-
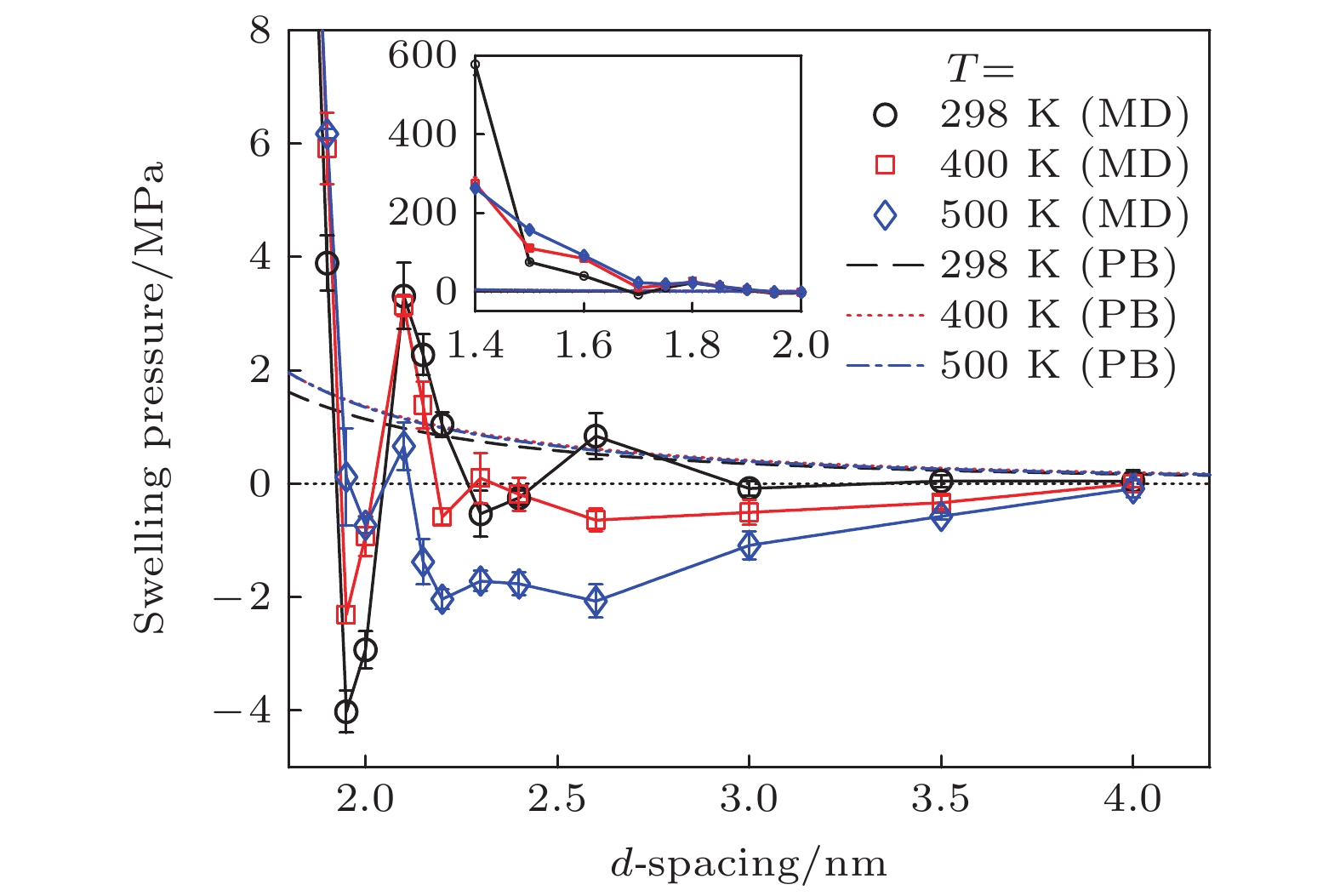
The swelling of Ca-montmorillonite at elevated temperatures is important for many applications including geological disposal of radioactive waste, subsurface carbon sequestration, and shale gas exploration. However, the experimentally observed swelling behaviors of Ca-montmorillonite contacting liquid water and the temperature effects on the swelling pressure are not well understood. In this work, molecular dynamics simulations are carried out to study the swelling of Wyoming Ca-montmorillonite with a d-spacing (d) range of 1.40–4.00 nm at 5 MPa and various temperatures (298–500 K). The ClayFF and SPC are adopted for modeling Ca-montmorillonite and water, respectively. The simulation box is measured to be 11.15, 3.66, and 28.00 nm in the x-, y-, and z-direction. Atomistic pistons are used to control the bulk pressure of the water environment, and the implicit walls are implemented for preventing the ions from leaking from the pore into the water environment. The clay atoms are fixed during the simulation and the swelling pressure is calculated through dividing the force by the area. The equilibrium time is at least 20 ns and the production time falls in a range of 50–88 ns. The swelling pressure results show that for small d, high temperature reduces the magnitude of the oscillating curve of swelling pressure and also reduces the range of d where hydration force dominates the swelling pressure. This temperature effect is due to the weakened hydration force as evidenced from the weakened water density distributions inside the pore. For large d, high temperature reduces the swelling pressure, which is consistent with the experimental result, and increases the range of d where double layer force dominates the swelling pressure. The reduction of the swelling pressure can be explained by the enhanced ion correlation that reduces the double layer force according to the strong coupling theory, given that the calculated coupling parameters at higher temperatures are smaller. The swelling pressures are negative at elevated temperatures and large d, which prevents the clay from further swelling. However, the classical Poisson-Boltzmann (PB) equation predicts the positive double layer force since the ion correlation effect is not considered in the PB equation. Furthermore, the calculated swelling free energy curve shows that at 298 K and 5 MPa, it is difficult for Ca-montmorillonite to swell beyond a d-spacing of around 1.9 nm, which is in good agreement with the experimental result. The energy barrier for Ca-montmorillonite to swell to large d is larger than that for Na-montmorillonite, which means that it is more difficult for Ca-montmorillonite to swell to large d. This behavior is consistent with experimental observation and can be explained by the larger ion correlation effect in the Ca-montmorillonite system. These findings enhance the understanding of swelling of Ca-montmorillonite at elevated temperatures and could help to engineer better barrier materials for nuclear waste storage.
-
Keywords:
- molecular dynamics simulation /
- swelling pressure /
- Ca-montmorillonite /
- ion correlation effect
[1] Yang Y, Qiao R, Wang Y, Sun S 2021 Appl. Clay Sci. 201 105924
 Google Scholar
Google Scholar
[2] Pusch R 1992 Clay Miner. 27 353
 Google Scholar
Google Scholar
[3] Higgo J 1987 Prog. Nucl. Energy 19 173
 Google Scholar
Google Scholar
[4] Morodome S, Kawamura K 2011 Clays Clay Miner. 59 165
 Google Scholar
Google Scholar
[5] Ferrage E, Lanson B, Sakharov B A, Geoffroy N, Jacquot E, Drits V A 2007 Am. Mineral. 92 1731
 Google Scholar
Google Scholar
[6] [7] Komine H, Ogata N 1996 Can. Geotech. J. 33 11
 Google Scholar
Google Scholar
[8] Norrish K 1954 Discuss. Faraday Soc. 18 120
 Google Scholar
Google Scholar
[9] Yong R 1999 Eng. Geol. 54 3
 Google Scholar
Google Scholar
[10] Posner A M, Quirk J P 1964 J. Colloid Sci. 19 798
 Google Scholar
Google Scholar
[11] Norrish K, Quirk J 1954 Nature 173 255
 Google Scholar
Google Scholar
[12] Pusch R, Karnland O, Hökmark H 1990 GMM-a General Microstructural Model for Qualitative and Quantitative Studies of Smectite Clays Report
[13] Villar M V, Lloret A 2004 Appl. Clay Sci. 26 337
 Google Scholar
Google Scholar
[14] Akinwunmi B, Hirvi J T, Kasa S, Pakkanen T A 2020 Chem. Phys. 528 110511
 Google Scholar
Google Scholar
[15] Akinwunmi B, Sun L, Hirvi J T, Kasa S, Pakkanen T A 2019 Chem. Phys. 516 177
 Google Scholar
Google Scholar
[16] Honorio T, Brochard L, Vandamme M 2017 Langmuir 33 12766
 Google Scholar
Google Scholar
[17] Yang Y, Narayanan Nair A K, Sun S 2019 ACS Earth Space Chem. 3 2635
 Google Scholar
Google Scholar
[18] Li Y, Narayanan Nair A K, Kadoura A, Yang Y, Sun S 2019 Ind. Eng. Chem. Res. 58 1396
 Google Scholar
Google Scholar
[19] 那平, 张帆, 李艳妮 2006 物理化学学报 22 1137
 Google Scholar
Google Scholar
Na P, Zhang F, Li Y N 2006 Acta Phys. -Chim. Sin. 22 1137
 Google Scholar
Google Scholar
[20] 王进, 王军霞, 曾凡桂, 吴秀玲 2010 化学学报 16 1653
Wang J, Wang J X, Zeng F G, Wu X L 2010 Acta Chim. Sin. 16 1653
[21] 王进, 曾凡柱, 王军霞 2006 化学学报 64 1654
 Google Scholar
Google Scholar
Wang J, Zeng F Z, Wang J X 2006 Acta Chim. Sin. 64 1654
 Google Scholar
Google Scholar
[22] 李丽丽, 张晓虹, 王玉龙, 国家辉, 张双 2016 65 19620201
 Google Scholar
Google Scholar
Li L L, Zhang X H, Wang Y L, Guo J H, Zhang S 2016 Acta Phys. Sin. 65 19620201
 Google Scholar
Google Scholar
[23] Plimpton S 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[24] Cygan R T, Liang J-J, Kalinichev A G 2004 J. Phys. Chem. B 108 1255
 Google Scholar
Google Scholar
[25] Berendsen H, Grigera J, Straatsma T 1987 J. Phys. Chem. 91 6269
 Google Scholar
Google Scholar
[26] Ryckaert J-P, Ciccotti G, Berendsen H J 1977 J. Comput. Phys. 23 327
 Google Scholar
Google Scholar
[27] Hockney R W, Eastwood J W 2021 Computer Simulation using Particles (Boca Raton: CRC Press)
[28] Shinoda W, Shiga M, Mikami M 2004 Phys. Rev. B 69 134103
 Google Scholar
Google Scholar
[29] Allen M P, Tildesley D J 2017 Computer Ssimulation of Liquids (Oxford University Press)
[30] Israelachvili J N 2015 Intermolecular and Surface Forces (Cambridge: Academic Press) p291
[31] Schlaich A, Dos Santos A P, Netz R R 2018 Langmuir 35 551
[32] Bourg I C, Sposito G 2011 J. Colloid Interface Sci. 360 701
 Google Scholar
Google Scholar
[33] Sposito G, Skipper N T, Sutton R, Park S H, Soper A K, Greathouse J A 1999 Proc. Natl. Acad. Sci. U. S. A. 96 3358
 Google Scholar
Google Scholar
[34] Fang C, Sun S, Qiao R 2019 Langmuir 35 10341
 Google Scholar
Google Scholar
[35] Moreira A G, Netz R R 2001 Phys. Rev. Lett. 87 78301
 Google Scholar
Google Scholar
[36] Netz R R 2001 Eur. Phys. J. E 5 557
 Google Scholar
Google Scholar
[37] Moreira A, Netz R 2002 Eur. Phys. J. E 8 33
 Google Scholar
Google Scholar
[38] Whitley H D, Smith D E 2004 J. Chem. Phys. 120 5387
 Google Scholar
Google Scholar
[39] Tambach T J, Bolhuis P G, Hensen E J, Smit B 2006 Langmuir 22 1223
 Google Scholar
Google Scholar
[40] Seppälä A, Puhakka E, Olin M 2016 Clay Miner. 51 197
 Google Scholar
Google Scholar
[41] Brochard L, Honório T, Vandamme M, Bornert M, Peigney M 2017 Acta Geotech. 12 1261
 Google Scholar
Google Scholar
-
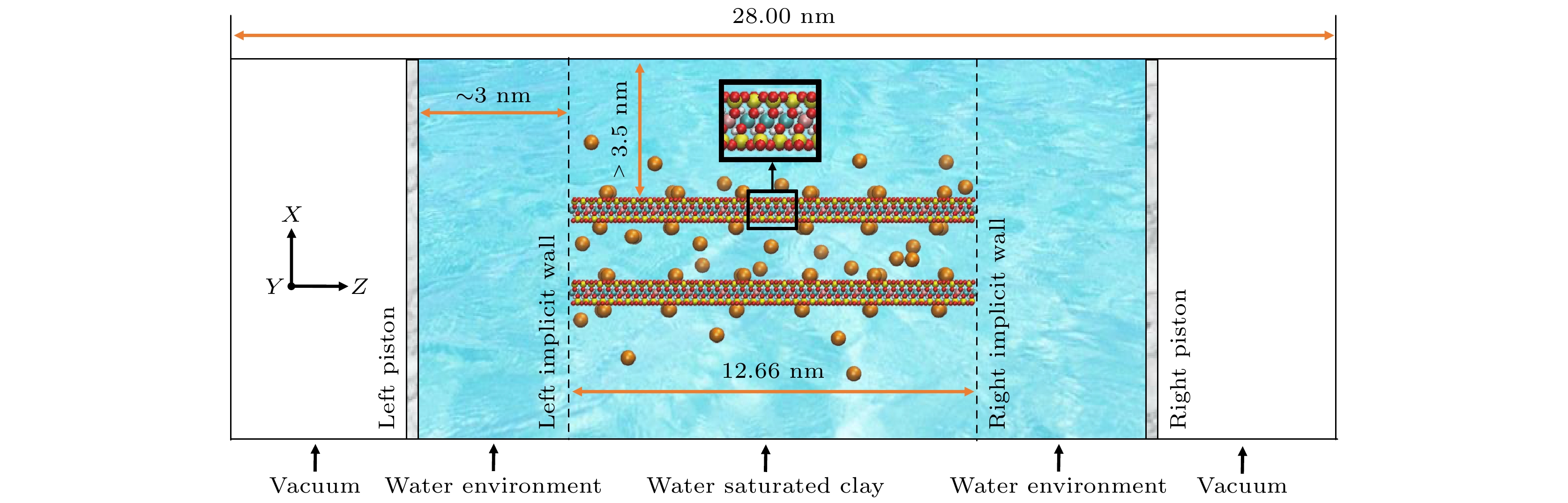
图 1 研究饱和钙蒙脱石膨胀压力的分子体系; 蒙脱石原子类型与颜色关系: 橙: 钙离子, 红: 氧, 白: 氢, 黄: 硅, 蓝: 铝, 粉: 镁; 体系中的水分子不作具体展示
Figure 1. Molecular system for studying the swelling pressure of Ca-montmorillonite in water. The color code for clay atoms: orange: Ca ion, red: O, white: H, yellow: Si, cyan: Al, pink: Mg. Water molecules are not explicitly shown.
图 3 在298 K和5 MPa下, 水在孤立钙蒙脱石表面(
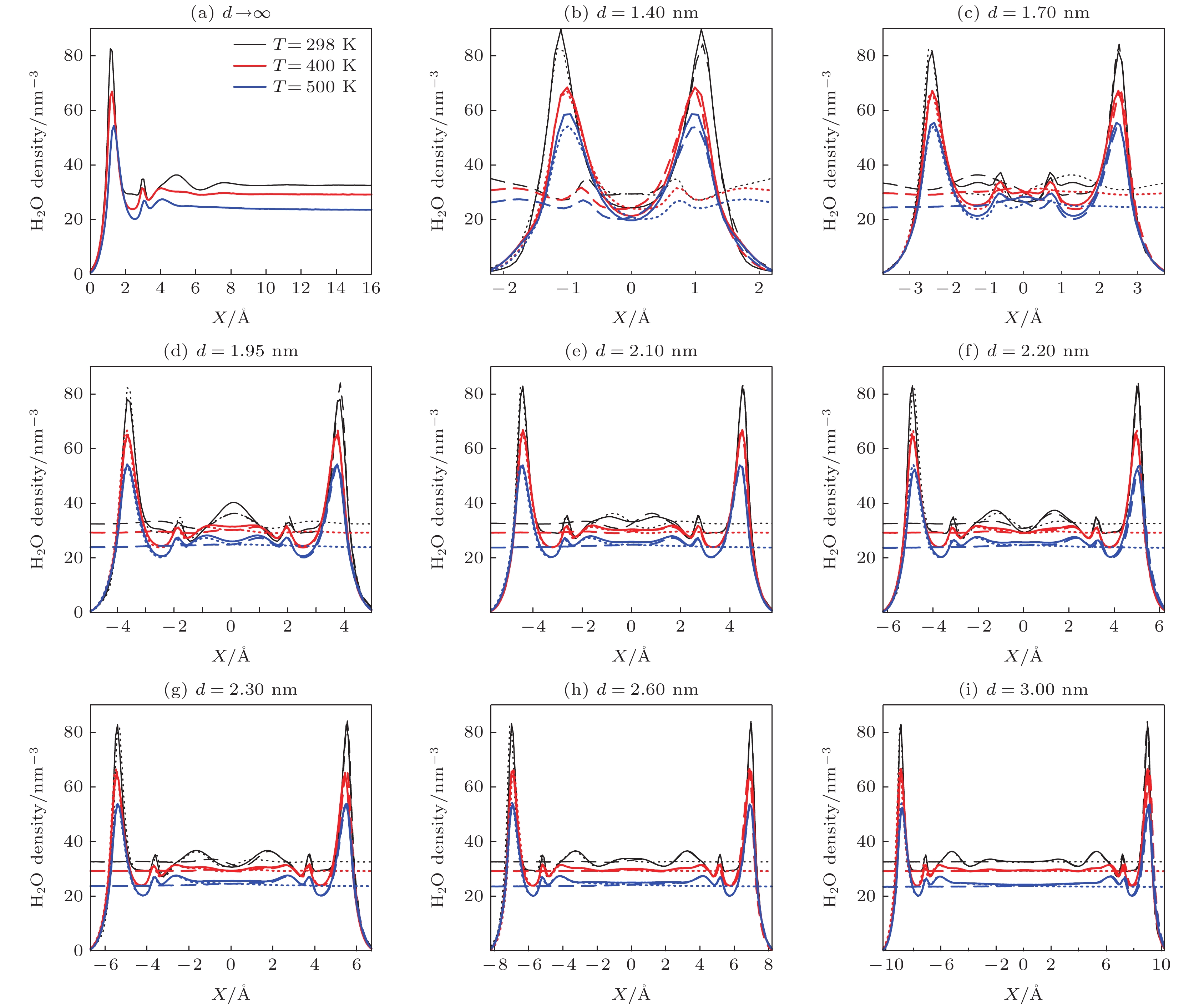
$ d\to \infty $ )的平衡密度分布(a)和钙蒙脱石层间水在不同晶面间距下的平衡密度分布((b)—(l)); 在((b)—(l))图中, 虚线(点线)为平移后的(a)图中的密度分布; x = 0 对应孔的中心Figure 3. Equilibrium density profiles of water near one quasi-isolated clay surface (a) and inside Ca-montmorillonite pore with various d-spacings ((b)–(l)) at 298 K and 5 MPa. In ((b)–(l)), the shifted density profile from (a) are plotted as dashed(dotted) lines. x = 0 corresponds to the center of the pore.
图 4 在298 K和5 MPa下, 钙离子在孤立钙蒙脱石表面(
$ d\to \infty $ )的平衡密度分布(a)和钙蒙脱石层间钙离子在不同晶面间距下的平衡密度分布((b)—(l)); 在((b)—(l))图中, 虚线(点线)为平移后的(a)图中的密度分布; x = 0 对应孔的中心Figure 4. Equilibrium density profiles of Ca ion near one quasi-isolated clay surface (a) and inside Ca-montmorillonite pore with various d-spacings ((b)–(l)) at 298 K and 5 MPa. In ((b)–(l)), the shifted density profile from (a) are plotted as dashed(dotted) lines. x = 0 corresponds to the center of the pore.
图 7 在5 MPa下, 水在孤立钙蒙脱石表面(
$ d\to \infty $ )的平衡密度分布(a)和钙蒙脱石层间水在不同晶面间距下的平衡密度分布((b)—(l)); 在((b)—(l))图中, 虚线(点线)为平移后的(a)图中的密度分布. x = 0 对应孔的中心Figure 7. Equilibrium density profiles of water near one quasi-isolated clay surface (a) and inside Ca-montmorillonite pore with various d-spacings ((b)–(l)) at 5 MPa. In ((b)–(l)), the shifted density profiles from (a) are plotted as dashed(dotted) lines. x = 0 corresponds to the center of the pore.
表 1 水、蒙脱石和离子的Lennard-Jones参数和电荷
Table 1. Lennard-Jones parameters and partial charges of water, montmorillonite, and ion.
原子种类 尺度参数$ {\sigma }_{i}/$nm 能量参数$ {\varepsilon }_{i}/$(kcal·mol–1) 电荷q/e 水 O 0.31656 1.554 × 10–1 –0.8200 H 0.00000 0.000 0.4100 蒙脱石 羟基O 0.31656 1.554 × 10–1 –0.9500 有取代的羟基O 0.31656 1.554 × 10–1 –1.0808 羟基H 0.00000 0.000 0.4250 桥联O 0.31656 1.554 × 10–1 –1.0500 有八面体取代的桥连O 0.31656 1.554 × 10–1 –1.1808 有四面体取代的桥连O 0.31656 1.554 × 10–1 –1.1688 四面体Si 0.33020 1.841 × 10–6 2.1000 四面体Al 0.33020 1.841 × 10–6 1.5750 八面体Al 0.42712 1.330 × 10–6 1.5750 八面体Mg 0.52643 9.030 × 10–7 1.3600 离子 Ca 0.28720 1.000 × 10–1 2.0000 表 2 在不同温度和晶面间距下, 分子动力学模拟计算的钙蒙脱石的膨胀压力及预测误差
Table 2. Swelling pressures of Ca-montmorillonite at different temperatures and d-spacings and the corresponding standard deviations obtained from molecular dynamics simulations.
晶面间距/nm 膨胀压力/MPa 膨胀压力的标准差/MPa T = 298 K T = 400 K T = 500 K T = 298 K T = 400 K T = 500 K 1.40 577.940 275.174 263.519 27.583 15.886 2.875 1.50 75.841 110.869 157.569 2.784 5.279 4.072 1.60 40.637 84.617 92.147 1.887 0.719 0.482 1.70 –6.800 10.744 23.476 0.689 0.155 0.609 1.75 10.575 16.781 20.106 0.349 0.408 0.445 1.80 22.749 25.890 23.449 0.195 0.286 0.401 1.85 13.275 14.241 13.884 0.285 1.186 0.364 1.90 3.893 5.913 6.174 0.482 0.629 0.077 1.95 –4.018 –2.307 0.121 0.369 0.149 0.858 2.00 –2.926 –0.923 –0.730 0.325 0.350 0.142 2.10 3.313 3.142 0.664 0.583 0.184 0.423 2.15 2.276 1.388 –1.376 0.361 0.411 0.401 2.20 1.044 –0.584 –2.034 0.220 0.144 0.177 2.30 –0.528 0.103 –1.714 0.404 0.442 0.176 2.40 –0.246 –0.184 –1.763 0.126 0.291 0.204 2.60 0.844 –0.639 –2.068 0.402 0.198 0.291 3.00 –0.082 –0.502 –1.084 0.130 0.223 0.244 3.50 0.050 –0.331 –0.572 0.107 0.125 0.060 4.00 0.048 0.003 –0.084 0.193 0.057 0.158 表 3 水在5 MPa和不同温度下的介电常数和耦合参数
Table 3. Water dielectric constants and coupling parameters at 5 MPa and various temperatures.
温度T/K 298 400 500 水介电常数 $ {\varepsilon }_{\mathrm{b}} $ 63.58a 40.01a 23.23a 耦合参数 ${\Xi }$ 30.38 42.58 80.84 a数据源于文献[1]. -
[1] Yang Y, Qiao R, Wang Y, Sun S 2021 Appl. Clay Sci. 201 105924
 Google Scholar
Google Scholar
[2] Pusch R 1992 Clay Miner. 27 353
 Google Scholar
Google Scholar
[3] Higgo J 1987 Prog. Nucl. Energy 19 173
 Google Scholar
Google Scholar
[4] Morodome S, Kawamura K 2011 Clays Clay Miner. 59 165
 Google Scholar
Google Scholar
[5] Ferrage E, Lanson B, Sakharov B A, Geoffroy N, Jacquot E, Drits V A 2007 Am. Mineral. 92 1731
 Google Scholar
Google Scholar
[6] [7] Komine H, Ogata N 1996 Can. Geotech. J. 33 11
 Google Scholar
Google Scholar
[8] Norrish K 1954 Discuss. Faraday Soc. 18 120
 Google Scholar
Google Scholar
[9] Yong R 1999 Eng. Geol. 54 3
 Google Scholar
Google Scholar
[10] Posner A M, Quirk J P 1964 J. Colloid Sci. 19 798
 Google Scholar
Google Scholar
[11] Norrish K, Quirk J 1954 Nature 173 255
 Google Scholar
Google Scholar
[12] Pusch R, Karnland O, Hökmark H 1990 GMM-a General Microstructural Model for Qualitative and Quantitative Studies of Smectite Clays Report
[13] Villar M V, Lloret A 2004 Appl. Clay Sci. 26 337
 Google Scholar
Google Scholar
[14] Akinwunmi B, Hirvi J T, Kasa S, Pakkanen T A 2020 Chem. Phys. 528 110511
 Google Scholar
Google Scholar
[15] Akinwunmi B, Sun L, Hirvi J T, Kasa S, Pakkanen T A 2019 Chem. Phys. 516 177
 Google Scholar
Google Scholar
[16] Honorio T, Brochard L, Vandamme M 2017 Langmuir 33 12766
 Google Scholar
Google Scholar
[17] Yang Y, Narayanan Nair A K, Sun S 2019 ACS Earth Space Chem. 3 2635
 Google Scholar
Google Scholar
[18] Li Y, Narayanan Nair A K, Kadoura A, Yang Y, Sun S 2019 Ind. Eng. Chem. Res. 58 1396
 Google Scholar
Google Scholar
[19] 那平, 张帆, 李艳妮 2006 物理化学学报 22 1137
 Google Scholar
Google Scholar
Na P, Zhang F, Li Y N 2006 Acta Phys. -Chim. Sin. 22 1137
 Google Scholar
Google Scholar
[20] 王进, 王军霞, 曾凡桂, 吴秀玲 2010 化学学报 16 1653
Wang J, Wang J X, Zeng F G, Wu X L 2010 Acta Chim. Sin. 16 1653
[21] 王进, 曾凡柱, 王军霞 2006 化学学报 64 1654
 Google Scholar
Google Scholar
Wang J, Zeng F Z, Wang J X 2006 Acta Chim. Sin. 64 1654
 Google Scholar
Google Scholar
[22] 李丽丽, 张晓虹, 王玉龙, 国家辉, 张双 2016 65 19620201
 Google Scholar
Google Scholar
Li L L, Zhang X H, Wang Y L, Guo J H, Zhang S 2016 Acta Phys. Sin. 65 19620201
 Google Scholar
Google Scholar
[23] Plimpton S 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[24] Cygan R T, Liang J-J, Kalinichev A G 2004 J. Phys. Chem. B 108 1255
 Google Scholar
Google Scholar
[25] Berendsen H, Grigera J, Straatsma T 1987 J. Phys. Chem. 91 6269
 Google Scholar
Google Scholar
[26] Ryckaert J-P, Ciccotti G, Berendsen H J 1977 J. Comput. Phys. 23 327
 Google Scholar
Google Scholar
[27] Hockney R W, Eastwood J W 2021 Computer Simulation using Particles (Boca Raton: CRC Press)
[28] Shinoda W, Shiga M, Mikami M 2004 Phys. Rev. B 69 134103
 Google Scholar
Google Scholar
[29] Allen M P, Tildesley D J 2017 Computer Ssimulation of Liquids (Oxford University Press)
[30] Israelachvili J N 2015 Intermolecular and Surface Forces (Cambridge: Academic Press) p291
[31] Schlaich A, Dos Santos A P, Netz R R 2018 Langmuir 35 551
[32] Bourg I C, Sposito G 2011 J. Colloid Interface Sci. 360 701
 Google Scholar
Google Scholar
[33] Sposito G, Skipper N T, Sutton R, Park S H, Soper A K, Greathouse J A 1999 Proc. Natl. Acad. Sci. U. S. A. 96 3358
 Google Scholar
Google Scholar
[34] Fang C, Sun S, Qiao R 2019 Langmuir 35 10341
 Google Scholar
Google Scholar
[35] Moreira A G, Netz R R 2001 Phys. Rev. Lett. 87 78301
 Google Scholar
Google Scholar
[36] Netz R R 2001 Eur. Phys. J. E 5 557
 Google Scholar
Google Scholar
[37] Moreira A, Netz R 2002 Eur. Phys. J. E 8 33
 Google Scholar
Google Scholar
[38] Whitley H D, Smith D E 2004 J. Chem. Phys. 120 5387
 Google Scholar
Google Scholar
[39] Tambach T J, Bolhuis P G, Hensen E J, Smit B 2006 Langmuir 22 1223
 Google Scholar
Google Scholar
[40] Seppälä A, Puhakka E, Olin M 2016 Clay Miner. 51 197
 Google Scholar
Google Scholar
[41] Brochard L, Honório T, Vandamme M, Bornert M, Peigney M 2017 Acta Geotech. 12 1261
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 7460
- PDF Downloads: 134
- Cited By: 0














 DownLoad:
DownLoad: