-
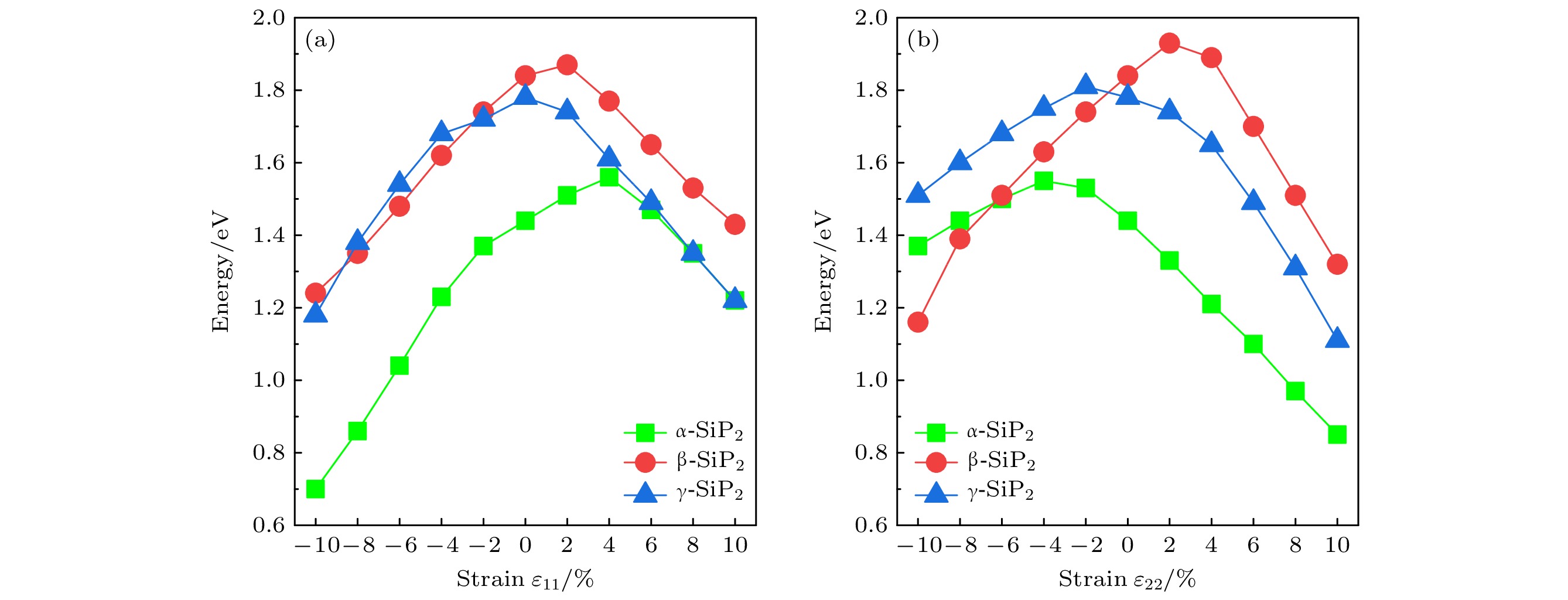
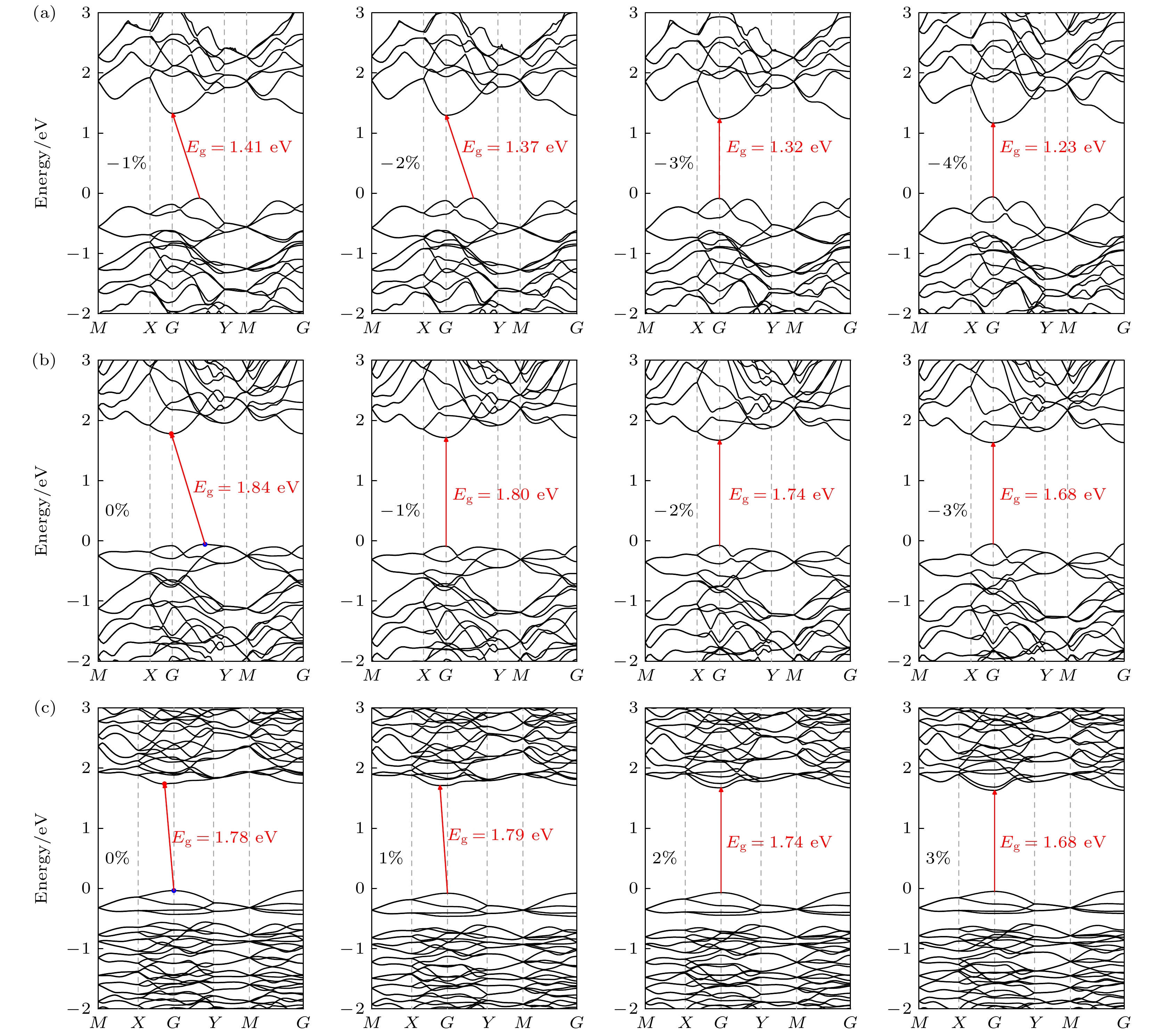
Since the successful preparation of single-layer graphene in 2004, the two-dimensional (2D) materials have received widespread attention. Driven by this research upsurge, many kinds of 2D compound materials with different properties have been discovered one after another, and some of these 2D materials have a variety of allotropes, showing more abundant properties. Our computational studies focus on searching for new stable 2D SiP2 allotropes, and studying their binding energy, phonon dispersions, electronic band structures, strain-dependent bandgap modulation behaviors, piezoelectric properties, etc. In this paper, three novel 2D SiP2 allotrope structures, i.e. α-SiP2, β-SiP2, and γ-SiP2, are found by the random prediction method of crystal structure based on group theory and graph theory (RG2). Their stabilities and electronic properties are investigated by using the first-principles method based on the density functional theory. The results show that the three novel SiP2 structures are stable thermodynamically, dynamically and mechanically. Using the GW calculations, three novel SiP2 structures possess indirect band gaps of 2.62, 2.99 and 3.00 eV, respectively. Their band gaps are feasible to modulate effectively by applying strain. The band gaps of the three novel SiP2 isomers are reduced significantly when subjected to a large strainused, and the three novel SiP2 isomers exhibit indirect-to-direct bandgap transitions when experienced by a certain strain along the x-axis direction. These properties make them potential materials that are suitable for serving as nanoscale photocatalysts. Moreover, three SiP2 isomers have non-centrosymmetric crystal structures, which enable them to exhibit their piezoelectricities. Therefore, we study their piezoelectric properties by combining the Berry phase theory. Our studies show that three novel 2D SiP2 allotropes have good piezoelectric properties. The piezoelectric coefficient of the α-SiP2 isomer and the β-SiP2 isomer are both larger than that of h-BN, and they are comparable to the counterpart of MoS2. These novel structures promise to be used to fabricate nano-electromechanical devices for micro- and nano-scaled electromechanical conversion and electromechanical sensing and controlling.
-
Keywords:
- SiP2 allotropes /
- structural prediction /
- electronic properties /
- first principles
[1] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[2] Zhang Y B, Tan Y W, Stormer H L, Kim P 2005 Nature 438 201
 Google Scholar
Google Scholar
[3] Mak K F, Lee C, Hone J, Shan J, Heinz T F 2010 Phys. Rev. Lett. 105 136805
 Google Scholar
Google Scholar
[4] Topsakal M, Aktuerk E, Ciraci S 2009 Phys. Rev. B 79 115442
 Google Scholar
Google Scholar
[5] Cahangirov S, Topsakal M, Akturk E, Sahin H, Ciraci S 2009 Phys. Rev. Lett. 102 236804
 Google Scholar
Google Scholar
[6] Li L, Yu Y, Ye G, Ge Q, Ou X, Wu H, Feng D, Chen, Xian H, Zhang Y B 2014 Nature Nanotechnology 9 372
 Google Scholar
Google Scholar
[7] Liu H, Neal A T, Zhu Z, Luo Z, Xu X F, Tomanek D, Ye P D 2014 ACS Nano 8 4033
 Google Scholar
Google Scholar
[8] Li T, Eugenio C, Daniele C, Carlo G, Marco F, Madan D, Alessandro M, Deji A 2015 Nat. Nanotechnol. 10 227
 Google Scholar
Google Scholar
[9] Duerloo K-A N, Ong M T, Reed E J 2012 J. Phys. Chem. Lett. 3 2871
 Google Scholar
Google Scholar
[10] Fazel S, Jae R H, Hong S K 2017 J. Mater. Chem. A 5 22146
 Google Scholar
Google Scholar
[11] Guo J, Liu Y, Ma Y, Zhu E B, Lee S, Lu Z X, Zhao Z P, Xu C H, Lee S J, Wu H, Kovnir K, Huang Y, Duan X F 2018 Adv. Mater. 30 e1705934
 Google Scholar
Google Scholar
[12] Yang S X, Yang Y H, Wu M H, Hu C G, Shen W F, Gong Y J, Huang L, Jiang C H, Zhang Y Z, Ajayan P M 2018 Adv. Funct. Mater. 28 1707379
 Google Scholar
Google Scholar
[13] Zhou L Q, Guo Y, Zhao J J 2018 Physica E Low Dimens. Syst. Nanostruct. 95 149
 Google Scholar
Google Scholar
[14] Li L, Gong P L, Sheng D P, Wang S A, Wang W K, Zhu X D, Shi X Q, Wang F K, Han W, Yang S J, Liu K L, Li H Q, Zhai T Y 2018 Adv. Mater. 30 e1804541
 Google Scholar
Google Scholar
[15] Wadsten T 1967 Acts Chem. Scand. 21 1374
[16] Matta S K, Zhang C, Jiao Y, O, Mullane A, Du A 2018 Nanoscale 10 6369
 Google Scholar
Google Scholar
[17] Xu Y, Li Z, He C, Li J, Ouyang T, Zhang C, Tang C, Zhong J 2020 Appl. Phys. Lett. 116 023103
 Google Scholar
Google Scholar
[18] Xi F, Yang H Y, Ling F, He C Z, Hou J R, Guo J Y, Li L M 2021 Chin. Chem. Lett. 32 1089
 Google Scholar
Google Scholar
[19] Shi X Z, He C Y, Pickard C J, Tang C, Zhong J X 2018 Phys. Rev. B 97 014104
 Google Scholar
Google Scholar
[20] King-Smith R D, Vanderbilt D 1993 Phys. Rev. B 47 1651
 Google Scholar
Google Scholar
[21] Vanderbilt D 2000 J. Phys. Chem. Solids 61 147
 Google Scholar
Google Scholar
[22] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[23] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[24] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[25] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[26] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[27] Grimme S, Antony J, Ehrlich S, Krieg H 2010 J. Chem. Phys. 132 154104
 Google Scholar
Google Scholar
[28] Gomes L C, Carvalho A, Neto A H C 2015 Phys. Rev. B 92 214103
 Google Scholar
Google Scholar
[29] Ni M, Leung M K H, Leung D Y C, Sumathy K 2006 Renew. Sust. Energ. Rev. 11 401
[30] Roldan R, Castellanos-Gomez A, Cappelluti E, Guinea F 2015 J. Phys. Condens. Matter 27 313201
 Google Scholar
Google Scholar
[31] Zhu C R, Wang G, Liu B L, Marie X, Qiao X F, Zhang X, Wu X X, Fan H, Tan P H, Amand T, Urbaszek B 2013 Phys. Rev. B 88 121301
 Google Scholar
Google Scholar
[32] Conley H J, Wang B, Ziegler J I, Haglund R F Jr, Pantelides S T, Bolotin K I 2013 Nano Lett. 13 3626
 Google Scholar
Google Scholar
[33] Fei R, Li W, Li J, Yang L 2015 Appl. Phys. Lett. 107 173104
 Google Scholar
Google Scholar
[34] Blonsky M N, Zhuang H L, Singh A K, Hennig R G 2015 ACS Nano 9 9885
 Google Scholar
Google Scholar
[35] Sevik C, Çakır D, Gülseren O, Peeters F M 2016 J. Phys. Chem. C 120 13948
 Google Scholar
Google Scholar
[36] Michel K H, Verberck B 2009 Phys. Rev. B 80 224301
 Google Scholar
Google Scholar
[37] Liu S, Cohen R 2017 Phys. Rev. Lett. 119 207601
 Google Scholar
Google Scholar
-
图 1 3种SiP2同素异构体及r-SiP2结构的俯视图(上图)和侧视图(下图) (a) α-SiP2; (b) β-SiP2; (c) γ-SiP2; (d) r-SiP2[14], 红色和绿色分别代表P和Si原子
Figure 1. Top view and side view of three SiP2 allotropes and r-SiP2 structure: (a) α-SiP2; (b) β-SiP2; (c) γ-SiP2; (d) r-SiP2[14]. Red and green balls represent P and Si atoms, respectively.
表 1 优化后的3种SiP2同素异构体及r-SiP2[16]结构晶格常数、形成能(Eb)和带隙值(Eg)
Table 1. Optimized lattice parameters, binding energy (Eb), and band gap values (Eg) of three SiP2 allotropes and r-SiP2[16] structures.
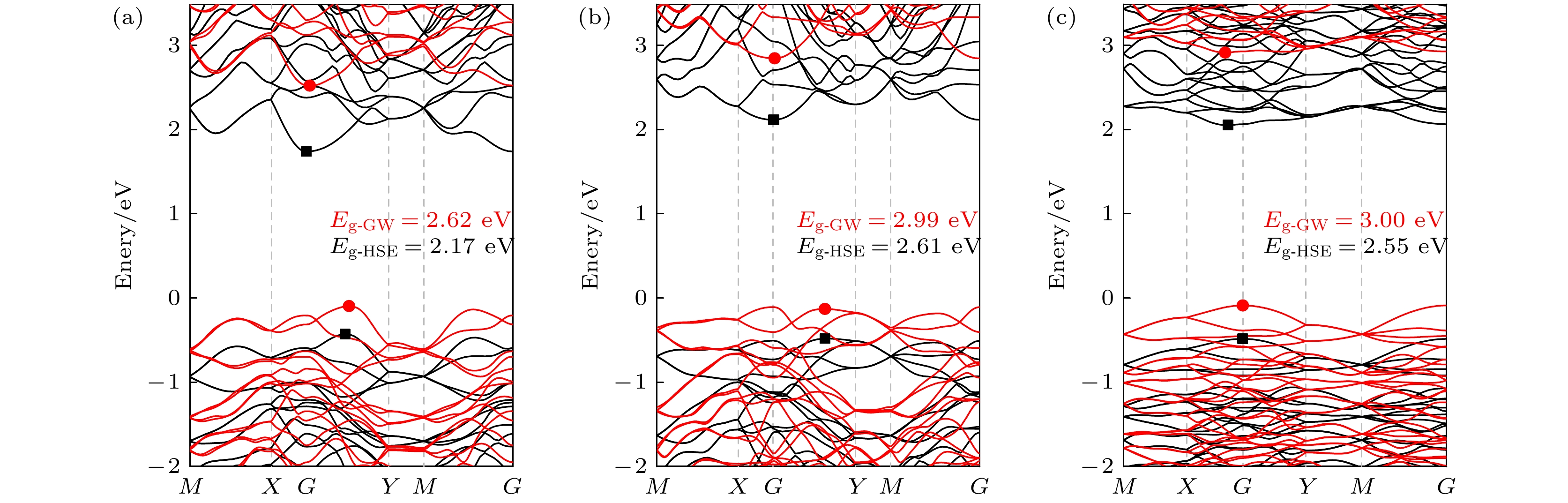
System A/Å b/Å ${ {E} }_{\text{b} }\text{/}\text{eV}$ ${ {E} }_{\text{g} }^{\text{PBE} }\text{/}\text{eV}$ ${ {E} }_{\text{g} }^{\text{HSE} }\text{/}\text{eV}$ ${ {E} }_{\text{g} }^{\text{GW} }\text{/}\text{eV}$ α-SiP2 6.13 13.89 –5.38 1.44 2.17 2.62 β-SiP2 6.09 13.73 –5.43 1.84 2.61 2.99 γ-SiP2 12.11 13.95 –5.45 1.78 2.55 3.00 r-SiP2 10.00 3.44 –5.47 1.47 2.25 2.63 表 2 3种SiP2异构体的弹性常数Cij (N/m)及压电系数eij (10–10 C/m)和dij (pm/V)
Table 2. Calculated elastic coefficients Cij (N/m) and piezoelectric coefficients eij (10–10 C/m) and dij (pm/V) of three SiP2 allotropes.
System $ {C}_{11} $ $ {C}_{22} $ $ {C}_{12} $ $ {e}_{11} $ $ {e}_{12} $ $ {d}_{11} $ $ {d}_{12} $ α-SiP2 69.69 65.74 14.79 –1.05 0.37 –1.71 0.95 β-SiP2 78.36 65.43 19.10 –0.94 –0.38 –1.13 –0.26 γ-SiP2 77.65 59.97 18.04 –0.13 –0.46 –0.01 –0.77 r-SiP2[17] 17.94 121.77 8.66 –1.93 0.17 –11.22 0.93 h-BN[9] 291 291 62 1.38 1.38 0.60 0.60 MoS2[9] 130 130 32 3.64 3.64 3.73 3.73 -
[1] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[2] Zhang Y B, Tan Y W, Stormer H L, Kim P 2005 Nature 438 201
 Google Scholar
Google Scholar
[3] Mak K F, Lee C, Hone J, Shan J, Heinz T F 2010 Phys. Rev. Lett. 105 136805
 Google Scholar
Google Scholar
[4] Topsakal M, Aktuerk E, Ciraci S 2009 Phys. Rev. B 79 115442
 Google Scholar
Google Scholar
[5] Cahangirov S, Topsakal M, Akturk E, Sahin H, Ciraci S 2009 Phys. Rev. Lett. 102 236804
 Google Scholar
Google Scholar
[6] Li L, Yu Y, Ye G, Ge Q, Ou X, Wu H, Feng D, Chen, Xian H, Zhang Y B 2014 Nature Nanotechnology 9 372
 Google Scholar
Google Scholar
[7] Liu H, Neal A T, Zhu Z, Luo Z, Xu X F, Tomanek D, Ye P D 2014 ACS Nano 8 4033
 Google Scholar
Google Scholar
[8] Li T, Eugenio C, Daniele C, Carlo G, Marco F, Madan D, Alessandro M, Deji A 2015 Nat. Nanotechnol. 10 227
 Google Scholar
Google Scholar
[9] Duerloo K-A N, Ong M T, Reed E J 2012 J. Phys. Chem. Lett. 3 2871
 Google Scholar
Google Scholar
[10] Fazel S, Jae R H, Hong S K 2017 J. Mater. Chem. A 5 22146
 Google Scholar
Google Scholar
[11] Guo J, Liu Y, Ma Y, Zhu E B, Lee S, Lu Z X, Zhao Z P, Xu C H, Lee S J, Wu H, Kovnir K, Huang Y, Duan X F 2018 Adv. Mater. 30 e1705934
 Google Scholar
Google Scholar
[12] Yang S X, Yang Y H, Wu M H, Hu C G, Shen W F, Gong Y J, Huang L, Jiang C H, Zhang Y Z, Ajayan P M 2018 Adv. Funct. Mater. 28 1707379
 Google Scholar
Google Scholar
[13] Zhou L Q, Guo Y, Zhao J J 2018 Physica E Low Dimens. Syst. Nanostruct. 95 149
 Google Scholar
Google Scholar
[14] Li L, Gong P L, Sheng D P, Wang S A, Wang W K, Zhu X D, Shi X Q, Wang F K, Han W, Yang S J, Liu K L, Li H Q, Zhai T Y 2018 Adv. Mater. 30 e1804541
 Google Scholar
Google Scholar
[15] Wadsten T 1967 Acts Chem. Scand. 21 1374
[16] Matta S K, Zhang C, Jiao Y, O, Mullane A, Du A 2018 Nanoscale 10 6369
 Google Scholar
Google Scholar
[17] Xu Y, Li Z, He C, Li J, Ouyang T, Zhang C, Tang C, Zhong J 2020 Appl. Phys. Lett. 116 023103
 Google Scholar
Google Scholar
[18] Xi F, Yang H Y, Ling F, He C Z, Hou J R, Guo J Y, Li L M 2021 Chin. Chem. Lett. 32 1089
 Google Scholar
Google Scholar
[19] Shi X Z, He C Y, Pickard C J, Tang C, Zhong J X 2018 Phys. Rev. B 97 014104
 Google Scholar
Google Scholar
[20] King-Smith R D, Vanderbilt D 1993 Phys. Rev. B 47 1651
 Google Scholar
Google Scholar
[21] Vanderbilt D 2000 J. Phys. Chem. Solids 61 147
 Google Scholar
Google Scholar
[22] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[23] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[24] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[25] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[26] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[27] Grimme S, Antony J, Ehrlich S, Krieg H 2010 J. Chem. Phys. 132 154104
 Google Scholar
Google Scholar
[28] Gomes L C, Carvalho A, Neto A H C 2015 Phys. Rev. B 92 214103
 Google Scholar
Google Scholar
[29] Ni M, Leung M K H, Leung D Y C, Sumathy K 2006 Renew. Sust. Energ. Rev. 11 401
[30] Roldan R, Castellanos-Gomez A, Cappelluti E, Guinea F 2015 J. Phys. Condens. Matter 27 313201
 Google Scholar
Google Scholar
[31] Zhu C R, Wang G, Liu B L, Marie X, Qiao X F, Zhang X, Wu X X, Fan H, Tan P H, Amand T, Urbaszek B 2013 Phys. Rev. B 88 121301
 Google Scholar
Google Scholar
[32] Conley H J, Wang B, Ziegler J I, Haglund R F Jr, Pantelides S T, Bolotin K I 2013 Nano Lett. 13 3626
 Google Scholar
Google Scholar
[33] Fei R, Li W, Li J, Yang L 2015 Appl. Phys. Lett. 107 173104
 Google Scholar
Google Scholar
[34] Blonsky M N, Zhuang H L, Singh A K, Hennig R G 2015 ACS Nano 9 9885
 Google Scholar
Google Scholar
[35] Sevik C, Çakır D, Gülseren O, Peeters F M 2016 J. Phys. Chem. C 120 13948
 Google Scholar
Google Scholar
[36] Michel K H, Verberck B 2009 Phys. Rev. B 80 224301
 Google Scholar
Google Scholar
[37] Liu S, Cohen R 2017 Phys. Rev. Lett. 119 207601
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 7860
- PDF Downloads: 127
- Cited By: 0















 DownLoad:
DownLoad: