-
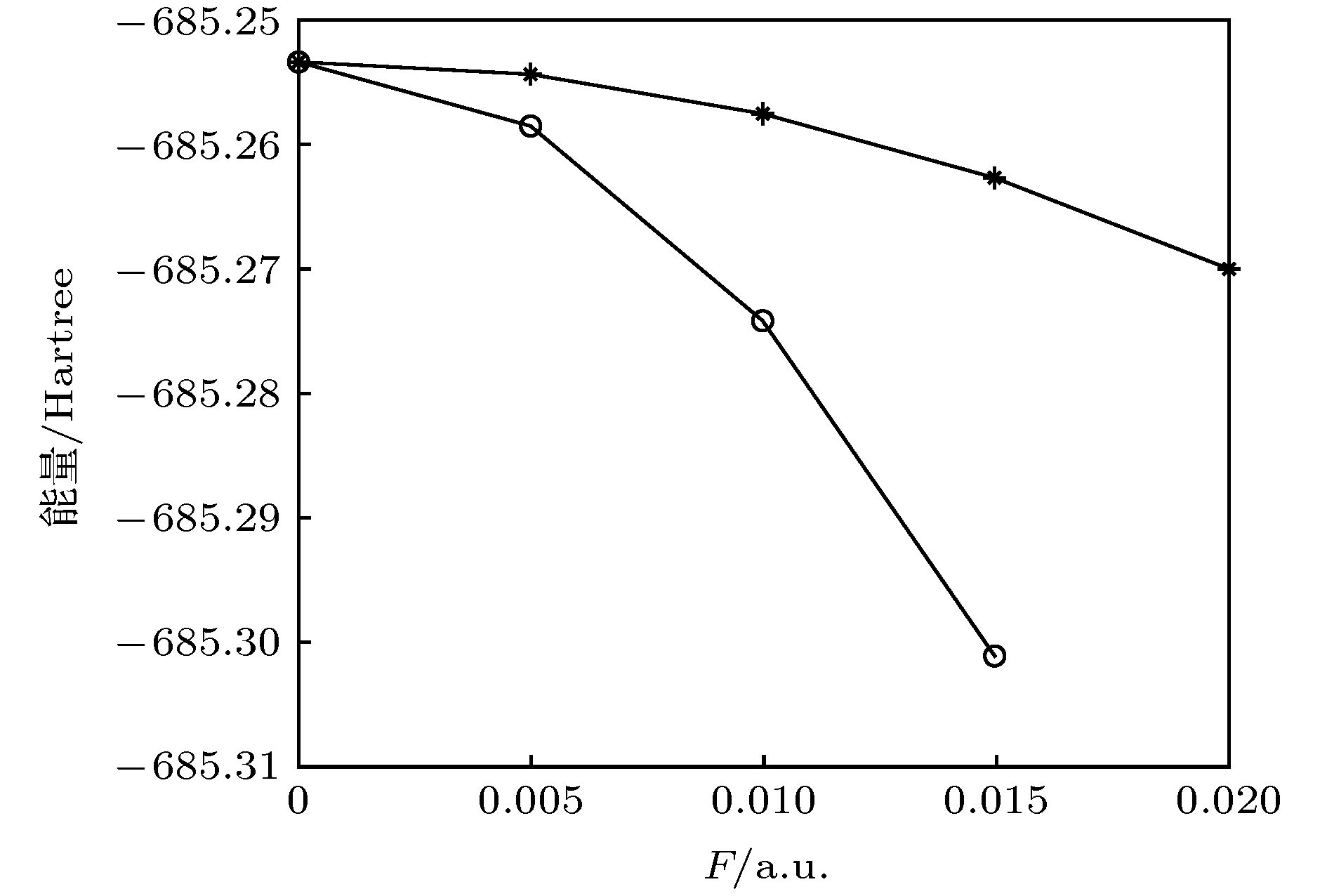
In this work, density functional theory method with the ωB97XD/def2-TZVP level is carried out to investigate the ground state structures, energy, electronic structures, aromaticity, infrared and Raman spectra of cyclo[18]carbon under different external electric field in the x, y and z direction of cyclo[18]carbon molecule. The excitation properties (the first 48 excited states containing excited energies, excited wavelengths and oscillator strengths) of cyclo[18]carbon are calculated by the time-dependent density functional theory method (TD-ωB97XD) with the def2-TZVP basis set under the same external electric field. The results show that cyclo[18]carbon can be elongated in the x or y direction under the electric field, and some C-C bond lengths can be elongated or shortened under the electric field. Meanwhile, the calculated results show that electric dipole moment is proved to be increasing with the increase of the external field intensity, but the total energy and LUMO-HOMO gap are proved to decrease with the increase of external field intensity. Moreover, addition of electric field can modify the electron delocalization and molecular aromaticity, such as external electric field in z direction can lower the electron delocalization and molecular aromaticity and external electric field in x or y direction can enhance the electron delocalization and molecular aromaticity. The addition of electric field can modify the infrared spectra, such as shift of vibrational frequencies and strengthening of infrared peaks. Furthermore, the calculated results indicate that the external electric field has significant effects on the excitation properties of cyclo[18]carbon. The increase of the electric field intensity can lead to the redshift of transition wavelengths (such as the first excited state). With the change of the electric field intensity, the stronger excited state (with the bigger oscillator strength) can become weak (with the small oscillator strength) or optically inactive (with the oscillator strength of zero). Meanwhile, the weak or optically inactive excited state can become stronger excited state by the external field. The ground state properties and excitation properties of cyclo[18]carbon can be modified by the external electric field. Our works can provide theoretical guidance for the application of cyclo[18]carbon in the nanotechnology such as molecular device.
-
Keywords:
- C18 /
- external electric field /
- ground state /
- excitation properties
[1] Kroto H W, Heath J R, O’Brien S C, Curl R F, Smalley R E 1985 Nature 318 162
 Google Scholar
Google Scholar
[2] Iijima S 1991 Nature 354 56
 Google Scholar
Google Scholar
[3] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[4] Parasuk V, Almlof J, Feyereisen M W 1991 J. Am. Chem. Soc. 113 1049
 Google Scholar
Google Scholar
[5] Torelli T, Mitas L 2000 Phys. Rev. Lett. 85 1702
 Google Scholar
Google Scholar
[6] Arulmozhiraja S, Ohno T 2008 J. Chem. Phys. 128 114301
 Google Scholar
Google Scholar
[7] Fowler P W, Mizoguchi N, Bean D E, Havenith R W 2009 Chemistry 15 6964
 Google Scholar
Google Scholar
[8] Kaiser K, Scriven L M, Schulz F, Gawel P, Gross L, Anderson H L 2019 Science 365 1299
 Google Scholar
Google Scholar
[9] Nandi A, Solel E, Kozuch S 2020 Chemistry 26 625
 Google Scholar
Google Scholar
[10] Pereira Z S, da Silva E Z 2020 J. Phys. Chem. A 124 1152
 Google Scholar
Google Scholar
[11] Pichierri F 2020 Chem. Phys. Lett. 738 136860
 Google Scholar
Google Scholar
[12] Shi B, Yuan L, Tang T, Yuan Y, Tang Y 2020 Chem. Phys. Lett. 741 136975
 Google Scholar
Google Scholar
[13] Stasyuk A J, Stasyuk O A, Solà M, Voityuk A A 2020 Chem. Commun. 56 352
 Google Scholar
Google Scholar
[14] 史丹丹, 张西沙, 张德清 2018 化学进展 30 658
 Google Scholar
Google Scholar
Shi D D, Zhang X S, Zhang D Q 2018 Progress in Chemistry 30 658
 Google Scholar
Google Scholar
[15] Yamaguchi Y, Takubo M, Ogawa K, Nakayama K, Koganezawa T, Katagiri H 2016 J. Am. Chem. Soc. 138 11335
 Google Scholar
Google Scholar
[16] Sluysmans D, Stoddart J F 2019 Trends in Chemistry 1 185
 Google Scholar
Google Scholar
[17] Perez E M 2017 Chemistry 23 12681
 Google Scholar
Google Scholar
[18] Castelvecchi D 2019 Nature 572 426
 Google Scholar
Google Scholar
[19] 杨涛, 刘代俊, 陈建钧 2016 65 053101
 Google Scholar
Google Scholar
Yang T, Liu D J, Chen J J 2016 Acta Phys. Sin. 65 053101
 Google Scholar
Google Scholar
[20] 李世雄, 张正平, 隆正文, 秦水介 2017 66 103102
 Google Scholar
Google Scholar
Li S X, Zhang Z P, Long Z W, Qin S J 2017 Acta Phys. Sin. 66 103102
 Google Scholar
Google Scholar
[21] 杜建宾, 冯志芳, 张倩, 韩丽君, 唐延林, 李奇峰 2019 68 173101
 Google Scholar
Google Scholar
Du J B, Feng Z F, Zhang Q, Han L J, Tang Y L, Li Q F 2019 Acta Phys. Sin. 68 173101
 Google Scholar
Google Scholar
[22] 李亚莎, 孙林翔, 周筱, 陈凯, 汪辉耀 2020 69 013101
 Google Scholar
Google Scholar
Li Y S, Sun L X, Zhou X, Chen K, Wang H Y 2020 Acta Phys. Sin. 69 013101
 Google Scholar
Google Scholar
[23] Shaik S, Mandal D, Ramanan R 2016 Nat. Chem. 8 1091
 Google Scholar
Google Scholar
[24] English N J, Waldron C J 2015 Phys. Chem. Chem. Phys. 17 12407
 Google Scholar
Google Scholar
[25] Foroutan-Nejad C, Andrushchenko V, Straka M 2016 Phys. Chem. Chem. Phys. 18 32673
 Google Scholar
Google Scholar
[26] Weigend F, Ahlrichs R 2005 Phys. Chem. Chem. Phys. 7 3297
 Google Scholar
Google Scholar
[27] Chai J D, Head-Gordon M 2008 Phys. Chem. Chem. Phys. 10 6615
 Google Scholar
Google Scholar
[28] Frisch M J et al. 2009 Gaussian 09 (Revision A.02) (Gaussian Inc., Wallingford, CT)
[29] Lu T, Chen F 2012 J. Comput. Chem. 33 580
 Google Scholar
Google Scholar
[30] Mayer I 1983 Chem. Phys. Lett. 97 270
 Google Scholar
Google Scholar
[31] Schmider H L, Becke A D 2000 J. Mol. Struct.THEOCHEM 527 51
 Google Scholar
Google Scholar
[32] Becke A D, Edgecombe K E 1990 J. Chem. Phys. 92 5397
 Google Scholar
Google Scholar
[33] Matito E 2016 Phys. Chem. Chem. Phys. 18 11839
 Google Scholar
Google Scholar
-
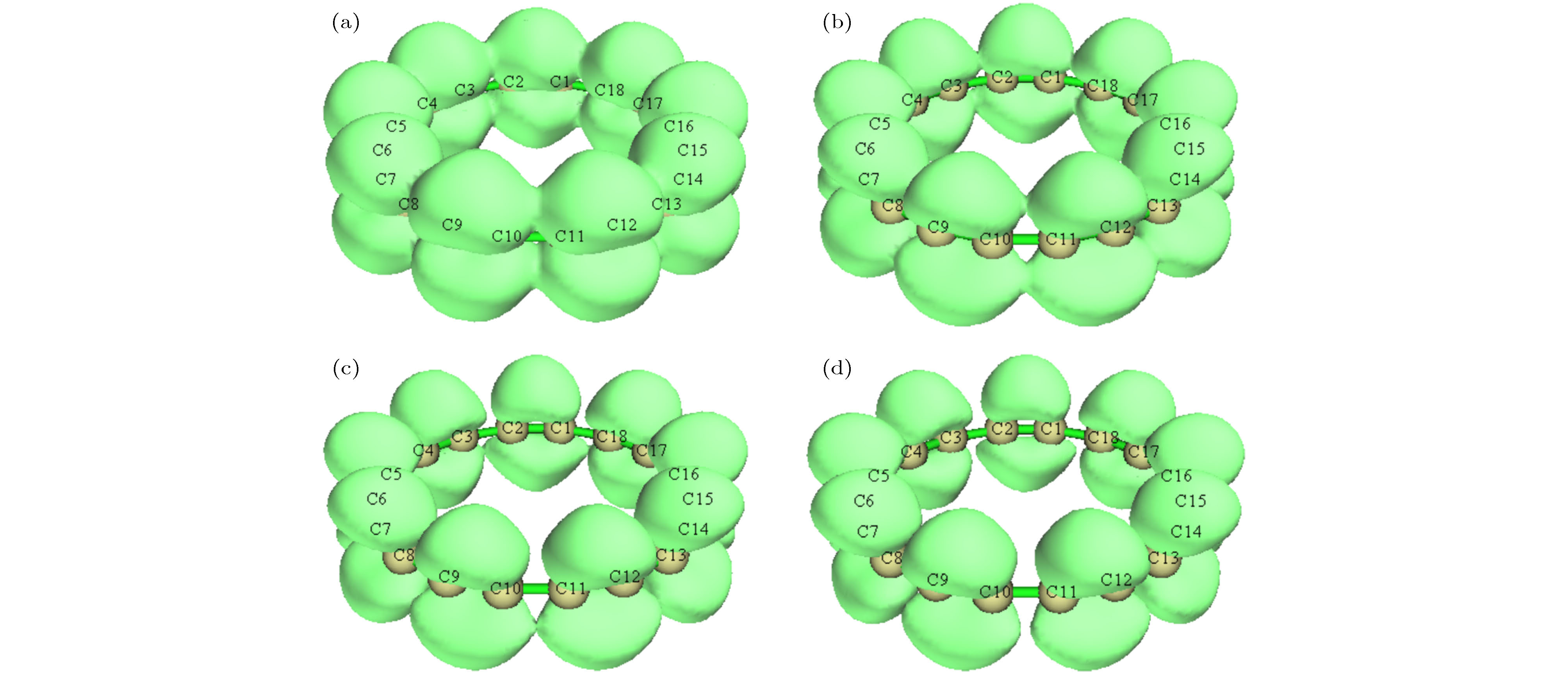
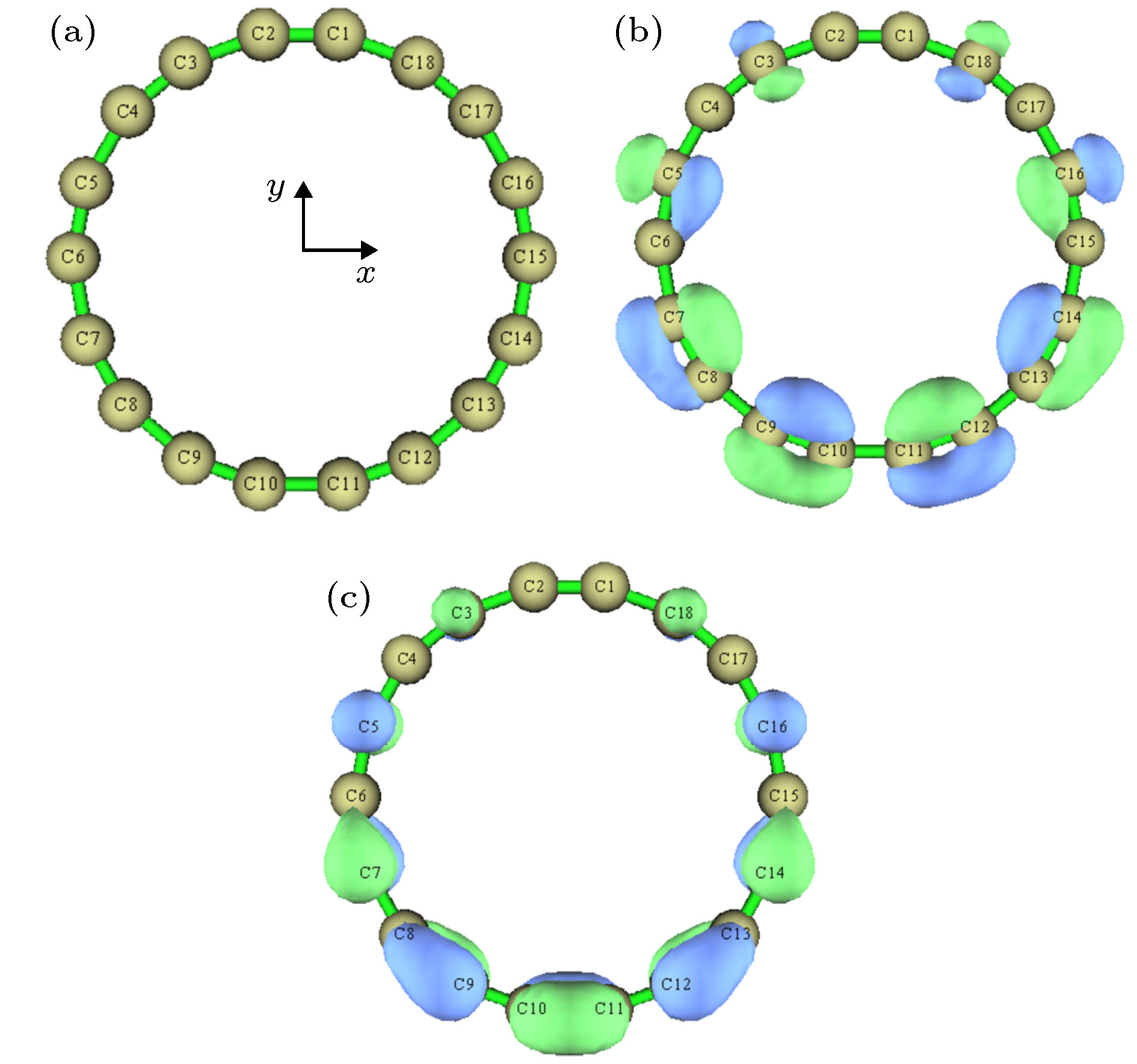
图 4 z方向外电场下环平面上/下(out-plane)π轨道的LOL, 等值面数值为0.38 (a) 0 a.u.; (b) 0.005 a.u.; (c) 0.01 a.u.; (d) 0.015 a.u.
Figure 4. Localized orbital locator calculated based on out-plane π orbitals under different external electric fields in the z direction, the isovalue is set to 0.38: (a) 0 a.u.; (b) 0.005 a.u.; (c) 0.01 a.u.; (d) 0.015 a.u..
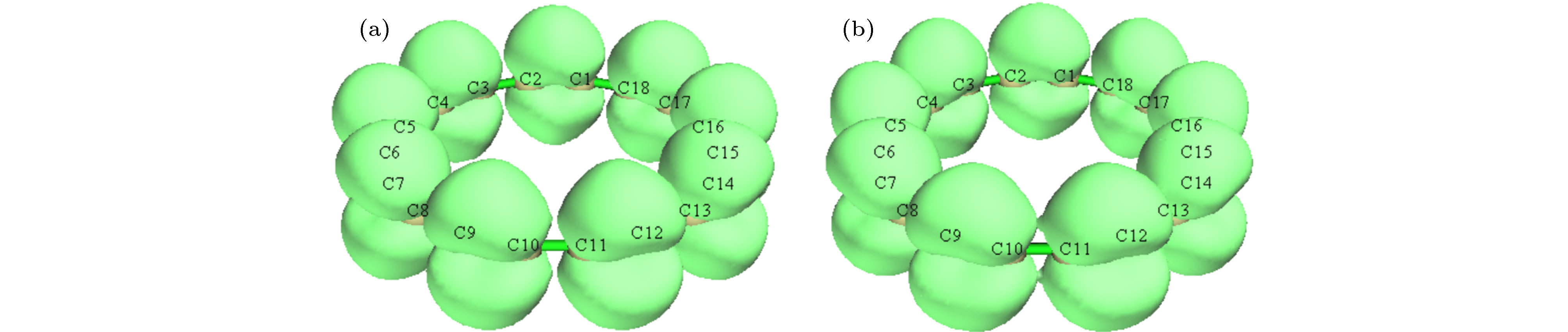
图 5 z方向外电场下环平面上/下(out-plane)π轨道的ELF, 等值面数值为0.38 (a) 0 a.u.; (b) 0.005 a.u.; (c) 0.01 a.u.; (d) 0.015 a.u.
Figure 5. Electron localization function calculated based on out-plane π orbitals under different external electric fields in the z direction, the isovalue is set to 0.38: (a) 0 a.u.; (b) 0.005 a.u.; (c) 0.01 a.u.; (d) 0.015 a.u..
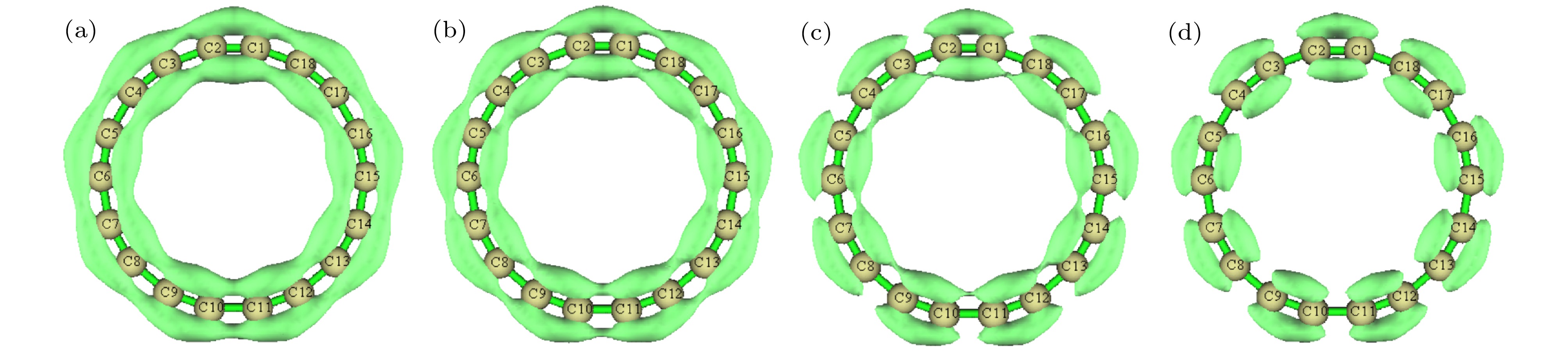
图 8 z方向外电场下环内/外(in-plane)π轨道的LOL, 等值面数值为0.37 (a) 0 a.u.; (b) 0.005 a.u.; (c) 0.01 a.u.; (d) 0.015 a.u.
Figure 8. LOL calculated based on in-plane π orbitals under different external electric fields in the z direction, the isovalue is set to 0.37: (a) 0 a.u.; (b) 0.005 a.u.; (c) 0.01 a.u.; (d) 0.015 a.u..
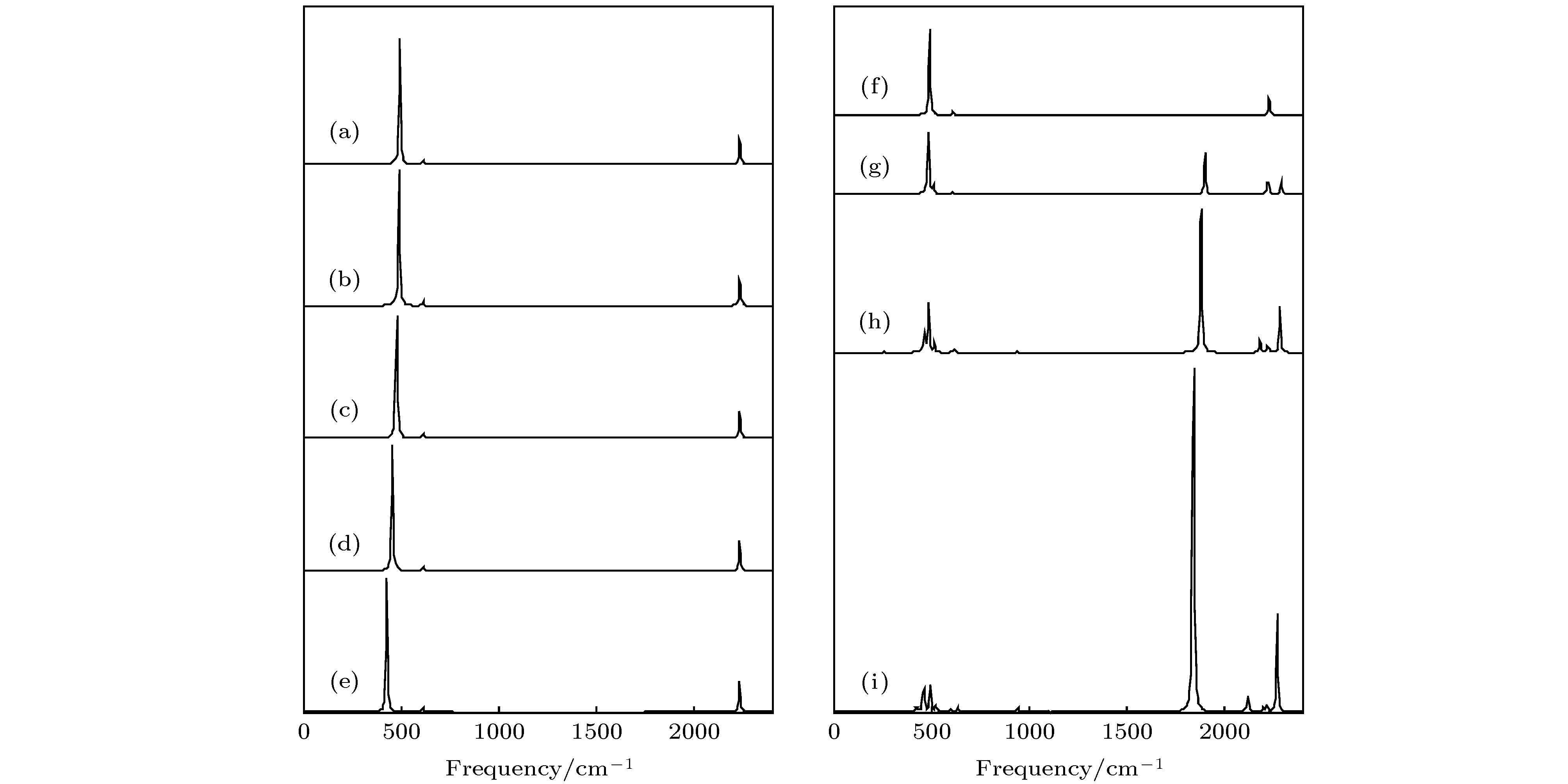
图 10 红外光谱随外电场的变化, 左边为随z方向电场变化, 右边是随y方向电场变化 (a) 0 a.u.; (b) 0.005 a.u.; (c) 0.01 a.u.; (d) 0.015 a.u.; (e) 0.02 a.u.; (f) 0 a.u.; (g) 0.005 a.u.; (h) 0.01 a.u.; (i) 0.015 a.u.
Figure 10. Calculated infrared spectra based on different external electric fields in the z direction (left side) and y direction (right side): (a) 0 a.u.; (b) 0.005 a.u.; (c) 0.01 a.u.; (d) 0.015 a.u.; (e) 0.02 a.u.; (f) 0 a.u.; (g) 0.005 a.u.;(h) 0.01 a.u.; (i) 0.015 a.u..
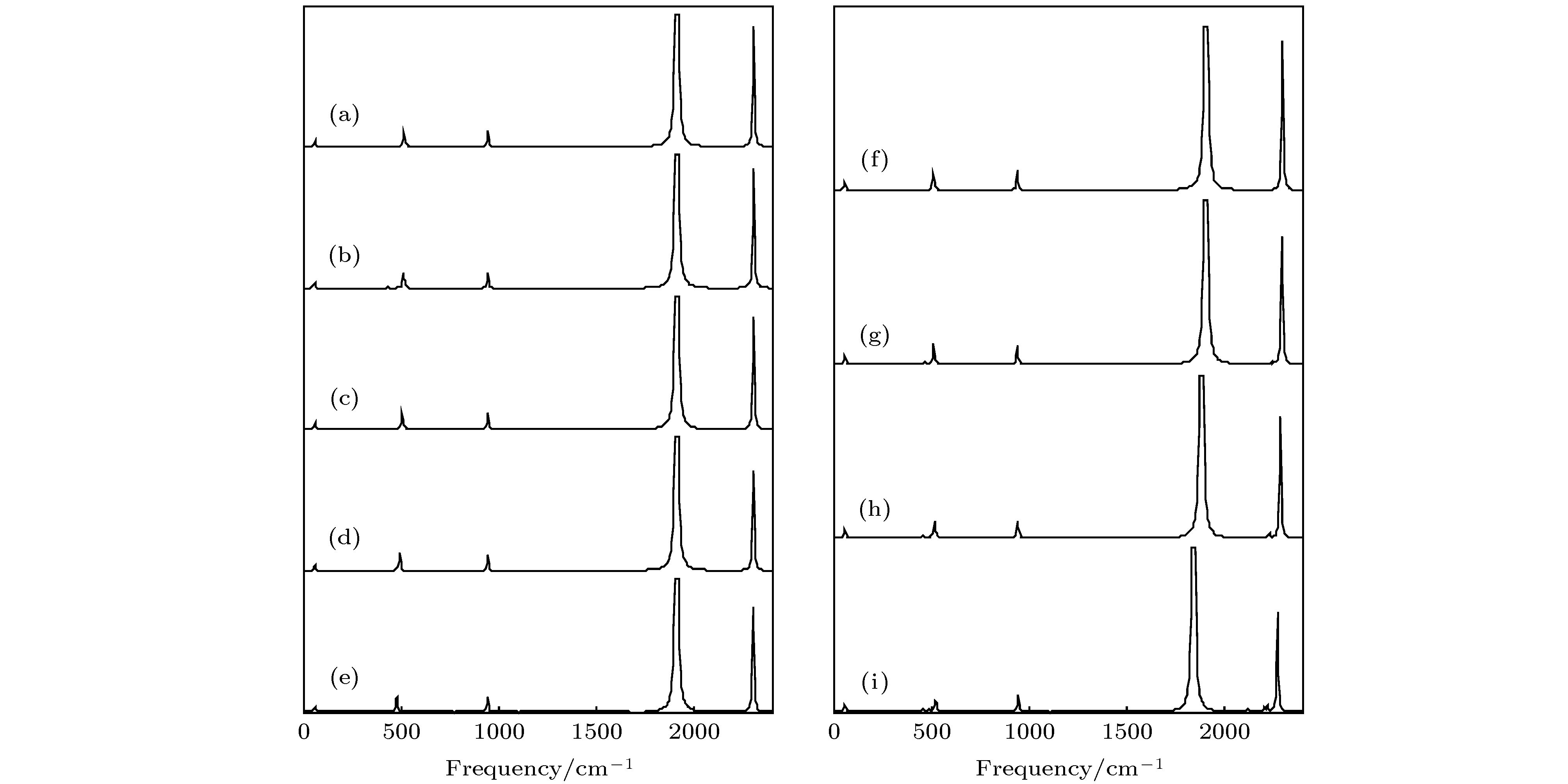
图 11 拉曼光谱随外电场的变化, 左边为z方向加电场, 右边是y方向加电场 (a) 0 a.u.; (b) 0.005 a.u.; (c) 0.01 a.u.; (d) 0.015 a.u.; (e) 0.02 a.u.; (f) 0 a.u.; (g) 0.005 a.u.; (h) 0.010 a.u.; (i) 0.015 a.u.
Figure 11. Calculated Raman spectra based on different external electric fields in the z direction (left side) and y direction (right side): (a) 0 a.u.; (b) 0.005 a.u.; (c) 0.01 a.u.; (d) 0.015 a.u.; (e) 0.02 a.u.; (f) 0 a.u.; (g) 0.005 a.u.;(h) 0.01 a.u.; (i) 0.015 a.u..
表 1 不同电场下的轨道能量EH, EL, EH–1, EH–2, EH–3, EL+1, EL+2, EL+3以及Eg. 轨道能量的单位是Hartree (1 Hartree = 2625.5 kJ/mol), 上标x, y, z分别表示x, y, z方向加电场
Table 1. The orbital energies EH, EL, EH–1, EH–2, EH–3, EL+1, EL+2, EL+3 and Eg of C18 under different external electric fields. The unit of orbital energy is hartree, the superscripts x, y and z denote thex, y and z direction, respectively.
F/a.u. EH – 3 EH – 2 EH – 1 EH EL EL + 1 EL + 2 EL + 3 Eg/eV 0 –8.5508 –8.5508 –8.4485 –8.4485 –1.7005 –1.7005 –1.6754 1.6754 6.7479 0.005z –8.5544 –8.5544 –8.4486 –8.4486 –1.7141 –1.7141 –1.6666 1.6666 6.7345 0.010z –8.5650 –8.5650 –8.4495 –8.4495 –1.7392 –1.7392 1.6547 1.6547 6.7102 0.015z –8.5819 –8.5819 –8.4518 –8.4518 –1.7700 –1.7700 –1.6461 –1.6461 6.6818 0.020z –8.6044 –8.6044 –8.4562 –8.4562 –1.8056 –1.8056 –1.6415 –1.6415 6.6505 0.005x –8.6369 –8.5205 –8.4629 –8.3831 –1.8002 –1.7217 –1.6612 –1.6317 6.5829 0.010x –8.7048 –8.5881 –8.3700 –8.3153 –1.9358 –1.8075 –1.6986 –1.6119 6.3794 0.015x –8.7516 –8.6519 –8.2846 –8.2518 –2.0999 –1.9295 –1.8027 –1.6493 6.1518 0.050y –8.6367 –8.5204 –8.4630 –8.3831 –1.8005 –1.7222 –1.6609 –1.6315 6.5825 0.010y –8.7046 –8.5879 –8.3700 –8.3153 –1.9359 –1.8075 –1.6988 –1.6120 6.3793 0.015y –8.7527 –8.6529 –8.2847 –8.2516 –2.0992 –1.9293 –1.8012 –1.6482 6.1524 表 2 不同电场下的AV1245指数
Table 2. The AV1245 of C18 under different external electric fields.
F/a.u. z x y 0 4.253 4.253 4.253 0.005 4.248 4.256 4.257 0.010 4.232 4.398 4.399 0.015 4.205 4.682 4.706 0.020 4.170 表 3 不同外电场(y方向)下C18分子部分激发态的激发能
Table 3. Excitation energy of C18 at different electric field in y direction.
F/a.u. E/eV n = 1 2 6 7 14 21 22 23 35 36 0 2.5076 2.6372 3.1285 3.1285 3.8301 5.6519 5.6519 5.7890 6.4794 6.4794 0.005 2.4993 2.6264 3.0978 3.1234 3.8130 5.6339 5.6558 5.7753 6.4573 6.4644 0.010 2.4560 2.5759 2.9933 3.0229 3.7358 5.5725 5.6564 5.7195 6.3833 6.4070 0.015 2.3747 2.4684 2.8621 2.9017 3.6094 5.4638 5.6232 5.6320 6.2782 6.3067 表 5 不同外电场(y方向)下C18分子部分激发态的振子强度
Table 5. Oscillator strength of C18 at different electric field in y direction.
F/a.u. f n = 1 2 6 7 14 21 22 23 35 36 0 0.0000 0.0000 0.0030 0.0030 0.0000 3.0216 3.0216 0.0000 0.3695 0.3695 0.005 0.0000 0.0003 0.0025 0.0028 0.0132 2.9676 3.0222 0.0000 0.3831 0.3838 0.010 0.0000 0.0011 0.0014 0.0002 0.0463 2.8009 2.9973 0.0000 0.4379 0.4337 0.015 0.0000 0.0000 0.0003 0.0000 0.0842 2.4917 0.0000 2.4509 0.5197 0.5373 表 4 不同外电场(y方向)下C18分子部分激发态的波长
Table 4. Excitation wavelength of C18 at different electric field in y direction.
F/a.u. $\lambda $/nm n = 1 2 6 7 14 21 22 23 35 36 0 494.43 470.14 396.30 396.30 323.71 219.37 219.37 214.17 191.35 191.35 0.005 496.08 472.07 400.24 396.96 325.17 220.07 219.21 214.68 192.01 191.80 0.010 504.82 481.32 414.20 410.15 331.88 222.49 219.19 216.77 194.23 193.51 0.015 522.11 502.28 433.20 427.29 343.51 226.92 220.49 220.14 197.48 196.59 -
[1] Kroto H W, Heath J R, O’Brien S C, Curl R F, Smalley R E 1985 Nature 318 162
 Google Scholar
Google Scholar
[2] Iijima S 1991 Nature 354 56
 Google Scholar
Google Scholar
[3] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[4] Parasuk V, Almlof J, Feyereisen M W 1991 J. Am. Chem. Soc. 113 1049
 Google Scholar
Google Scholar
[5] Torelli T, Mitas L 2000 Phys. Rev. Lett. 85 1702
 Google Scholar
Google Scholar
[6] Arulmozhiraja S, Ohno T 2008 J. Chem. Phys. 128 114301
 Google Scholar
Google Scholar
[7] Fowler P W, Mizoguchi N, Bean D E, Havenith R W 2009 Chemistry 15 6964
 Google Scholar
Google Scholar
[8] Kaiser K, Scriven L M, Schulz F, Gawel P, Gross L, Anderson H L 2019 Science 365 1299
 Google Scholar
Google Scholar
[9] Nandi A, Solel E, Kozuch S 2020 Chemistry 26 625
 Google Scholar
Google Scholar
[10] Pereira Z S, da Silva E Z 2020 J. Phys. Chem. A 124 1152
 Google Scholar
Google Scholar
[11] Pichierri F 2020 Chem. Phys. Lett. 738 136860
 Google Scholar
Google Scholar
[12] Shi B, Yuan L, Tang T, Yuan Y, Tang Y 2020 Chem. Phys. Lett. 741 136975
 Google Scholar
Google Scholar
[13] Stasyuk A J, Stasyuk O A, Solà M, Voityuk A A 2020 Chem. Commun. 56 352
 Google Scholar
Google Scholar
[14] 史丹丹, 张西沙, 张德清 2018 化学进展 30 658
 Google Scholar
Google Scholar
Shi D D, Zhang X S, Zhang D Q 2018 Progress in Chemistry 30 658
 Google Scholar
Google Scholar
[15] Yamaguchi Y, Takubo M, Ogawa K, Nakayama K, Koganezawa T, Katagiri H 2016 J. Am. Chem. Soc. 138 11335
 Google Scholar
Google Scholar
[16] Sluysmans D, Stoddart J F 2019 Trends in Chemistry 1 185
 Google Scholar
Google Scholar
[17] Perez E M 2017 Chemistry 23 12681
 Google Scholar
Google Scholar
[18] Castelvecchi D 2019 Nature 572 426
 Google Scholar
Google Scholar
[19] 杨涛, 刘代俊, 陈建钧 2016 65 053101
 Google Scholar
Google Scholar
Yang T, Liu D J, Chen J J 2016 Acta Phys. Sin. 65 053101
 Google Scholar
Google Scholar
[20] 李世雄, 张正平, 隆正文, 秦水介 2017 66 103102
 Google Scholar
Google Scholar
Li S X, Zhang Z P, Long Z W, Qin S J 2017 Acta Phys. Sin. 66 103102
 Google Scholar
Google Scholar
[21] 杜建宾, 冯志芳, 张倩, 韩丽君, 唐延林, 李奇峰 2019 68 173101
 Google Scholar
Google Scholar
Du J B, Feng Z F, Zhang Q, Han L J, Tang Y L, Li Q F 2019 Acta Phys. Sin. 68 173101
 Google Scholar
Google Scholar
[22] 李亚莎, 孙林翔, 周筱, 陈凯, 汪辉耀 2020 69 013101
 Google Scholar
Google Scholar
Li Y S, Sun L X, Zhou X, Chen K, Wang H Y 2020 Acta Phys. Sin. 69 013101
 Google Scholar
Google Scholar
[23] Shaik S, Mandal D, Ramanan R 2016 Nat. Chem. 8 1091
 Google Scholar
Google Scholar
[24] English N J, Waldron C J 2015 Phys. Chem. Chem. Phys. 17 12407
 Google Scholar
Google Scholar
[25] Foroutan-Nejad C, Andrushchenko V, Straka M 2016 Phys. Chem. Chem. Phys. 18 32673
 Google Scholar
Google Scholar
[26] Weigend F, Ahlrichs R 2005 Phys. Chem. Chem. Phys. 7 3297
 Google Scholar
Google Scholar
[27] Chai J D, Head-Gordon M 2008 Phys. Chem. Chem. Phys. 10 6615
 Google Scholar
Google Scholar
[28] Frisch M J et al. 2009 Gaussian 09 (Revision A.02) (Gaussian Inc., Wallingford, CT)
[29] Lu T, Chen F 2012 J. Comput. Chem. 33 580
 Google Scholar
Google Scholar
[30] Mayer I 1983 Chem. Phys. Lett. 97 270
 Google Scholar
Google Scholar
[31] Schmider H L, Becke A D 2000 J. Mol. Struct.THEOCHEM 527 51
 Google Scholar
Google Scholar
[32] Becke A D, Edgecombe K E 1990 J. Chem. Phys. 92 5397
 Google Scholar
Google Scholar
[33] Matito E 2016 Phys. Chem. Chem. Phys. 18 11839
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 10119
- PDF Downloads: 176
- Cited By: 0














 DownLoad:
DownLoad: