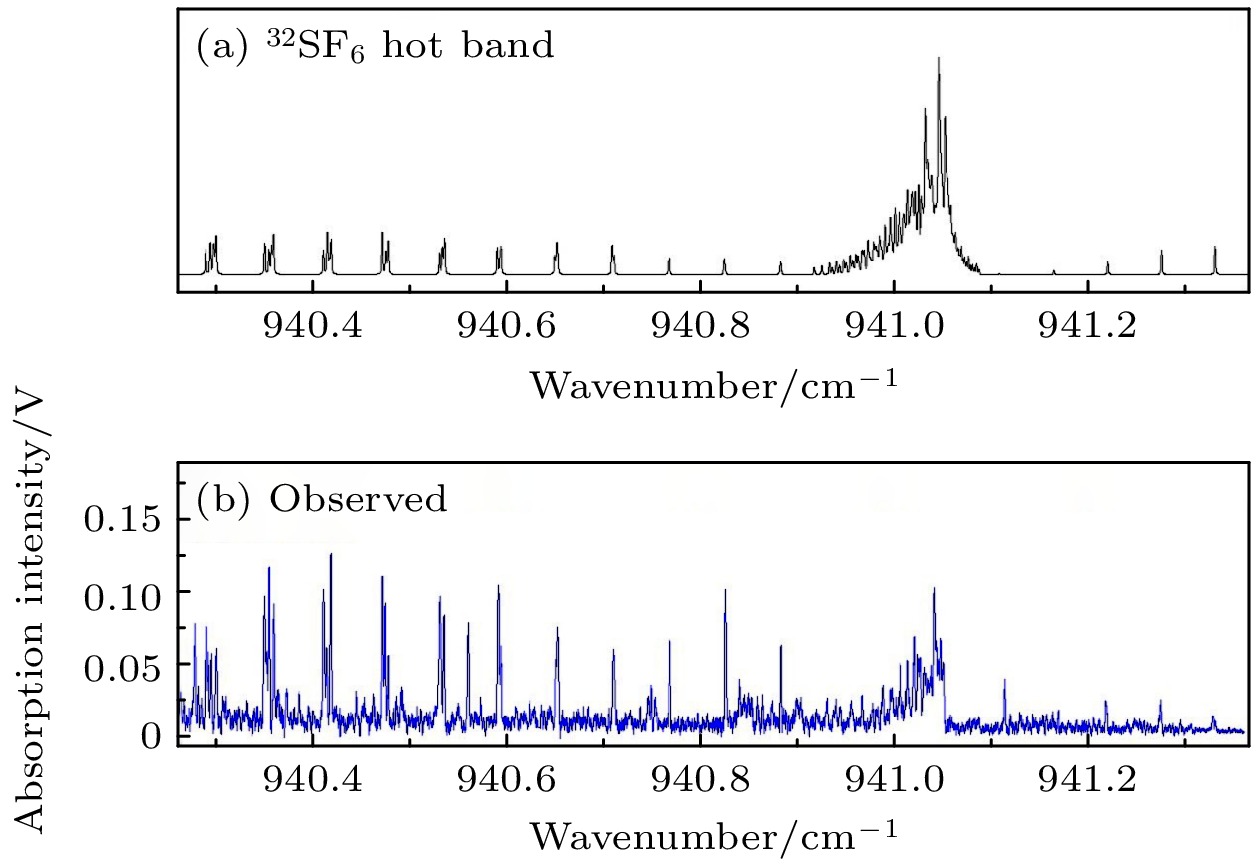
图 4 32SF6单体热带的吸收光谱 (a) 32SF6单体热带的模拟光谱, 线宽0.0008 cm–1 (~24 MHz), 转动温度50 K; (b)实验测量光谱
Fig. 4. Absorption spectrum of the tentatively assigned hot band of 32SF6. (a) The simulated spectrum of the hot band of 32SF6 with a linewidth of 0.0008 cm–1 (~24 MHz) and a rotational temperature of 50 K; (b) the experimental spectrum.
表 1 32SF6的热带的分子参数
Table 1. Molecular parameters for the hot band of 32SF6
Order ${ {\varOmega } }(K, n{ {\varGamma } })$ $ \left\{s\right\} $ $ \left\{s'\right\} $ Valuesb/cm–1 GSa 0 2(0, 0 A1g) 000000 A1g 000000 A1g 9.10756$ \times {10}^{-3} $ 2 4(0, 0 A1g) 000000 A1g 000000 A1g –7.2689$ \times {10}^{-9} $ 2 4(4, 0 A1g) 000000 A1g 000000 A1g 1.2227$ \times {10}^{-10} $ Excited 0 0(0, 0 A1g) 001000 F1u 001000 F1u 941.1785(21) 1 1(1, 0 F1g) 001000 F1u 001000 F1u 0.26651(10) 2 2(0, 0 A1g) 001000 F1u 001000 F1u –1.149(32)$ \times {10}^{-4} $ 2 2(0, 0 E1g) 001000 F1u 001000 F1u –1.1162(33)$ \times {10}^{-4} $ 注: a 基态分子参数固定于文献[9]的值; b 括号中的数字为标准偏差, 与参数值的最后两位对齐. 表 2 33SF6的v3基频带和32SF6的热带的部分谱线频率
Table 2. A part of observed transition frequencies of the v3 fundamental band of 33SF6 and the hot band of 32SF6
频率/cm–1 33SF6 32SF6 R(2) 939.181 P(3) 940.883 R(3) 939.238 P(4) 940.826 R(4) 939.294 P(5) 940.768 R(5) 939.350 P(6) 940.709 -
[1] Geller L, Elkins J, Lobert J, Clarke A, Hurst D, Butler J, Myers R 1997 Geophys. Res. Lett. 24 675
 Google Scholar
Google Scholar
[2] Makarov G N 2005 Phys. Usp. 48 37
 Google Scholar
Google Scholar
[3] Zellweger J M, Philippoz J M, Melinon P, Monot, van den Bergh H 1984 Phys. Rev. Lett. 52 522
 Google Scholar
Google Scholar
[4] Eerkens J W 1998 Laser Part. Beams 16 295
 Google Scholar
Google Scholar
[5] Makarov G N 2015 Phys. Usp. 58 670
 Google Scholar
Google Scholar
[6] Sai Prasad M B, Padma Nilaya J, Ghosh A, Biswas D J 2020 Chem. Phys. 538 110831
 Google Scholar
Google Scholar
[7] Faye M, Boudon V, Loët M, Roy P, Manceron L 2017 J. Quant. Spectrosc. Radiat. Transfer 190 38
 Google Scholar
Google Scholar
[8] Faye M, Manceron L, Roy P, Boudon V, Loët M 2018 J. Mol. Spectrosc. 348 37
 Google Scholar
Google Scholar
[9] Ke H, Boudon V, Richard C, Madhur, Faye M, Manceron L 2020 J. Mol. Spectrosc. 368 111251
 Google Scholar
Google Scholar
[10] Boudon V, Hepp M, Herman M, Pak I, Pierre G 1998 J. Mol. Spectrosc. 192 359
 Google Scholar
Google Scholar
[11] Boudon V, Doménech J L, Bermejo D, Willner H 2004 J. Mol. Spectrosc. 228 392
 Google Scholar
Google Scholar
[12] Boudon V, Doménech J L, Ramos A, Bermejo D, Willner H 2006 Mol. Phys. 104 2653
 Google Scholar
Google Scholar
[13] Faye M, Manceron L, Roy P, Boudon V, Loëte M 2018 J. Mol. Spectrosc. 346 23
 Google Scholar
Google Scholar
[14] Luo W, Zhang Y L, Li W G, Duan C X 2017 J. Mol. Spectrosc. 334 22
 Google Scholar
Google Scholar
[15] Liu Z, Luo W, Duan C X 2019 J. Chem. Phys. 150 064302
 Google Scholar
Google Scholar
[16] Li X, Liu Z, Duan C X 2021 J. Mol. Spectrosc. 377 111424
 Google Scholar
Google Scholar
[17] Gordon I E, Rothman L S, Hargreaves R J, et al. 2022 J. Quant. Spectrosc. Radiat. Transfer 277 107949
 Google Scholar
Google Scholar
[18] Western C M 2017 J. Quant. Spectrosc. Radiat. Transfer. 186 221
 Google Scholar
Google Scholar
[19] Asselin P, Turner A C, Bruel L, Brenner V, Gaveau M A, Mons M 2018 Phys. Chem. Chem. Phys. 20 28105
 Google Scholar
Google Scholar
[20] Rey M M, Chizhmakova I S, Nikitin A V, Tyuterev V G 2021 Phys. Chem. Chem. Phys. 23 12115
 Google Scholar
Google Scholar
[21] Champion J P, Loëte M, Pierre G 1992 Spectroscopy of the Earth's Atmosphere and Interstellar Medium (San Diego: Academic Press) pp339–422
[22] Boudon V, Champion J P, Gabard T, et al. 2004 J. Mol. Spectrosc. 228 620
 Google Scholar
Google Scholar
[23] Wenger C, Boudon V, Rotger M, Sanzharov J P, Champion J P 2008 J. Mol. Spectrosc. 251 102
 Google Scholar
Google Scholar
计量
- 文章访问数: 6728
- PDF下载量: 114
- 被引次数: 0














 下载:
下载: