-
MoSSe material is a very promising photoelectric material, and its application environment is aqueous solution. However, there is no research of the electrochemical stability of MoSSe materials in aqueous solution. In this work, the Pourbaix diagrams of monolayer MoSSe with different atomic ratios of molybdenum, sulfur and selenium are constructed based on density functional theory, and the thermodynamic stabilities and electrochemical corrosion behaviors under different pH values and electrode potentials are studied. The study of the pourbaix diagram of MoSSe shows that part of the corrosion-free region of MoSSe exists within the stable region of water in the Pourbaix diagram, indicating that the MoSSe can exist stably in the water environment. Compared with alkaline solutions, MoSSe has good corrosion resistance in acidic solution and neutral solution. The Pourbaix diagram of Mo4S2Se6, Mo4S6Se2, Mo4S7Se and Mo4SSe7 show that in the case of high molar fraction of sulfur in monolayer MoSSe with different atomic ratios of molybdenum, sulfur and selenium, the conditions for the stable existence of materials in aqueous solution can have a larger range, and the corrosion resistance becomes better. In the case of high molar fractions of selenium in monolayer MoSSe with different atomic ratios of molybdenum, sulfur and selenium, the range of conditions for the stable existence of materials in aqueous solution becomes smaller, and the corrosion resistance becomes worse. In this work, the stabilities and corrosion behaviors of monolayer MoSSe with different atomic ratios of molybdenum, sulfur and selenium in aqueous solution are predicted, and the degradation behaviors of MoSSe materials are further explored, which can provide theoretical guidance for the application of MoSSe materials in the field of optoelectronics.
-
Keywords:
- first principles /
- Pourbaix diagram /
- MoSSe material /
- stability
[1] Lu A Y, Zhu H, Xiao J, Chuu C P, Han Y, Chiu M H, Cheng C C, Yang C W, Wei K H, Yang Y, Wang Y, Sokaras D, Nordlund D, Yang P, Muller D A, Chou M Y, Zhang X, Li L J 2017 Nat. Nanotechnol. 12 744
 Google Scholar
Google Scholar
[2] Yin W J, Liu Y, Wen B, Li X B, Chai Y F, Wei X L, Ma S, Teobaldi G 2021 Dalton Trans. 50 10252
 Google Scholar
Google Scholar
[3] Zhang J, Jia S, Kholmanov I, Dong L, Er D, Chen W B, Guo H, Jin Z H, Shenoy V B, Shi L, Lou J 2017 ACS Nano 11 8192
 Google Scholar
Google Scholar
[4] Paez-Ornelas J I, Ponce-Pérez R, Fernández-Escamilla H N, Hoat D M, Murillo-Bracamontes E A, Moreno-Armenta M G, Galván D H, Guerrero-Sánchez J 2021 Sci. Rep. 11 1
 Google Scholar
Google Scholar
[5] Guan Z Y, Ni S, Hu S L 2018 J. Phys. Chem. C 122 6209
 Google Scholar
Google Scholar
[6] Singh A, Jain M, Bhattacharya S 2021 Nanoscale Adv. 3 2837
 Google Scholar
Google Scholar
[7] Teets T S, Nocera D G 2011 Chem. Commun. 47 9268
 Google Scholar
Google Scholar
[8] Hansen H A, Rossmeisl J, Nørskov J K 2008 Phys. Chem. Chem. Phys. 10 3722
 Google Scholar
Google Scholar
[9] 彭少方, 张昭 1992 新疆有色金属 1 28
Peng S F, Zhang Z 1992 Xinjiang Youse Jinshu 1 28
[10] Beverskog B, Puigdomenech I 1997 Corros. Sci. 39 969
 Google Scholar
Google Scholar
[11] Ding R, Shang J X, Wang F H, Chen Y 2018 Comput. Mater. Sci. 143 431
 Google Scholar
Google Scholar
[12] Huang L F, Rondinelli J M 2015 Phys. Rev. B 92 245126
 Google Scholar
Google Scholar
[13] Dong X X, Wei B, Legut D, Zhang H J, Zhang R F 2021 Phys. Chem. Chem. Phys. 23 19602
 Google Scholar
Google Scholar
[14] Perry S C, Gateman S M, Stephens L I, Lacasse R, Schulz R, Mauzaroll J 2019 J. Electrochem. Soc. 166 3186
 Google Scholar
Google Scholar
[15] Bajdich M, García-Mota M, Vojvodic A, Nørskov J K, Bell A T 2013 J. Am. Chem. Soc. 135 13521
 Google Scholar
Google Scholar
[16] Persson K A, Waldwick B, Lazic P, Ceder G 2012 Phys. Rev. B 85 235438
 Google Scholar
Google Scholar
[17] Chen J, Selloni A 2013 J. Phys. Chem. C 117 20002
 Google Scholar
Google Scholar
[18] Wang L, Maxisch T, Ceder G 2006 Phys. Rev. B 73 195107
 Google Scholar
Google Scholar
[19] Zeng Z H, Chan M K Y, Zhao Z J, Kubal J, Fan D X, Greeley J 2015 J. Phys. Chem. C 119 18177
 Google Scholar
Google Scholar
[20] Exner K S 2017 ChemElectroChem 4 3231
 Google Scholar
Google Scholar
[21] Perdew J P, Burke K, Ernzerhof M 1998 Phys. Rev. Lett. 80 891
 Google Scholar
Google Scholar
[22] 林洪斌, 林春, 陈越, 钟克华, 张健敏, 许桂贵, 黄志高 2021 70 138201
 Google Scholar
Google Scholar
Lin H B, Lin C, Chen Y, Zhong K H, Zhang J M, Xu G G, Huang Z G 2021 Acta Phys. Sin. 70 138201
 Google Scholar
Google Scholar
[23] Zunger A, Wei S H, Ferreira L G, Bernard J E 1990 Phys. Rev. Lett. 65 353
 Google Scholar
Google Scholar
[24] Grau-Crespo R, Hamad S, Catlow C R A, Leeuw N H D 2007 J. Phys. Condens. Matter 19 256201
 Google Scholar
Google Scholar
[25] Sen S, Ghosh H 2016 Eur. Phys. J. B 89 277
 Google Scholar
Google Scholar
[26] Binder K 1981 Phys. Rev. Lett. 47 693
 Google Scholar
Google Scholar
[27] Gale J D 1997 J. Chem. Soc. Faraday Trans. 93 629
 Google Scholar
Google Scholar
[28] Huang L F, Rondinelli J M 2015 Physical Review B 92 245126
[29] Barry T I 1980 ACS Symp. Ser. 133 681
[30] Lee J B 1981 Corrosion 37 467
 Google Scholar
Google Scholar
[31] Muñoz-Portero M J, García-Antón J, Guiñón J L, Pérez-Herranz V 2009 Corros. Sci. 51 807
 Google Scholar
Google Scholar
[32] Nikolaychuk P A, Tyurin A G 2011 Mater. Sci. 24 101
[33] Protopopoff E, Marcus P 2012 Electrochim. Acta 63 22
 Google Scholar
Google Scholar
[34] Wagman D D, Evans W H, Parker V B, Schemm R H, Halow I 1982 J. Phys. Chem. Ref. Data 11 2
[35] Beverskog B, Puigdomenech I 1997 Corrosion Science 39 969
[36] 吴雄伟, 彭穗, 冯必钧, 山村朝雄, 矢野贵, 佐藤伊佐務, 刘素琴, 黄可龙 2011 无机化学学报 26 535
Wu X W, Peng S, Feng B J, Tomoo Y, Yano T, Isamu S, Liu S Q, Huang K L 2011 Chinese J. Inorg. Chem. 26 535
[37] Gana S J, Egiebor N, Ankumah R O 2011 Mater. Sci. Appl. 2 81
[38] Nishimoto M, Muto I, Sugawara Y, Hara N 2019 J. Electrochem. Soc. 166 3081
[39] Choudhary L, Macdonald D D, Alfantazi A 2015 Corrosion 71 1147
 Google Scholar
Google Scholar
[40] Alhasan R, Nasim M J, Jacob C, Gaucher C 2019 Curr. Pharmacol. Rep. 5 163
 Google Scholar
Google Scholar
-
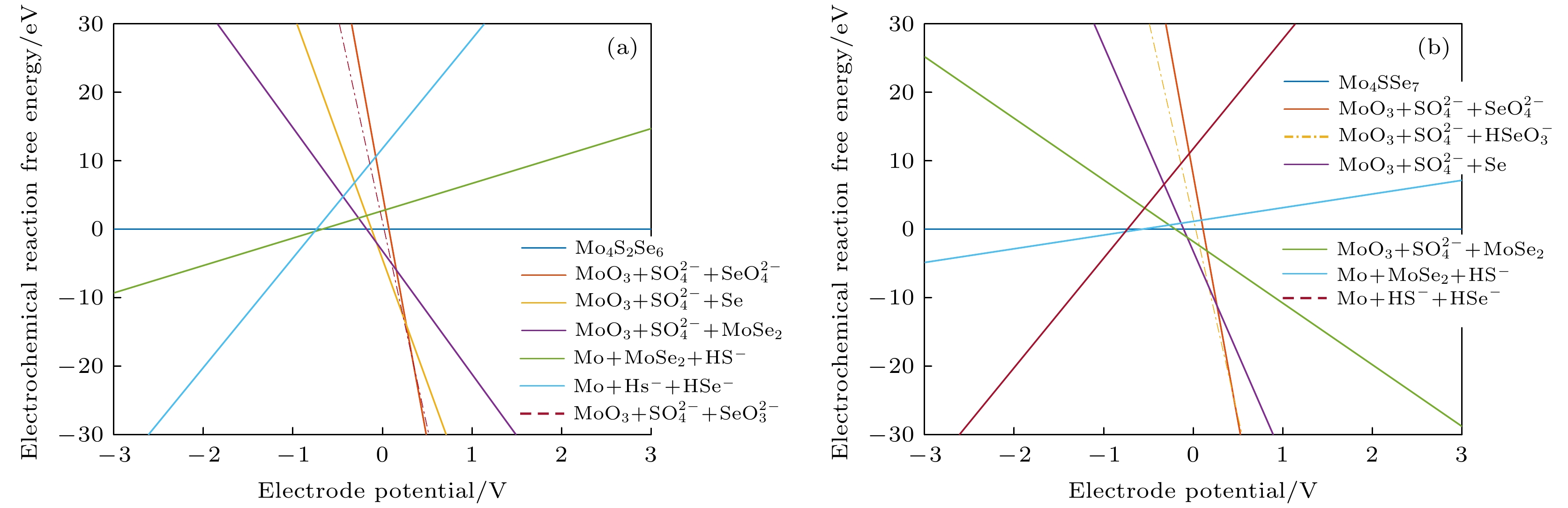
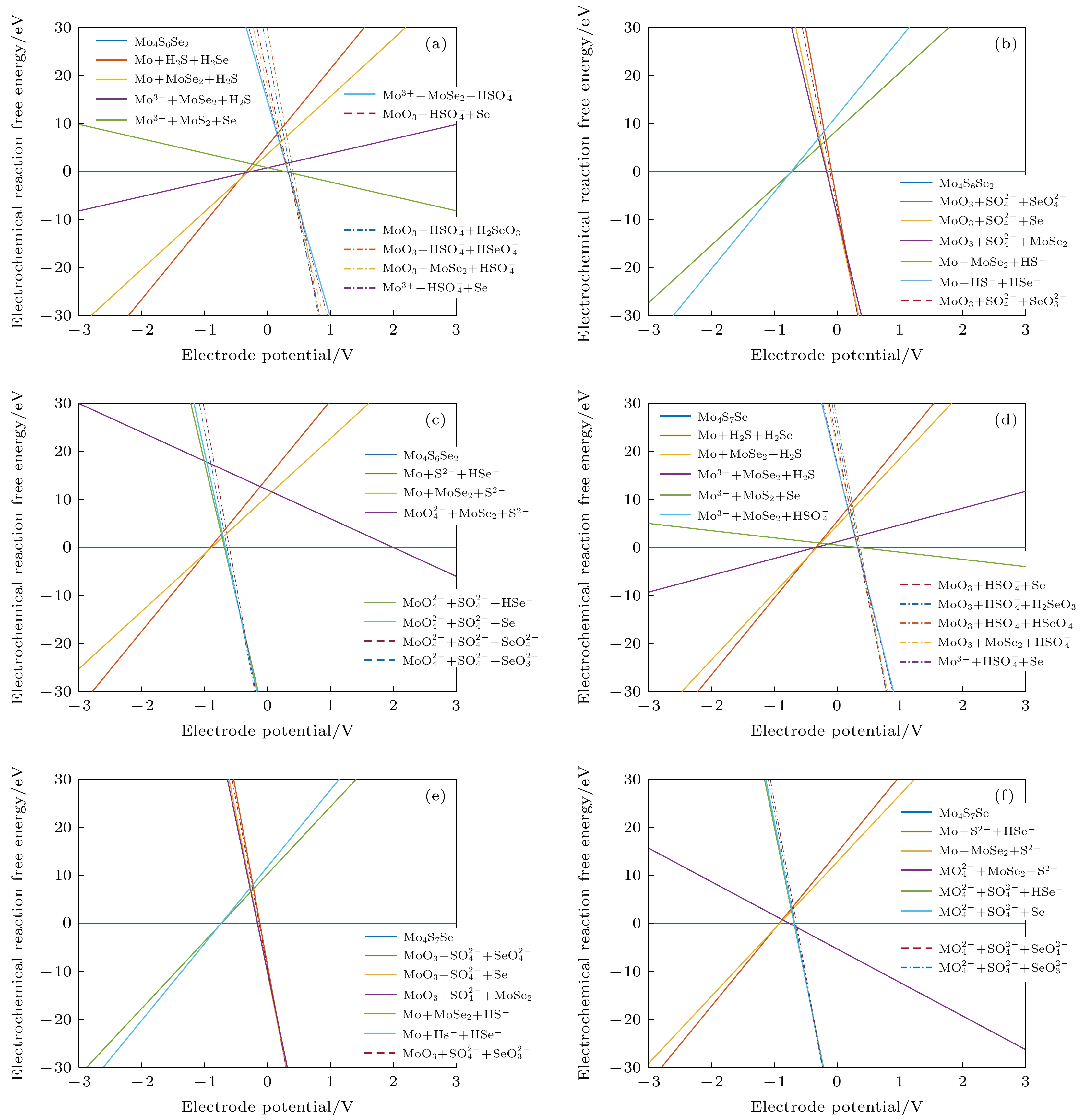
图 7 不同条件下的吉布斯自由能随电极电位变化图 (a) Mo4S6Se2材料在pH = 0时; (b) Mo4S6Se2材料在pH = 7时; (c) Mo4S6Se2材料在pH = 14时; (d) Mo4S7Se材料在pH = 0时; (e) Mo4S7Se材料在pH = 7时; (f) Mo4S7Se材料在pH = 14时
Figure 7. Diagram of Gibbs free energy variation with electrode potential variation under different conditions: (a) Mo4S6Se2 material at pH=0; (b) Mo4S6Se2 material at pH = 7; (c) Mo4S6Se2 material at pH = 14; (d) Mo4S7Se material at pH = 0; (e) Mo4S7Se material at pH = 7; (f) Mo4S7Se material at pH = 14.
表 1 不同比例MoSSe材料的标准化学势
Table 1. Standard chemical potential of MoSSe materials with different proportions.
物质 标准化学势 μ0/eV MoSSe –2.21 Mo4S2Se6 –8.357 Mo4S6Se2 –9.721 Mo4SSe7 –7.990 Mo4S7Se –10.038 表 2 Mo, S和Se元素在水溶液中可能形成的离子状态物质及其标准化学势
Table 2. The possible ionic state substance of Mo, S and Se elements in aqueous solution and their standard chemical potentials.
表 3 Mo, S和Se元素形成的固态物质的空间群和理论化学势
Table 3. The space group and theoretical chemical potentials of solid substances formed by Mo, S and Se elements.
物质 标准化学势μ0/eV 空间群 Mo 0 ${Im} \bar 3m$ MoO2 –5.53 ${ {{P} }{4_2}/{{mnm} } }$ MoO3 –6.92 $P2_1/c$ S 0 $P2/c$ Se 0 $P2/c$ MoSe –0.23 $P \bar 6 m2$ MoSe2 –6.92 $P\bar 3 m1$ MoS2 –2.34 $P6_3/mmc$ 表 4 Mo, S和Se元素在水溶液中可能形成的溶液状态物质及其标准化学势
Table 4. The The possible aqueous state substance of Mo, S and Se elements in aqueous solution and their standard chemical potentials.
表 5 编号对照表
Table 5. Numbering reference table.
编号 物质(溶液中) 1 MoO3 + HSO4– + HSeO4– 2 MoO3 + HSO4– + SeO42– 3 MoO3 + SO42– + SeO42– 4 MoO42– + SO42– + SeO42– 5 MoO42– + SO42– + SeO32– 6 MoO3 + SO42– + SeO32– 7 MoO3 + SO42– + HSeO3– 8 MoO3 + SO42– + H2SeO3 9 MoO3 + HSO4– + H2SeO3 10 MoO3 + HSO4– + Se 11 MoO3 + SO42– + Se 12 MoO42– + SO42– + Se 13 MoO42– + SO42– + HSe– 14 MoO42– + SO42– + Se2– 15 MoO42– + S2– + Se2– 16 MoO42– + S2– + HSe– 17 MoO42– + MoSe2 + S2– 18 MoO42– + MoSe2 + SO42– 19 MoO3 + MoSe2 + SO42– 20 MoO3 + MoSe2 + HSO4– 21 Mo3+ + HSO4– + Se 22 Mo3+ + MoS2 + Se 23 Mo3+ + MoSe2 + H2S 24 MoSSe 25 Mo + MoSe2 + S2– 26 Mo + MoSe2 + HS– 27 Mo + MoSe2 + H2S 28 Mo + H2S + H2Se 29 Mo + H2S + HSe– 30 Mo + HS– + HSe– 31 Mo + S2– + HSe– 32 Mo + S2– + Se2– 33 Mo3+ + MoSe2 + HSO4– 34 MoO42– + HSe– + Mo4S5Se3 35 Mo3+ + Se + Mo4S5Se3 36 Mo + HSe– + Mo4S5Se3 37 Mo3+ + Se + Mo4S6Se2 38 MoO3 + Se + Mo4S5Se3 39 Mo4S2Se6 40 MoO42– + MoSe2 + HS– 41 MoO2 + MoSe2 + HS– 42 MoO3 + MoSe2 + HS– 43 Mo4SSe7 44 Mo4S6Se2 45 Mo4S7Se 46 Mo4S5Se3 -
[1] Lu A Y, Zhu H, Xiao J, Chuu C P, Han Y, Chiu M H, Cheng C C, Yang C W, Wei K H, Yang Y, Wang Y, Sokaras D, Nordlund D, Yang P, Muller D A, Chou M Y, Zhang X, Li L J 2017 Nat. Nanotechnol. 12 744
 Google Scholar
Google Scholar
[2] Yin W J, Liu Y, Wen B, Li X B, Chai Y F, Wei X L, Ma S, Teobaldi G 2021 Dalton Trans. 50 10252
 Google Scholar
Google Scholar
[3] Zhang J, Jia S, Kholmanov I, Dong L, Er D, Chen W B, Guo H, Jin Z H, Shenoy V B, Shi L, Lou J 2017 ACS Nano 11 8192
 Google Scholar
Google Scholar
[4] Paez-Ornelas J I, Ponce-Pérez R, Fernández-Escamilla H N, Hoat D M, Murillo-Bracamontes E A, Moreno-Armenta M G, Galván D H, Guerrero-Sánchez J 2021 Sci. Rep. 11 1
 Google Scholar
Google Scholar
[5] Guan Z Y, Ni S, Hu S L 2018 J. Phys. Chem. C 122 6209
 Google Scholar
Google Scholar
[6] Singh A, Jain M, Bhattacharya S 2021 Nanoscale Adv. 3 2837
 Google Scholar
Google Scholar
[7] Teets T S, Nocera D G 2011 Chem. Commun. 47 9268
 Google Scholar
Google Scholar
[8] Hansen H A, Rossmeisl J, Nørskov J K 2008 Phys. Chem. Chem. Phys. 10 3722
 Google Scholar
Google Scholar
[9] 彭少方, 张昭 1992 新疆有色金属 1 28
Peng S F, Zhang Z 1992 Xinjiang Youse Jinshu 1 28
[10] Beverskog B, Puigdomenech I 1997 Corros. Sci. 39 969
 Google Scholar
Google Scholar
[11] Ding R, Shang J X, Wang F H, Chen Y 2018 Comput. Mater. Sci. 143 431
 Google Scholar
Google Scholar
[12] Huang L F, Rondinelli J M 2015 Phys. Rev. B 92 245126
 Google Scholar
Google Scholar
[13] Dong X X, Wei B, Legut D, Zhang H J, Zhang R F 2021 Phys. Chem. Chem. Phys. 23 19602
 Google Scholar
Google Scholar
[14] Perry S C, Gateman S M, Stephens L I, Lacasse R, Schulz R, Mauzaroll J 2019 J. Electrochem. Soc. 166 3186
 Google Scholar
Google Scholar
[15] Bajdich M, García-Mota M, Vojvodic A, Nørskov J K, Bell A T 2013 J. Am. Chem. Soc. 135 13521
 Google Scholar
Google Scholar
[16] Persson K A, Waldwick B, Lazic P, Ceder G 2012 Phys. Rev. B 85 235438
 Google Scholar
Google Scholar
[17] Chen J, Selloni A 2013 J. Phys. Chem. C 117 20002
 Google Scholar
Google Scholar
[18] Wang L, Maxisch T, Ceder G 2006 Phys. Rev. B 73 195107
 Google Scholar
Google Scholar
[19] Zeng Z H, Chan M K Y, Zhao Z J, Kubal J, Fan D X, Greeley J 2015 J. Phys. Chem. C 119 18177
 Google Scholar
Google Scholar
[20] Exner K S 2017 ChemElectroChem 4 3231
 Google Scholar
Google Scholar
[21] Perdew J P, Burke K, Ernzerhof M 1998 Phys. Rev. Lett. 80 891
 Google Scholar
Google Scholar
[22] 林洪斌, 林春, 陈越, 钟克华, 张健敏, 许桂贵, 黄志高 2021 70 138201
 Google Scholar
Google Scholar
Lin H B, Lin C, Chen Y, Zhong K H, Zhang J M, Xu G G, Huang Z G 2021 Acta Phys. Sin. 70 138201
 Google Scholar
Google Scholar
[23] Zunger A, Wei S H, Ferreira L G, Bernard J E 1990 Phys. Rev. Lett. 65 353
 Google Scholar
Google Scholar
[24] Grau-Crespo R, Hamad S, Catlow C R A, Leeuw N H D 2007 J. Phys. Condens. Matter 19 256201
 Google Scholar
Google Scholar
[25] Sen S, Ghosh H 2016 Eur. Phys. J. B 89 277
 Google Scholar
Google Scholar
[26] Binder K 1981 Phys. Rev. Lett. 47 693
 Google Scholar
Google Scholar
[27] Gale J D 1997 J. Chem. Soc. Faraday Trans. 93 629
 Google Scholar
Google Scholar
[28] Huang L F, Rondinelli J M 2015 Physical Review B 92 245126
[29] Barry T I 1980 ACS Symp. Ser. 133 681
[30] Lee J B 1981 Corrosion 37 467
 Google Scholar
Google Scholar
[31] Muñoz-Portero M J, García-Antón J, Guiñón J L, Pérez-Herranz V 2009 Corros. Sci. 51 807
 Google Scholar
Google Scholar
[32] Nikolaychuk P A, Tyurin A G 2011 Mater. Sci. 24 101
[33] Protopopoff E, Marcus P 2012 Electrochim. Acta 63 22
 Google Scholar
Google Scholar
[34] Wagman D D, Evans W H, Parker V B, Schemm R H, Halow I 1982 J. Phys. Chem. Ref. Data 11 2
[35] Beverskog B, Puigdomenech I 1997 Corrosion Science 39 969
[36] 吴雄伟, 彭穗, 冯必钧, 山村朝雄, 矢野贵, 佐藤伊佐務, 刘素琴, 黄可龙 2011 无机化学学报 26 535
Wu X W, Peng S, Feng B J, Tomoo Y, Yano T, Isamu S, Liu S Q, Huang K L 2011 Chinese J. Inorg. Chem. 26 535
[37] Gana S J, Egiebor N, Ankumah R O 2011 Mater. Sci. Appl. 2 81
[38] Nishimoto M, Muto I, Sugawara Y, Hara N 2019 J. Electrochem. Soc. 166 3081
[39] Choudhary L, Macdonald D D, Alfantazi A 2015 Corrosion 71 1147
 Google Scholar
Google Scholar
[40] Alhasan R, Nasim M J, Jacob C, Gaucher C 2019 Curr. Pharmacol. Rep. 5 163
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 7659
- PDF Downloads: 95
- Cited By: 0














 DownLoad:
DownLoad: