-
Ionization energy (IE) is an important characteristic parameter of atoms or molecules. It plays an important role in the process of photophysics and photochemistry. The precise ionization energy is very important for relevant research. Especially, it is very useful for adjusting the signal of the zero-kinetic energy (ZEKE) spectrum, and it also plays a key role in judging the number of rotamers and molecular configuration. In linear time-of-flight mass spectrometers, pulsed electric fields are usually used to drive photo-ionized ions to the detector to produce the photoionization efficiency (PIE) spectrum. The ionization energy is directly obtained from the PIE curve. The uncertainty of the measured IE is usually greater than or equal to ± 10 cm–1. The ZEKE spectroscopy is based on the long-lived Rydberg state field ionization technology. In the ZEKE experiments, the laser excites molecules to the Rydberg state and then a pulsed field ionization (PFI) is used for measurement. A peak with high signal-to-noise ratio and narrow linewidth signal appears near the ionization threshold. Therefore, the more accurate ionization energy can be obtained, and the uncertainty of the measured value is about ± 5 cm–1. The 1,3-diethoxybenzene is an important benzene derivative, and experiments have confirmed that there are two rotamers, i.e. I (down-up) and III (down-down) in the supersonic molecular beam. In this paper, a linear time-of-flight mass spectrometer is used to measure the photoionization efficiency curves of 1,3-diethoxybenzene in electrostatic fields. From the linear fitting of the ionization energy values measured under different electric fields (Stark effect) to the square root of the field strengths, the precise ionization energy values of rotamer I and rotamer III are determined to be (62419 ± 2) cm–1 and (63378 ± 2) cm–1, respectively. Compared with the accuracies of the values measured by the usual pulsed electric field acceleration mechanism and the ZEKE spectroscopy, the accuracy is improved from about ± 10 and ± 5 to ± 2 cm–1, respectively. The physical mechanism, advantages and disadvantages of different methods are analyzed and discussed. The present research results show that the ionization energy measured in the electrostatic field is more accurate, the physical meaning of the measurement process is clear, and the threshold data are easy to collect. This is the first report on the precise ionization energy of 1,3-diethoxybenzene rotamers.
-
Keywords:
- ionization energy /
- photoionization efficiency /
- Stark effect /
- 1,3-diethoxybenzene
[1] Zhang L J, Yu D, Dong C W, Cheng M, Hu L L, Zhou Z M, Du Y K, Zhu Q H, Zhang C H 2013 Spectrochim. Acta, Part A 104 235
 Google Scholar
Google Scholar
[2] Yang S C, Huang H W, Tzeng W B 2010 J. Phys. Chem. A 114 11144
 Google Scholar
Google Scholar
[3] Qin C, Tzeng S Y, Zhang B, Tzeng W B 2019 J. Mol. Spectrosc. 355 26
 Google Scholar
Google Scholar
[4] Xu Y Q, Tzeng S Y, Zhang B, Tzeng W B 2013 Spectrochim. Acta, Part A 102 365
 Google Scholar
Google Scholar
[5] Lee Y R, Kim M H, Kim H L, Kwon C H 2018 J. Chem. Phys. 149 174302
 Google Scholar
Google Scholar
[6] Wu P Y, Tzeng W B 2015 J. Mol. Spectrosc. 316 72
 Google Scholar
Google Scholar
[7] Tsai C Y, Tzeng W B 2013 J. Photoch. Photobio. A 270 53
 Google Scholar
Google Scholar
[8] Dai W S, Zhang Z, Du Y K 2020 Spectrochim. Acta, Part A 224 117398
 Google Scholar
Google Scholar
[9] Xiao D Q, Yu D, Xu X L, Yu Z J, Cheng M, Du Y K, Zheng W J, Zhu Q H 2009 Phys. Chem. Chem. Phys. 11 3532
 Google Scholar
Google Scholar
[10] Xu Y Q, Tzeng S Y, Shivatare V, Takahashi K, Zhang B, Tzeng W B 2015 J. Chem. Phys. 142 124314
 Google Scholar
Google Scholar
[11] Huang J H, Huang K L, Liu S Q, Luo Q, Tzeng W B 2007 J. Photoch. Photobio. A 188 252
 Google Scholar
Google Scholar
[12] Li C Y, Lin J L, Tzeng W B 2005 J. Chem. Phys. 122 044311
 Google Scholar
Google Scholar
[13] Zhang L J, Dong C W, Cheng M, Hu L L, Du Y K, Zhu Q H, Zhang C H 2012 Spectrochim. Acta, Part A 96 578
 Google Scholar
Google Scholar
[14] Li C Y, Pradhan M, Tzeng W B 2005 Chem. Phys. Lett. 411 506
 Google Scholar
Google Scholar
[15] Lin J L, Li C Y, Tzeng W B 2004 J. Chem. Phys. 120 10513
 Google Scholar
Google Scholar
[16] Qin C, Tzeng S Y, Zang B, Tzeng W B 2014 Acta Phys. Chim. Sin. 30 1416
 Google Scholar
Google Scholar
[17] Wu P Y, Tzeng S Y, Hsu Y C, Tzeng W B 2017 J. Mol. Spectrosc. 332 3
 Google Scholar
Google Scholar
[18] Lin J L, Tzeng W B 2000 Phys. Chem. Chem. Phys 2 3759
 Google Scholar
Google Scholar
[19] Shivatare V, Tzeng W B 2014 Bull. Korean Chem. Soc. 35 815
 Google Scholar
Google Scholar
[20] Lin J L, Li Y C, Tzeng W B 2007 Chem. Phys. 334 189
 Google Scholar
Google Scholar
[21] Ketkov S Y, Tzeng S Y, Wu P Y, Markin G V, Tzeng W B 2017 Chem. Eur. J. 23 1
 Google Scholar
Google Scholar
[22] Zhang L J, Li D Z, Cheng M, Du Y K, Zhu Q H 2017 Spectrochim. Acta, Part A 183 177
 Google Scholar
Google Scholar
[23] Hao J Y, Duan C Y, Yang Y G, Li C Y, Jia S T 2020 J. Mol. Spectrosc. 369 111258
 Google Scholar
Google Scholar
[24] Jin Y H, Zhao Y, Yang Y G, Wang L R, Li C Y, Jia S T 2018 Chem. Phys. Lett. 692 395
 Google Scholar
Google Scholar
[25] 李鑫, 赵岩, 靳颖辉, 王晓锐, 余谢秋, 武媚, 韩昱行, 杨勇刚, 李昌勇, 贾锁堂 2017 66 093301
 Google Scholar
Google Scholar
Li X, Zhao Y, Jin Y H, Wang X R, Yu X Q, Wu M, Han Y Y, Yang Y G, Li C Y, Jia S T 2017 Acta Phys. Sin. 66 093301
 Google Scholar
Google Scholar
[26] Zhao Y, Jin Y H, Hao J Y, Yang Y G, Wang L R, Li C Y, Jia S T 2019 Spectrochim. Acta, Part A 207 328
 Google Scholar
Google Scholar
[27] Ullrich S, Geppert W D, Dessent C E H, Mu1ler-Dethlefs K 2000 J. Phys. Chem. A 104 11864
[28] Wilke M, Schneider M, Wilke J, Ruiz-Santoyo J A, Campos-Amador J J, Gonzalez-Medina M. E, Alvarez-Valtierra L, Schmitt M 2017 J. Mol. Struct. 1140 59
 Google Scholar
Google Scholar
[29] 李昌勇, 张临杰, 赵建明, 贾锁堂 2012 61 163202
 Google Scholar
Google Scholar
Li C Y, Zhang L J, Zhao J M, Jia S T 2012 Acta Phys. Sin. 61 163202
 Google Scholar
Google Scholar
[30] Li C Y, Hao T, Zgang H, Zhu X B, TAO G Q, Zhang L J, Zhao J M, Jia S T 2012 J. Phys. Soc. Jpn. 81 044302
 Google Scholar
Google Scholar
[31] Dong H, Wang T, Li C Y, Zhao J M, Zhang L J 2013 Chin. Phys. B 22 073201
 Google Scholar
Google Scholar
[32] Dong H, Hang K S, Li C Y, Zhao J M, Zhang L, Jia S T 2014 Chin. Phys. B 23 093202
 Google Scholar
Google Scholar
[33] 董慧杰, 王新宇, 李昌勇, 贾锁堂 2015 64 093201
 Google Scholar
Google Scholar
Gong H J, Wang X Y, Li C Y, Jia S T 2015 Acta Phys. Sin. 64 093201
 Google Scholar
Google Scholar
[34] Wang L M, Li C Y, Zhang H, Zhang L J, Yang Y G, Man Y, Zhao J M, Jia S T 2016 Phys. Rev. A 93 033416
 Google Scholar
Google Scholar
[35] Chupka W A 1993 J. Chem. Phys. 98 4520
 Google Scholar
Google Scholar
[36] Zhang B, Li C Y, Su H W, Lin J L, Tzeng W B 2004 Chem. Phys. Lett. 390 65
 Google Scholar
Google Scholar
[37] Choi K W, Choi S, Baek S J, Kim S K 2007 J. Chem. Phys. 126 034308
 Google Scholar
Google Scholar
-
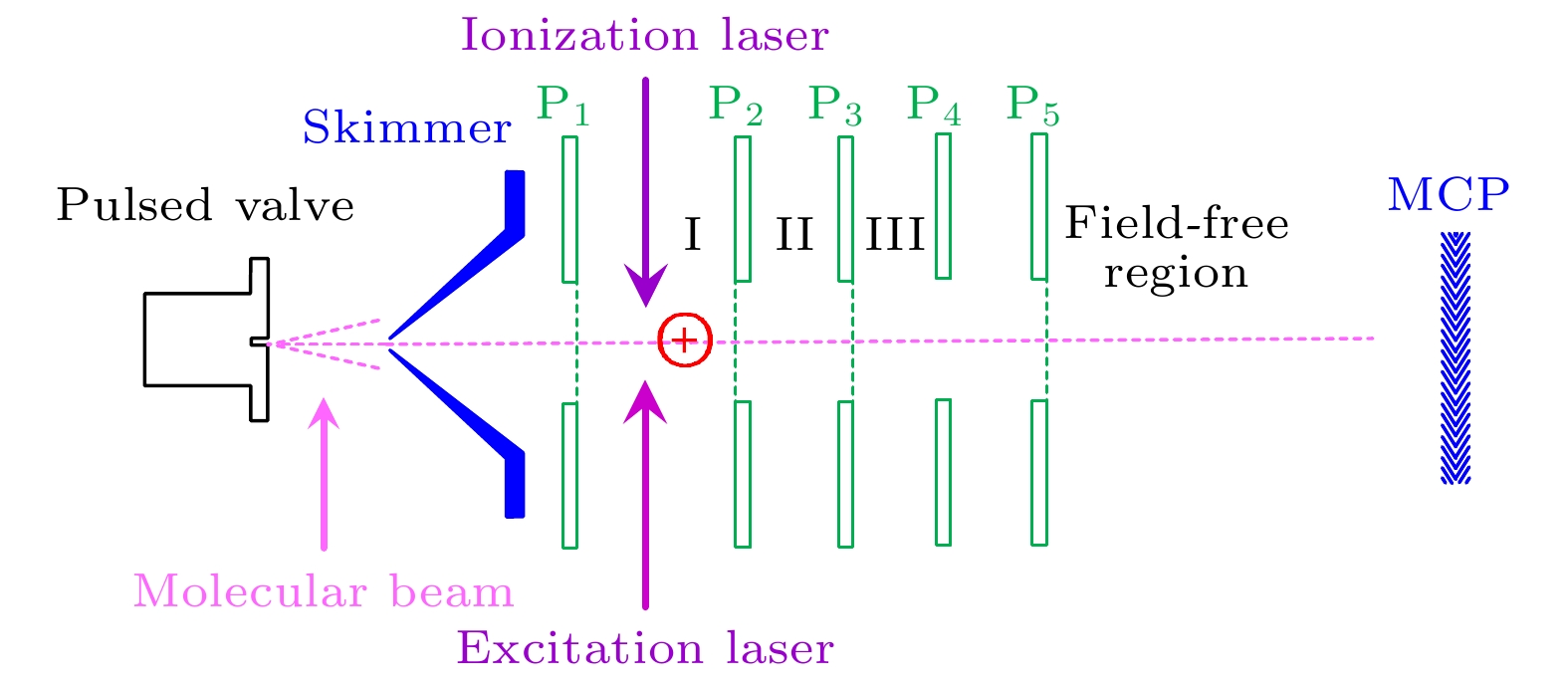
图 1 直线式飞行时间质谱仪原理图. P1, P2, P3, P4, P5为离子透镜的片状电极; P1与P2间区域I为激光和分子束相互作用区. MCP为微通道板探测器
Figure 1. Schematic diagram of a linear time-of-flight mass spectrometer. P1, P2, P3, P4, P5 are the electrodes of the electrostatic lens; the region I between P1 and P2 is the interaction area between lasers and molecular beam. MCP is a microchannel plate detector.
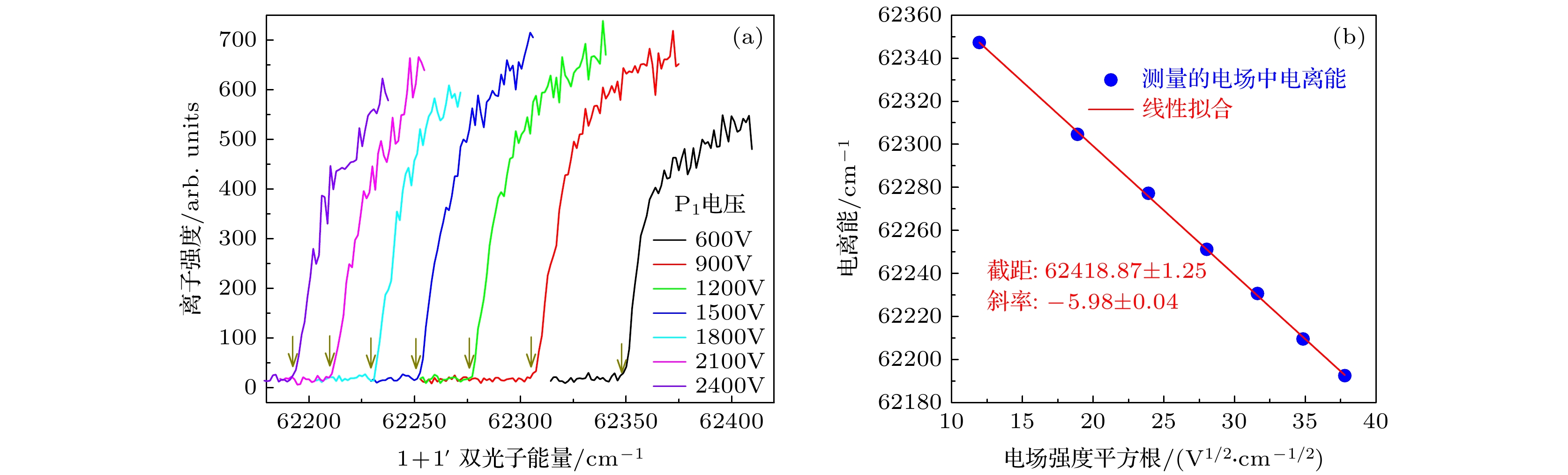
图 3 异构物I在不同电场中的光电离效率曲线(a)及其测量的电离能对电场强度平方根的线性拟合(b). 图(a)中箭头指向了电场中电离阈值的取值点
Figure 3. The photoionization efficiency curves of isomer I (down-up) in different electric fields (a), and the linear fitting of the measured ionization energy to the square root of the electric field intensity (b). The arrows in figure (a) point to the ionization thresholds in the electric fields.
图 4 异构物III在不同电场中的光电离效率曲线(a)及其测量的电离能对电场强度平方根的线性拟合(b). 图(a)中箭头指向了电场中电离阈值的采集点
Figure 4. The photoionization efficiency curves of isomer III (down-down) in different electric fields (a), and the linear fitting of the measured ionization energy to the square root of the electric field intensity (b). The arrows in figure (a) point to the ionization thresholds in the electric fields.
-
[1] Zhang L J, Yu D, Dong C W, Cheng M, Hu L L, Zhou Z M, Du Y K, Zhu Q H, Zhang C H 2013 Spectrochim. Acta, Part A 104 235
 Google Scholar
Google Scholar
[2] Yang S C, Huang H W, Tzeng W B 2010 J. Phys. Chem. A 114 11144
 Google Scholar
Google Scholar
[3] Qin C, Tzeng S Y, Zhang B, Tzeng W B 2019 J. Mol. Spectrosc. 355 26
 Google Scholar
Google Scholar
[4] Xu Y Q, Tzeng S Y, Zhang B, Tzeng W B 2013 Spectrochim. Acta, Part A 102 365
 Google Scholar
Google Scholar
[5] Lee Y R, Kim M H, Kim H L, Kwon C H 2018 J. Chem. Phys. 149 174302
 Google Scholar
Google Scholar
[6] Wu P Y, Tzeng W B 2015 J. Mol. Spectrosc. 316 72
 Google Scholar
Google Scholar
[7] Tsai C Y, Tzeng W B 2013 J. Photoch. Photobio. A 270 53
 Google Scholar
Google Scholar
[8] Dai W S, Zhang Z, Du Y K 2020 Spectrochim. Acta, Part A 224 117398
 Google Scholar
Google Scholar
[9] Xiao D Q, Yu D, Xu X L, Yu Z J, Cheng M, Du Y K, Zheng W J, Zhu Q H 2009 Phys. Chem. Chem. Phys. 11 3532
 Google Scholar
Google Scholar
[10] Xu Y Q, Tzeng S Y, Shivatare V, Takahashi K, Zhang B, Tzeng W B 2015 J. Chem. Phys. 142 124314
 Google Scholar
Google Scholar
[11] Huang J H, Huang K L, Liu S Q, Luo Q, Tzeng W B 2007 J. Photoch. Photobio. A 188 252
 Google Scholar
Google Scholar
[12] Li C Y, Lin J L, Tzeng W B 2005 J. Chem. Phys. 122 044311
 Google Scholar
Google Scholar
[13] Zhang L J, Dong C W, Cheng M, Hu L L, Du Y K, Zhu Q H, Zhang C H 2012 Spectrochim. Acta, Part A 96 578
 Google Scholar
Google Scholar
[14] Li C Y, Pradhan M, Tzeng W B 2005 Chem. Phys. Lett. 411 506
 Google Scholar
Google Scholar
[15] Lin J L, Li C Y, Tzeng W B 2004 J. Chem. Phys. 120 10513
 Google Scholar
Google Scholar
[16] Qin C, Tzeng S Y, Zang B, Tzeng W B 2014 Acta Phys. Chim. Sin. 30 1416
 Google Scholar
Google Scholar
[17] Wu P Y, Tzeng S Y, Hsu Y C, Tzeng W B 2017 J. Mol. Spectrosc. 332 3
 Google Scholar
Google Scholar
[18] Lin J L, Tzeng W B 2000 Phys. Chem. Chem. Phys 2 3759
 Google Scholar
Google Scholar
[19] Shivatare V, Tzeng W B 2014 Bull. Korean Chem. Soc. 35 815
 Google Scholar
Google Scholar
[20] Lin J L, Li Y C, Tzeng W B 2007 Chem. Phys. 334 189
 Google Scholar
Google Scholar
[21] Ketkov S Y, Tzeng S Y, Wu P Y, Markin G V, Tzeng W B 2017 Chem. Eur. J. 23 1
 Google Scholar
Google Scholar
[22] Zhang L J, Li D Z, Cheng M, Du Y K, Zhu Q H 2017 Spectrochim. Acta, Part A 183 177
 Google Scholar
Google Scholar
[23] Hao J Y, Duan C Y, Yang Y G, Li C Y, Jia S T 2020 J. Mol. Spectrosc. 369 111258
 Google Scholar
Google Scholar
[24] Jin Y H, Zhao Y, Yang Y G, Wang L R, Li C Y, Jia S T 2018 Chem. Phys. Lett. 692 395
 Google Scholar
Google Scholar
[25] 李鑫, 赵岩, 靳颖辉, 王晓锐, 余谢秋, 武媚, 韩昱行, 杨勇刚, 李昌勇, 贾锁堂 2017 66 093301
 Google Scholar
Google Scholar
Li X, Zhao Y, Jin Y H, Wang X R, Yu X Q, Wu M, Han Y Y, Yang Y G, Li C Y, Jia S T 2017 Acta Phys. Sin. 66 093301
 Google Scholar
Google Scholar
[26] Zhao Y, Jin Y H, Hao J Y, Yang Y G, Wang L R, Li C Y, Jia S T 2019 Spectrochim. Acta, Part A 207 328
 Google Scholar
Google Scholar
[27] Ullrich S, Geppert W D, Dessent C E H, Mu1ler-Dethlefs K 2000 J. Phys. Chem. A 104 11864
[28] Wilke M, Schneider M, Wilke J, Ruiz-Santoyo J A, Campos-Amador J J, Gonzalez-Medina M. E, Alvarez-Valtierra L, Schmitt M 2017 J. Mol. Struct. 1140 59
 Google Scholar
Google Scholar
[29] 李昌勇, 张临杰, 赵建明, 贾锁堂 2012 61 163202
 Google Scholar
Google Scholar
Li C Y, Zhang L J, Zhao J M, Jia S T 2012 Acta Phys. Sin. 61 163202
 Google Scholar
Google Scholar
[30] Li C Y, Hao T, Zgang H, Zhu X B, TAO G Q, Zhang L J, Zhao J M, Jia S T 2012 J. Phys. Soc. Jpn. 81 044302
 Google Scholar
Google Scholar
[31] Dong H, Wang T, Li C Y, Zhao J M, Zhang L J 2013 Chin. Phys. B 22 073201
 Google Scholar
Google Scholar
[32] Dong H, Hang K S, Li C Y, Zhao J M, Zhang L, Jia S T 2014 Chin. Phys. B 23 093202
 Google Scholar
Google Scholar
[33] 董慧杰, 王新宇, 李昌勇, 贾锁堂 2015 64 093201
 Google Scholar
Google Scholar
Gong H J, Wang X Y, Li C Y, Jia S T 2015 Acta Phys. Sin. 64 093201
 Google Scholar
Google Scholar
[34] Wang L M, Li C Y, Zhang H, Zhang L J, Yang Y G, Man Y, Zhao J M, Jia S T 2016 Phys. Rev. A 93 033416
 Google Scholar
Google Scholar
[35] Chupka W A 1993 J. Chem. Phys. 98 4520
 Google Scholar
Google Scholar
[36] Zhang B, Li C Y, Su H W, Lin J L, Tzeng W B 2004 Chem. Phys. Lett. 390 65
 Google Scholar
Google Scholar
[37] Choi K W, Choi S, Baek S J, Kim S K 2007 J. Chem. Phys. 126 034308
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 8424
- PDF Downloads: 91
- Cited By: 0














 DownLoad:
DownLoad: