-
以铯铅氯为代表的全无机金属卤化物钙钛矿因为优异的光电性质与缺陷容忍度成为发展高性能光伏和光电子器件的重要候选材料. 然而, 较差的结构稳定性成为限制其商业化应用的瓶颈问题. 本文提出范德瓦耳斯集成薄层的氧氯化铋(BiOCl)在铯铅氯钙钛矿(CsPbCl3)表面形成范德瓦耳斯异质结, 结合第一性原理计算与从头算分子动力学模拟系统研究了BiOCl/CsPbCl3范德瓦耳斯异质结的环境稳定性以及界面效应对其光电性质的影响. 计算结果表明, 范德瓦耳斯集成BiOCl在CsPbCl3的表面能够极大地改善其环境稳定性, 这源于高稳定的BiOCl层隔绝了水和氧分子与钙钛矿晶格的反应. 并且, 两种不同终端的BiOCl/CsPbCl3范德瓦耳斯异质结显示II型的能带结构, 这有助于促进载流子分离; 但是, CsCl终端的异质结表现出比PbCl2终端异质结更大的能带带阶, 导致CsCl终端的异质结具有更高的开路电压和更低的暗电流. 由于BiOCl和CsPbCl3不同的禁带宽度使得异质结在可见到紫外光区域都显示出较高的光学吸收系数. 本工作为提高CsPbCl3钙钛矿材料的结构稳定性以及在高性能光电器件的应用提供了一个新的思路和理论基础.All-inorganic metal halide perovskites represented by cesium lead chloride have become important candidates for the development of high-performance photovoltaic and optoelectronic devices due to their excellent optoelectronic properties and defect tolerance. However, poor structural stability has become a bottleneck for its commercial applications. In this work, we propose to integrate thin layers of bismuth oxychloride (BiOCl) on the surface of cesium lead chloride perovskite (CsPbCl3) to form a van der Waals heterojunction. And we systematically study the environmental stability of BiOCl/CsPbCl3 van der Waals heterojunction and the influence of interfacial effects on its optoelectronic properties by combining first-principles calculations and ab initio molecular dynamics simulations. The calculated results show that the van der Waals integrated BiOCl on the surface of CsPbCl3 can greatly improve its environmental stability, which is due to the highly stable BiOCl layer isolating the reaction of water and oxygen molecules with the perovskite lattice. Moreover, the two BiOCl/CsPbCl3 van der Waals heterojunctions show a type-II band structure, which conduces to promoting the carrier separation. At the same time, the two heterojunctions have small effective carrier mass, which well preserves the excellent carrier transport properties of CsPbCl3 and BiOCl. However, CsCl-terminated heterojunctions exhibit larger band orders than PbCl2-terminated heterojunctions, which can lead to higher open-circuit voltages and lower dark currents in CsCl-terminated heterojunctions. Owing to the different band gaps of BiOCl and CsPbCl3, the heterojunctions show high optical absorption coefficients in the visible-to-ultraviolet region. This work provides a new idea and theoretical basis for improving the structural stability of CsPbCl3 perovskite materials and their applications in high-performance optoelectronic devices.
-
Keywords:
- all-inorganic halide perovskites /
- stability /
- Van der Waals heterojunction /
- optoelectronic properties /
- first principles
[1] 姚鑫, 丁艳丽, 张晓丹, 赵颖 2015 64 038805
 Google Scholar
Google Scholar
Yao X, Ding Y L, Zhang X D, Zhao Y 2015 Acta Phys. Sin. 64 038805
 Google Scholar
Google Scholar
[2] Jeong J, Kim M, Seo J, Lu H, Ahlawat P, Mishra A, Yang Y, Hope M A, Eickemeyer F T, Kim M, Yoon Y J, Choi I W, Darwich B P, Choi S J, Jo Y, Lee J H, Walker B, Zakeeruddin S M, Emsley L, Rothlisberger U, Hagfeldt A, Kim D S, Gratzel M, Kim J Y 2021 Nature 592 381
 Google Scholar
Google Scholar
[3] Valverde-Chávez D A, Ponseca C S, Stoumpos C C, Yartsev A, Kanatzidis M G, Sundström V, Cooke D G 2015 Energy Environ. Sci. 8 3700
 Google Scholar
Google Scholar
[4] Wang Y P, Gong C, Rao G, Hu K, Wang X, Yan C, Dai L, Wu C, Xiong J 2018 Adv. Opt. Mater. 6 1701302
 Google Scholar
Google Scholar
[5] Conings B, Drijkoningen J, Gauquelin N, Babayigit A, D'Haen J, D’Olieslaeger L, Ethirajan A, Verbeeck J, Manca J, Mosconi E, Angelis F D, Boyen H G 2015 Adv. Energy Mater. 5 1500477
 Google Scholar
Google Scholar
[6] Bai F, Qi J, Li F, Fang Y, Han W, Wu H, Zhang Y 2018 Adv. Mater. Interfaces 5 1701275
 Google Scholar
Google Scholar
[7] Ouedraogo N A N, Chen Y, Xiao Y Y, Meng Q, Han C B, Yan H, Zhang Y 2020 Nano Energy 67 104249
 Google Scholar
Google Scholar
[8] Yang J, Zhang Q, Xu J, Liu H, Qin R, Zhai H, Chen S, Yuan M 2019 Nanomaterials 9 1666
 Google Scholar
Google Scholar
[9] Liu C, Li W, Li H, Wang H, Zhang C, Yang Y, Gao X, Xue Q, Yip H L, Fan J, Schropp R E I, Mai Y 2019 Adv. Energy Mater. 9 1803572
 Google Scholar
Google Scholar
[10] Liu X, Wang X, Zhang T, Miao Y, Qin Z, Chen Y, Zhao Y 2021 Angew Chem. Int Ed. 60 12351
 Google Scholar
Google Scholar
[11] Wu L, Chen K, Huang W, Lin Z, Zhao J, Jiang X, Ge Y, Zhang F, Xiao Q, Guo Z, Xiang Y, Li J, Bao Q, Zhang H 2018 Adv. Opt. Mater. 6 1800400
 Google Scholar
Google Scholar
[12] Lee J W, Dai Z, Han T H, Choi C, Chang S Y, Lee S J, De Marco N, Zhao H, Sun P, Huang Y, Yang Y 2018 Nat. Commun. 9 3021
 Google Scholar
Google Scholar
[13] 刘晓敏, 李亦回, 王兴涛, 赵一新 2019 68 158805
 Google Scholar
Google Scholar
Liu X M, Li Y H, Wang X T, Zhao Y X 2019 Acta Phys. Sin. 68 158805
 Google Scholar
Google Scholar
[14] Chen P, Bai Y, Wang S, Lyu M, Yun J H, Wang L 2018 Adv. Funct. Mater. 28 1706923
 Google Scholar
Google Scholar
[15] Schlipf J, Hu Y, Pratap S, Bießmann L, Hohn N, Porcar L, Bein T, Docampo P, Müller B P 2019 ACS Appl. Energy Mater. 2 1011
 Google Scholar
Google Scholar
[16] Zhang T, Long M, Qin M, Lu X, Chen S, Xie F, Gong L, Chen J, Chu M, Miao Q, Chen Z, Xu W, Liu P, Xie W, Xu J B 2018 Joule 2 2706
 Google Scholar
Google Scholar
[17] Lin D, Zhang T, Wang J, Long M, Xie F, Chen J, Wu B, Shi T, Yan K, Xie W, Liu P, Xu J 2019 Nano Energy 59 619
 Google Scholar
Google Scholar
[18] Loi H L, Cao J, Guo X, Liu C K, Wang N, Song J, Tang G, Zhu Y, Yan F 2020 Adv. Sci. 7 2000776
 Google Scholar
Google Scholar
[19] 王其, 延玲玲, 陈兵兵, 李仁杰, 王三龙, 王鹏阳, 黄茜, 许盛之, 侯国付, 陈新亮, 李跃龙, 丁毅, 张德坤, 王广才, 赵颖, 张晓丹 2021 70 057802
 Google Scholar
Google Scholar
Wang Q, Yan L L, Chen B B, Li R J, Wang S L, Wang P Y, Huang Q, Xu S Z, Hou G F, Chen X L, Li Y L, Ding Y, Zhang D K, Wang G C, Zhao Y, Zhang X D 2021 Acta Phys. Sin. 70 057802
 Google Scholar
Google Scholar
[20] He J, Su J, Lin Z, Zhang S, Qin Y, Zhang J, Chang J, Hao Y 2019 J. Phys. Chem. C 123 7158
 Google Scholar
Google Scholar
[21] Liu Z, Chang J, Lin Z, Zhou L, Yang Z, Chen D, Zhang C, Liu S F, Hao Y 2018 Adv. Energy Mater. 8 1703432
 Google Scholar
Google Scholar
[22] Zhang H, Liu L, Zhou Z 2012 RSC Adv. 2 9224
 Google Scholar
Google Scholar
[23] Gnayem H, Sasson Y 2013 ACS Catalysis 3 186
 Google Scholar
Google Scholar
[24] Zhang X, Ai Z, Jia F, Zhang L J 2008 J. Phys. Chem. C 112 747
 Google Scholar
Google Scholar
[25] Ye L, Zan L, Tian L, Peng T, Zhang J 2011 Chem. Commun. 47 6951
 Google Scholar
Google Scholar
[26] Zhang K, Liang J, Wang S, Liu J, Ren K, Zheng X, Luo H, Peng Y, Zou X, Bo X, Li J, Yu X 2012 Cryst. Growth Des. 12 793
 Google Scholar
Google Scholar
[27] Liu X, Su Y, Zhao Q, Du C, Liu Z 2016 Sci. Rep. 6 28689
 Google Scholar
Google Scholar
[28] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[29] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[30] Blochl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[31] Payne M C, Teter M P, Allan D C, Arias T A, Joannopoulos J D 1992 Rev. Mod. Phys. 64 1045
 Google Scholar
Google Scholar
[32] Krukau A V, Vydrov O A, Izmaylov A F, Scuseria G E 2006 J. Chem. Phys. 125 224106
 Google Scholar
Google Scholar
[33] Wang N, Cao D, Wang J, Liang P, Chen X, Shu H 2017 J. Mater. Chem. C 5 9687
 Google Scholar
Google Scholar
[34] Boyd C C, Cheacharoen R, Leijtens T, McGehee M D 2019 Chem. Rev. 119 3418
 Google Scholar
Google Scholar
[35] Berhe T A, Su W N, Chen C H, Pan C J, Cheng J H, Chen H M, Tsai M C, Chen L Y, Dubale A A, Hwang B J 2016 Energy Environ. Sci. 9 323
 Google Scholar
Google Scholar
[36] Lu Z H, Lockwood D J, Baribeau J M 1995 Nature 378 258
 Google Scholar
Google Scholar
[37] Murtaza G, Ahmad I 2011 Physica B 406 3222
 Google Scholar
Google Scholar
[38] Zheng B, Ma C, Li D, Lan J, Zhang Z, Sun X, Zheng W, Yang T, Zhu C, Ouyang G, Xu G, Zhu X, Wang X, Pan A 2018 J. Am. Chem. Soc. 140 11193
 Google Scholar
Google Scholar
[39] Duan X, Wang C, Shaw J C, Cheng R, Chen Y, Li H, Wu X, Tang Y, Zhang Q, Pan A, Jiang J, Yu R, Huang Y, Duan X 2014 Nat. Nanotechnol. 9 1024
 Google Scholar
Google Scholar
[40] Zheng B, Ma C, Li D, Lan J, Zhang Z, Sun X, Zheng W, Yang T, Zhu C, Ouyang G, Xu G, Zhu X, Wang X, Pan A 2018 JACS 140 11193
[41] Roy T, Tosun M, Hettick M, Ahn G H, Hu C, Javey A 2016 Appl. Phys. Lett. 108 083111
 Google Scholar
Google Scholar
[42] Liao C S, Yu Z L, He P B, Zhao Y Q, Liu B, Cai M Q 2020 J. Power Sources 478 229078
 Google Scholar
Google Scholar
-
图 1 BiOCl/CsPbCl3异质结构建过程 (a) CsCl终端CsPbCl3, (b) BiOCl和(c) PbCl2终端CsPbCl3结构俯视图(蓝框为初始晶胞, 黄框为构建异质结所选晶格大小, CsPbCl3材料黄蓝框相重合); (d) CsCl和(e) PbCl2终端的异质结构型
Fig. 1. The construction process of the BiOCl/CsPbCl3 heterostructure: The top views of (a) CsCl-terminated, (b) BiOCl and (c) PbCl2-terminated of CsPbCl3 structure (the blue box is the initial unit cell, and the yellow box is the lattice selected for the construction of the heterojunction; (d) CsCl-terminated and (e)PbCl2-terminated heterojunction structures.
图 2 (a)表面覆盖了水氧分子层的2L-CsPbCl3结构以及BiOCl/CsPbCl3异质结在AIMD模拟中的能量随模拟时间的变化曲线图(上方曲线为2L-CsPbCl3结构, 下方为HIT-CsCl异质结); (b) 2L-CsPbCl3和(c)BiOCl/CsPbCl3异质结在温度为300 K下, AIMD轨迹在时间为0, 2, 4和6 ps时的快照
Fig. 2. The total energies of (a) 2L-CsPbCl3 and (b) BiOCl/CsPbCl3 as a function of AIMD simulation time; (b), (c) the snapshots of AIMD trajectories for PNMA-3 and DPA-3 at the simulation time of 0, 2, 4 and 6 ps.
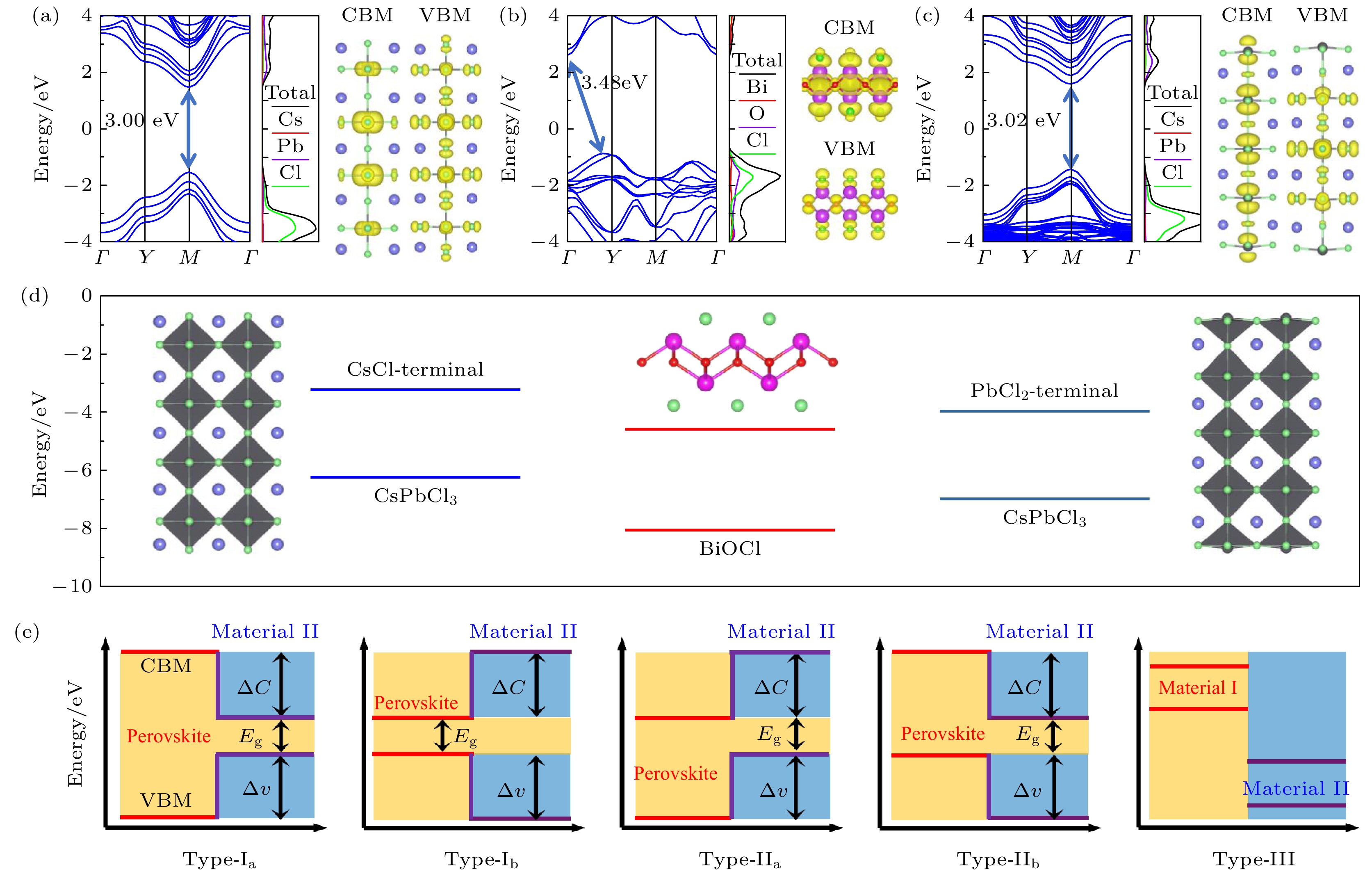
图 3 异质结带阶类型预测图 (a) CsCl终端CsPbCl3, (b) BiOCl与(c)PbCl2终端CsPbCl3的能带、态密度以及电荷密度等值面(CDI)分布图; (d)三种结构带边相对真空能级的位置与分子构型图; (e)五种带阶类型示意图
Fig. 3. Heterogeneous band alignment type predictive diagram: Energy band, density of states and charge density isosurface (CDI) distributions of (a) CsCl-terminated CsPbCl3, (b) BiOCl and (c) PbCl2-terminated CsPbCl3; (d) position comparison diagram of three structural strip positions relative vacuum level, wherein the illustrations are a molecular configuration diagram of three structures; (e) tive lead types that can be formed by constructing heterojunction.
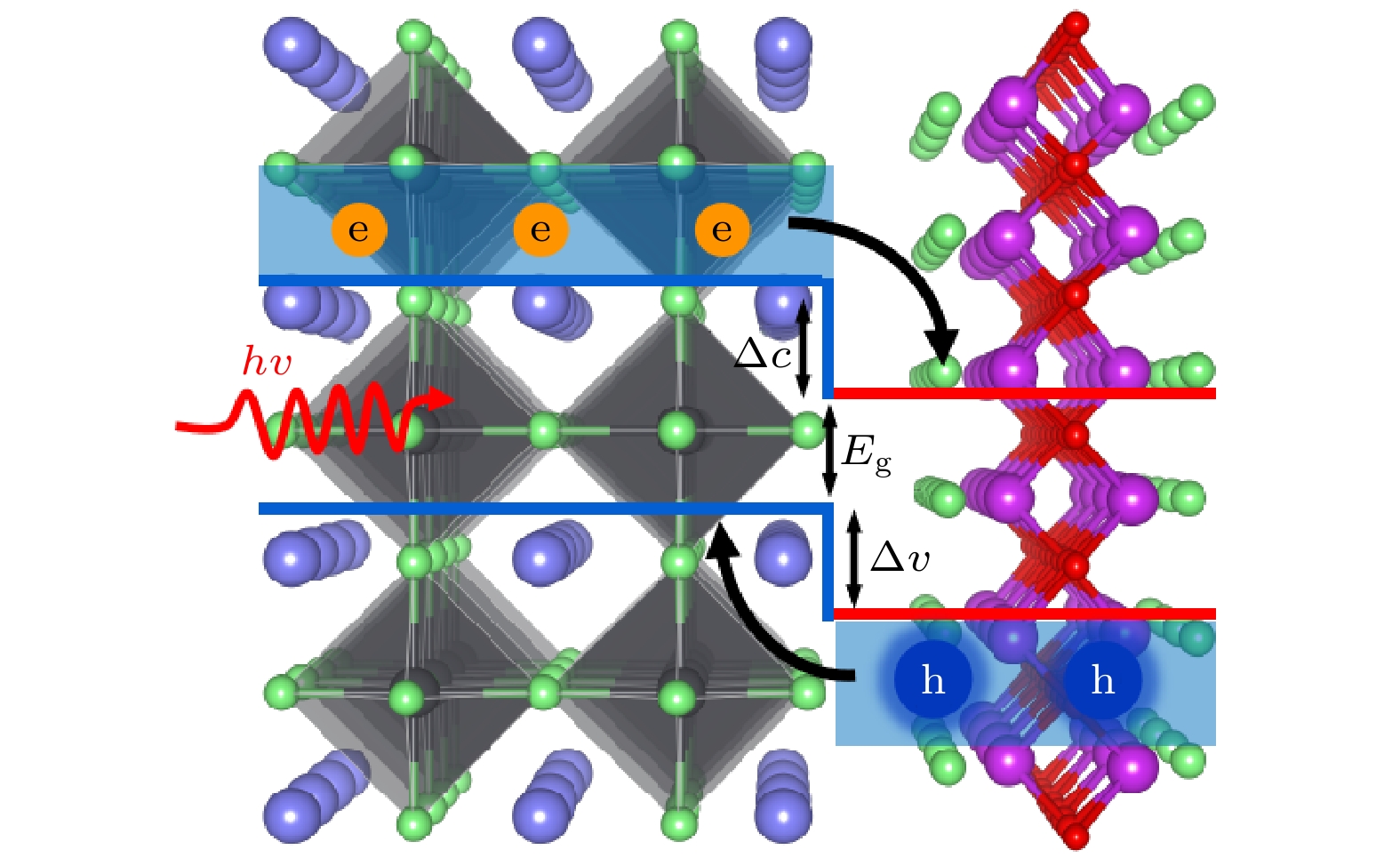
图 4 BiOCl/CsPbCl3异质结的电子性质 (a)II型带阶类型的载流子输运示意图; (b)载流子有效质量柱状图; (c) CsCl终端与(d)PbCl2终端异质结的投影能带图、组分之间带边差异、态密度图以及CDI分布图
Fig. 4. Electronic properties of BiOCl/CsPbCl3 heterojunctions: (a) Schematic diagram of carrier transport of type II band-order type; (b) effective carrier mass; (c) CsCl-terminated and (d) PbCl2-terminated heterojunctions Projected energy band diagrams, band edge differences between components, density of states diagrams, and CDI distribution diagrams.
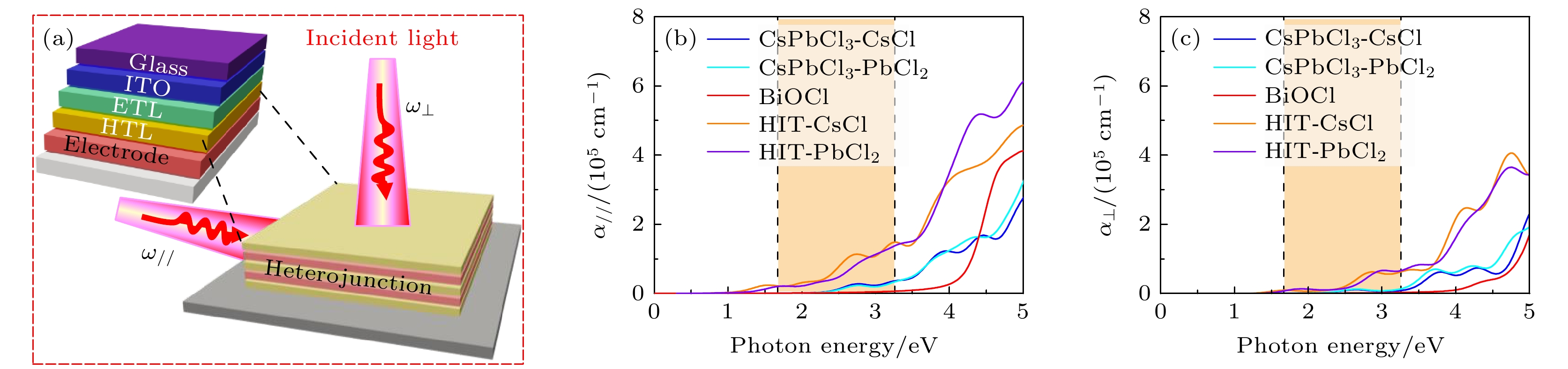
图 5 BiOCl/CsPbCl3异质结及其原料的光吸收图 (a)异质结器件沿面内 (ω//) 和面外 (ω⊥) 方向的光照射示意图; 不同终端异质结及其原料沿(b)面内(α//)和(c)面外(α⊥)方向的光吸收光谱(虚线范围对应于波长范围为390—780 nm的可见光区域)
Fig. 5. Optical absorption diagram of BiOCl/CsPbCl3 heterojunction and its components: (a) Schematic illustration of light illumination in the in-plane (ω//) and out-of-plane (ω⊥) directions of the heterojunction device; optical absorption spectra of different terminated heterojunctions and their components along (b) in-plane (α//) and (c) out-of-plane (α⊥) directions (the dotted line range corresponds to the visible region of wavelengths from 390 to 780 nm).
图 A1 8种异质结构型图 (a)—(d)CsCl终端CsPbCl3与BiOCl构建的4种异质结, 原子对齐位置如红线所标注; (e)—(h) PbCl2终端CsPbCl3与BiOCl构建的4种异质结, 原子对齐位置与CsCl终端相同
Fig. A1. Figures of 8 types of heterostructures: (a)–(d) Four types of heterojunctions constructed by CsCl-terminated CsPbCl3 and BiOCl, and the atomic alignment positions are marked by red lines; (e)–(h) four types of heterojunctions constructed by PbCl2-terminated CsPbCl3 and BiOCl, with atoms aligned The position is the same as the CsCl terminal.
表 A1 不同终端的8种BiOCl/CsPbCl3异质结构型的能量和层间距
Table A1. Energy comparison and layer spacing of eight BiOCl/CsPbCl3 heterojunctions types with different terminations.
Structures Method Energy/eV Layer
Spacing/ ÅHIT-CsCl PBE S1 –134.47 3.26 S2 –134.46 3.68 S3 –134.43 3.41 S4 –134.41 3.44 HIT-PbCl2 S1 –138.55 3.35 S2 –138.57 3.28 S3 –138.54 3.28 S4 –138.54 3.29 表 B1 1 L-BiOCl、两种终端4 L-CsPbCl3和两种终端HIT的总原子数 (Ntot)、异质结层间距、优化后的晶格参数 (a, b和c)、带隙(Eg)、形成能 (Ef)和载流子有效质量(m*)
Table B1. 1 L-BiOCl, 4 L-CsPbCl3 with two terminations, and HIT with two terminations: total atomic number (Ntot), heterojunction interlayer spacing, optimized lattice parameters (a, b and c), band gap (Eg), formation energy (Ef) and carrier effective mass (m*).
Ntot Layer spacing
/ÅLattice constant /Å Eg/eV Ef/eV m* a b c me mh 1L-BiOCl 12 5.73 5.73 42.96 3.48 0.38 1.71 4L-CsPbCl3-CsCl 22 5.72 5.72 42.86 3.00 0.21 0.22 4L-CsPbCl3-PbCl2 23 5.72 5.72 42.86 3.02 0.30 0.22 HIT-CsCl 22 3.17 5.70 5.71 42.77 1.51 8.6×10–3 0.44 0.22 HIT-PbCl2 23 3.35 5.71 5.71 42.78 1.60 8.9×10–3 0.34 0.23 -
[1] 姚鑫, 丁艳丽, 张晓丹, 赵颖 2015 64 038805
 Google Scholar
Google Scholar
Yao X, Ding Y L, Zhang X D, Zhao Y 2015 Acta Phys. Sin. 64 038805
 Google Scholar
Google Scholar
[2] Jeong J, Kim M, Seo J, Lu H, Ahlawat P, Mishra A, Yang Y, Hope M A, Eickemeyer F T, Kim M, Yoon Y J, Choi I W, Darwich B P, Choi S J, Jo Y, Lee J H, Walker B, Zakeeruddin S M, Emsley L, Rothlisberger U, Hagfeldt A, Kim D S, Gratzel M, Kim J Y 2021 Nature 592 381
 Google Scholar
Google Scholar
[3] Valverde-Chávez D A, Ponseca C S, Stoumpos C C, Yartsev A, Kanatzidis M G, Sundström V, Cooke D G 2015 Energy Environ. Sci. 8 3700
 Google Scholar
Google Scholar
[4] Wang Y P, Gong C, Rao G, Hu K, Wang X, Yan C, Dai L, Wu C, Xiong J 2018 Adv. Opt. Mater. 6 1701302
 Google Scholar
Google Scholar
[5] Conings B, Drijkoningen J, Gauquelin N, Babayigit A, D'Haen J, D’Olieslaeger L, Ethirajan A, Verbeeck J, Manca J, Mosconi E, Angelis F D, Boyen H G 2015 Adv. Energy Mater. 5 1500477
 Google Scholar
Google Scholar
[6] Bai F, Qi J, Li F, Fang Y, Han W, Wu H, Zhang Y 2018 Adv. Mater. Interfaces 5 1701275
 Google Scholar
Google Scholar
[7] Ouedraogo N A N, Chen Y, Xiao Y Y, Meng Q, Han C B, Yan H, Zhang Y 2020 Nano Energy 67 104249
 Google Scholar
Google Scholar
[8] Yang J, Zhang Q, Xu J, Liu H, Qin R, Zhai H, Chen S, Yuan M 2019 Nanomaterials 9 1666
 Google Scholar
Google Scholar
[9] Liu C, Li W, Li H, Wang H, Zhang C, Yang Y, Gao X, Xue Q, Yip H L, Fan J, Schropp R E I, Mai Y 2019 Adv. Energy Mater. 9 1803572
 Google Scholar
Google Scholar
[10] Liu X, Wang X, Zhang T, Miao Y, Qin Z, Chen Y, Zhao Y 2021 Angew Chem. Int Ed. 60 12351
 Google Scholar
Google Scholar
[11] Wu L, Chen K, Huang W, Lin Z, Zhao J, Jiang X, Ge Y, Zhang F, Xiao Q, Guo Z, Xiang Y, Li J, Bao Q, Zhang H 2018 Adv. Opt. Mater. 6 1800400
 Google Scholar
Google Scholar
[12] Lee J W, Dai Z, Han T H, Choi C, Chang S Y, Lee S J, De Marco N, Zhao H, Sun P, Huang Y, Yang Y 2018 Nat. Commun. 9 3021
 Google Scholar
Google Scholar
[13] 刘晓敏, 李亦回, 王兴涛, 赵一新 2019 68 158805
 Google Scholar
Google Scholar
Liu X M, Li Y H, Wang X T, Zhao Y X 2019 Acta Phys. Sin. 68 158805
 Google Scholar
Google Scholar
[14] Chen P, Bai Y, Wang S, Lyu M, Yun J H, Wang L 2018 Adv. Funct. Mater. 28 1706923
 Google Scholar
Google Scholar
[15] Schlipf J, Hu Y, Pratap S, Bießmann L, Hohn N, Porcar L, Bein T, Docampo P, Müller B P 2019 ACS Appl. Energy Mater. 2 1011
 Google Scholar
Google Scholar
[16] Zhang T, Long M, Qin M, Lu X, Chen S, Xie F, Gong L, Chen J, Chu M, Miao Q, Chen Z, Xu W, Liu P, Xie W, Xu J B 2018 Joule 2 2706
 Google Scholar
Google Scholar
[17] Lin D, Zhang T, Wang J, Long M, Xie F, Chen J, Wu B, Shi T, Yan K, Xie W, Liu P, Xu J 2019 Nano Energy 59 619
 Google Scholar
Google Scholar
[18] Loi H L, Cao J, Guo X, Liu C K, Wang N, Song J, Tang G, Zhu Y, Yan F 2020 Adv. Sci. 7 2000776
 Google Scholar
Google Scholar
[19] 王其, 延玲玲, 陈兵兵, 李仁杰, 王三龙, 王鹏阳, 黄茜, 许盛之, 侯国付, 陈新亮, 李跃龙, 丁毅, 张德坤, 王广才, 赵颖, 张晓丹 2021 70 057802
 Google Scholar
Google Scholar
Wang Q, Yan L L, Chen B B, Li R J, Wang S L, Wang P Y, Huang Q, Xu S Z, Hou G F, Chen X L, Li Y L, Ding Y, Zhang D K, Wang G C, Zhao Y, Zhang X D 2021 Acta Phys. Sin. 70 057802
 Google Scholar
Google Scholar
[20] He J, Su J, Lin Z, Zhang S, Qin Y, Zhang J, Chang J, Hao Y 2019 J. Phys. Chem. C 123 7158
 Google Scholar
Google Scholar
[21] Liu Z, Chang J, Lin Z, Zhou L, Yang Z, Chen D, Zhang C, Liu S F, Hao Y 2018 Adv. Energy Mater. 8 1703432
 Google Scholar
Google Scholar
[22] Zhang H, Liu L, Zhou Z 2012 RSC Adv. 2 9224
 Google Scholar
Google Scholar
[23] Gnayem H, Sasson Y 2013 ACS Catalysis 3 186
 Google Scholar
Google Scholar
[24] Zhang X, Ai Z, Jia F, Zhang L J 2008 J. Phys. Chem. C 112 747
 Google Scholar
Google Scholar
[25] Ye L, Zan L, Tian L, Peng T, Zhang J 2011 Chem. Commun. 47 6951
 Google Scholar
Google Scholar
[26] Zhang K, Liang J, Wang S, Liu J, Ren K, Zheng X, Luo H, Peng Y, Zou X, Bo X, Li J, Yu X 2012 Cryst. Growth Des. 12 793
 Google Scholar
Google Scholar
[27] Liu X, Su Y, Zhao Q, Du C, Liu Z 2016 Sci. Rep. 6 28689
 Google Scholar
Google Scholar
[28] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[29] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[30] Blochl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[31] Payne M C, Teter M P, Allan D C, Arias T A, Joannopoulos J D 1992 Rev. Mod. Phys. 64 1045
 Google Scholar
Google Scholar
[32] Krukau A V, Vydrov O A, Izmaylov A F, Scuseria G E 2006 J. Chem. Phys. 125 224106
 Google Scholar
Google Scholar
[33] Wang N, Cao D, Wang J, Liang P, Chen X, Shu H 2017 J. Mater. Chem. C 5 9687
 Google Scholar
Google Scholar
[34] Boyd C C, Cheacharoen R, Leijtens T, McGehee M D 2019 Chem. Rev. 119 3418
 Google Scholar
Google Scholar
[35] Berhe T A, Su W N, Chen C H, Pan C J, Cheng J H, Chen H M, Tsai M C, Chen L Y, Dubale A A, Hwang B J 2016 Energy Environ. Sci. 9 323
 Google Scholar
Google Scholar
[36] Lu Z H, Lockwood D J, Baribeau J M 1995 Nature 378 258
 Google Scholar
Google Scholar
[37] Murtaza G, Ahmad I 2011 Physica B 406 3222
 Google Scholar
Google Scholar
[38] Zheng B, Ma C, Li D, Lan J, Zhang Z, Sun X, Zheng W, Yang T, Zhu C, Ouyang G, Xu G, Zhu X, Wang X, Pan A 2018 J. Am. Chem. Soc. 140 11193
 Google Scholar
Google Scholar
[39] Duan X, Wang C, Shaw J C, Cheng R, Chen Y, Li H, Wu X, Tang Y, Zhang Q, Pan A, Jiang J, Yu R, Huang Y, Duan X 2014 Nat. Nanotechnol. 9 1024
 Google Scholar
Google Scholar
[40] Zheng B, Ma C, Li D, Lan J, Zhang Z, Sun X, Zheng W, Yang T, Zhu C, Ouyang G, Xu G, Zhu X, Wang X, Pan A 2018 JACS 140 11193
[41] Roy T, Tosun M, Hettick M, Ahn G H, Hu C, Javey A 2016 Appl. Phys. Lett. 108 083111
 Google Scholar
Google Scholar
[42] Liao C S, Yu Z L, He P B, Zhao Y Q, Liu B, Cai M Q 2020 J. Power Sources 478 229078
 Google Scholar
Google Scholar
计量
- 文章访问数: 6177
- PDF下载量: 109
- 被引次数: 0














 下载:
下载: