-
Brain is a typical complex system with characteristics such as self-adaptation, self-organization, and multistability. The activity of the default mode network (DMN), a crucial functional subnetwork of the human brain in resting state, obeys typical non-equilibrium statistical mechanical processes in which the system continually switches among multiple metastable states. Revealing the underlying dynamical mechanism of these processes has important scientific significance and clinical application prospects. In this paper, according to the blood oxygen level dependent (BOLD) signals obtained from functional magnetic resonance imaging (fMRI), we build an energy landscape, disconnectivity graph and transition network to explore the non-equilibrium processes of DMN switching among different attractors in resting state. Taking the activities of high-level visual and auditory cortices for examples, we verify the intimate relationship between the dynamics of DMN and the activity modes of these external brain regions, through comparing the distributions in state space and the algorithms such as XGBoost and deep neural networks. In addition, we analyze the interaction between various DMN regions in the resting state by using the techniques such as compressive-sensing-based partial correlation and convergence cross mapping. The results in this paper may presnt new insights into revealing the dynamics of the intrinsic non-equilibrium processes of brain in resting state, and putting forward clinically significant biomarkers for brain dysfunction from the viewpoint of dynamics.
-
Keywords:
- brain functional network /
- resting state /
- default mode network /
- energy landscape /
- state transition
[1] Sarraf S, Sun J 2016 arXiv: 1602.02225 [physics.med-ph]
[2] Liu C, Zhou C, Wang J, Loparo K 2018 IEEE Trans. Neural Syst. Rehabil. Eng. 26 1649
 Google Scholar
Google Scholar
[3] Lei Y, Song B, Chen L, Su J, Zhang X, Ni W, Yu Y, Xu B, Yu L, Gu Y, Mao Y 2018 Brain Imaging Behav. 11682
 Google Scholar
Google Scholar
[4] Chen J E, Glover G H, Greicius M D, Chang C 2017 Hum. Brain. Mapp. 38 2454
 Google Scholar
Google Scholar
[5] Chen B, Li X 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC) Banff, Canada, October 1–4, 2017 p2820
[6] Roberto T, Fox P Tomás P J 2008 Cereb. Cortex 18 2553
 Google Scholar
Google Scholar
[7] Raichle M E, Macleod A M, Snyder A Z, Powers W J, Gusnard D A, d Shulman G L 2001 Proc. Natl. Acad. Sci. U.S.A. 98 676
 Google Scholar
Google Scholar
[8] Greicius M D, Ben K, Reiss A L, Vinod M 2003 Proc. Natl. Acad. Sci. U.S.A. 100 253
 Google Scholar
Google Scholar
[9] Greicius M D, Kaustubh S, Vinod M, Dougherty R F 2009 Cereb. Cortex 19 72
 Google Scholar
Google Scholar
[10] Lin P, Yang Y, Jovicich J, Pisapia N D, Wang X, Zuo C S, Levitt J J 2016 Brain Imaging Behav. 10 212
 Google Scholar
Google Scholar
[11] Gusnard D A, Raichle M E 2001 Nat. Rev. Neurosci. 2 685
 Google Scholar
Google Scholar
[12] Li Y, Yao H, Lin P, Zheng L, Li C, Zhou B, Wang P, Zhang Z, Wang L, An N 2017 Front. Ag. Neurosci. 9 259
 Google Scholar
Google Scholar
[13] Anticevic A, Cole M W, Murray J D, Corlett P R, Wang X J, Krystal J H 2012 Trends Cogn. Sci. 16 584
 Google Scholar
Google Scholar
[14] Wang J, Wang Y, Wu X, Huang H, Jia Y, Zhong S, Wu X, Zhao L, He Y, Huang L, Huang R 2020 Brain Imaging Behav. 14 186
 Google Scholar
Google Scholar
[15] Zhao Q, Swati Z N, Metmer H, Sang X, Lu J 2019 Neurosci. Lett. 701 154
 Google Scholar
Google Scholar
[16] Greicius M D, Srivastava G, Reiss A L, Menon V 2004 Proc. Natl. Acad. Sci. U.S.A. 101 4637
 Google Scholar
Google Scholar
[17] Fassbender C, Zhang H, Buzy W M, Cortes C R, Mizuiri D, Beckett L, Schweitzer J B 2009 Brain Res. 1273 114
 Google Scholar
Google Scholar
[18] Uddin L Q, Kelly A M, Biswal B B, Margulies D S, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler L A, Castellanos F X, Milhama M P 2008 J. Neurosci. Methods 169 249
 Google Scholar
Google Scholar
[19] Manoliu A, Riedl V, Zherdin A, Mühlau M, Schwerthöffer D, Scherr M, Peters H, Zimmer C, Förstl H, Bäuml J, Wohlschläger A M, Sorg C 2014 Schizophrenia Bull. 40 428
 Google Scholar
Google Scholar
[20] Supekar K, Cai W, Krishnadas R, Palaniyappan L, Menon V 2019 Biol. Psychiatry 85 60
 Google Scholar
Google Scholar
[21] Bonnelle V, Ham T E, Leech R, Kinnunen K M, Mehta M A, Greenwood R J, Sharp D J 2012 Proc. Natl. Acad. Sci. U.S.A. 109 4690
 Google Scholar
Google Scholar
[22] Cui Y, Yu S, Zhang T, Zhang Y, Xia Y, Yao D, Guo D 2018 Brain Res. 1696 71
 Google Scholar
Google Scholar
[23] Wang S J, Ouyang G, Guang J, Zhang M, Wong K M, Zhou C 2016 Phys. Rev. Lett. 116 018101
 Google Scholar
Google Scholar
[24] Guo D, Guo F, Zhang Y, Li F, Xia Y, Xu P, Yao D 2018 Front. Comput. Neurosci. 12 21
 Google Scholar
Google Scholar
[25] Watanabe T, Rees G 2017 Nat. Commun. 8 1
 Google Scholar
Google Scholar
[26] Ashourvan A, Gu S, Mattar M G, Vettel J M, Bassett D S 2017 Neuroimage 157 364
 Google Scholar
Google Scholar
[27] Lee H, Lee D S, Kang H, Kim B N, Chung M K 2011 IEEE Trans. Med. Imaging 30 1154
 Google Scholar
Google Scholar
[28] Sugihara G, May R, Ye H, Hsieh C H, Deyle E, Fogarty M, Munch S 2012 Science 338 496
 Google Scholar
Google Scholar
[29] Finn E S, Shen X, Scheinost D, Rosenberg M D, Huang J, Chun M M, Papademetris X, Constable R T 2015 Nat. Neurosci. 18 1664
 Google Scholar
Google Scholar
[30] Qian J, Diez I, Ortiz-Terán L, Bonadio C, Liddell T, Goñi J, Sepulcre J 2018 Front. Neurosci. 12 38
 Google Scholar
Google Scholar
-
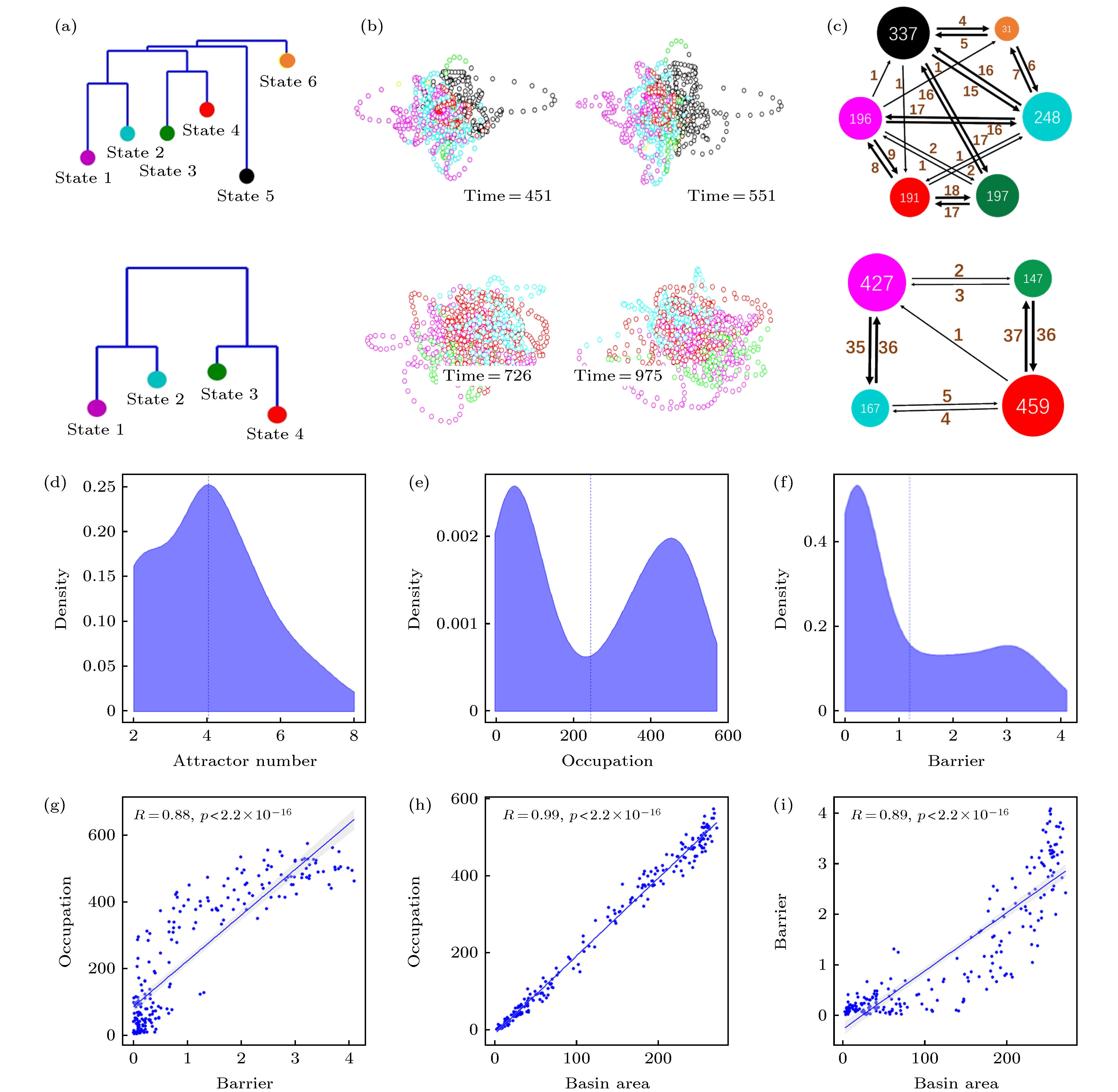
图 3 默认模式网络(DMN)激活行为的动力学特征 (a) dDMN状态能量图景中吸引子状态的非联通图(disconnectivity graph); (b)根据吸引子吸引域标签进行着色的DMN状态轨迹在3维空间的投影, 左右两图显示了同一被试轨迹不同角度的观察; (c)吸引子间跳转网络, 节点大小表示对应吸引域的访问次数, 点间有向边的权重表示对应的单向跳转频次, 总时长T = 1200. (a)−(c)中上下两行分别对应于被试1和被试2的结果; (d)−(i)为55个被试能量图景的统计分布特征: (d)吸引子数目的统计分布; (e)吸引域占据率(访问次数)的统计分布; (f)能量壁垒(势阱深度)的统计分布; (g)吸引域占据率与能量壁垒的关系; (h) 吸引域占据率与吸引域面积的关系; (i) 能量壁垒和吸引域面积的关系
Figure 3. Dynamic characteristics of the resting state default mode network (DMN): (a) Disconnectivity graph of the energy landscape for the dDMN; (b) projections of the DMN state trajectories, colored according to the labels of attraction basins, in a 3-dimensional space. The left and right panels show the trajectory of the same subject from different viewing angles, respectively; (c) attractors transition networks. The nodal size indicates the visiting frequency in the corresponding attracting domain, and the weight of directed edge indicates the corresponding directed switching frequency, with the recording time T = 1200. The upper and lower rows in the panels (a)−(c) correspond to the results of test subjects 1 and 2, respectively. Panels (d)−(i) are the statistical results of energy landscapes for 55 subjects: (d) The distribution of attractor numbers; (e) the distribution of basin occupations; (f) the distribution of energy barriers; (g) dependence of basin occupation and energy barrier; (h) dependence of basin occupation and area; (i) dependence of energy barrier and area.
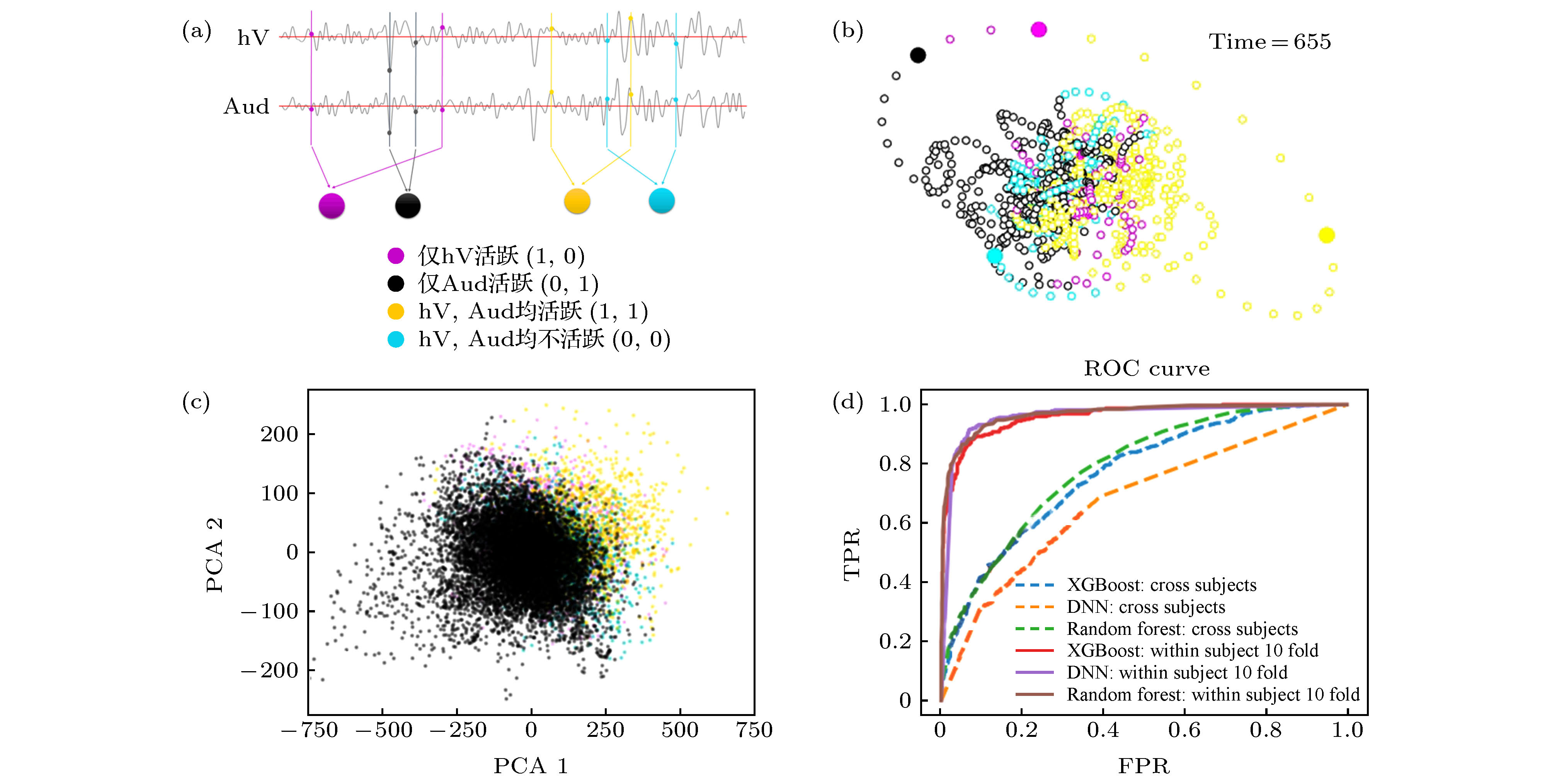
图 4 默认模式网络(DMN)状态与其他脑区激活行为的共变 (a)对高级视觉皮层(hV)和听觉皮层(Aud)活动二值化, 得到4种激活状态的(hV, Aud)标签序列; (b)根据(hV, Aud)标签序列着色的DMN状态轨道; (c) DMN的23个脑区激活行为在PCA1-PAC2空间的投影; (d)对DMN的激活行为数据按照同时刻(hV, Aud)标签进行4分类训练所得ROC曲线, 其中包括XGBoost, DNN, Random Forest三种方法及两种训练方式
Figure 4. Covariation of the default mode network (DMN) states with activity mode in other brain regions: (a) Binarizing the activities of high-level visual cortex (hV) and auditory cortex (Aud) to obtain (hV, Aud) label sequence; (b) DMN state orbit colored according to (hV, Aud) label sequence; (c) projection of the activity states of the 23 brain regions in the PCA1-PAC2 space; (d) the ROC curve obtained by performing 4-classes training on the (hV, Aud) labels of DMN activity data by XGBoost, DNN, Random Forest with two training schemes.
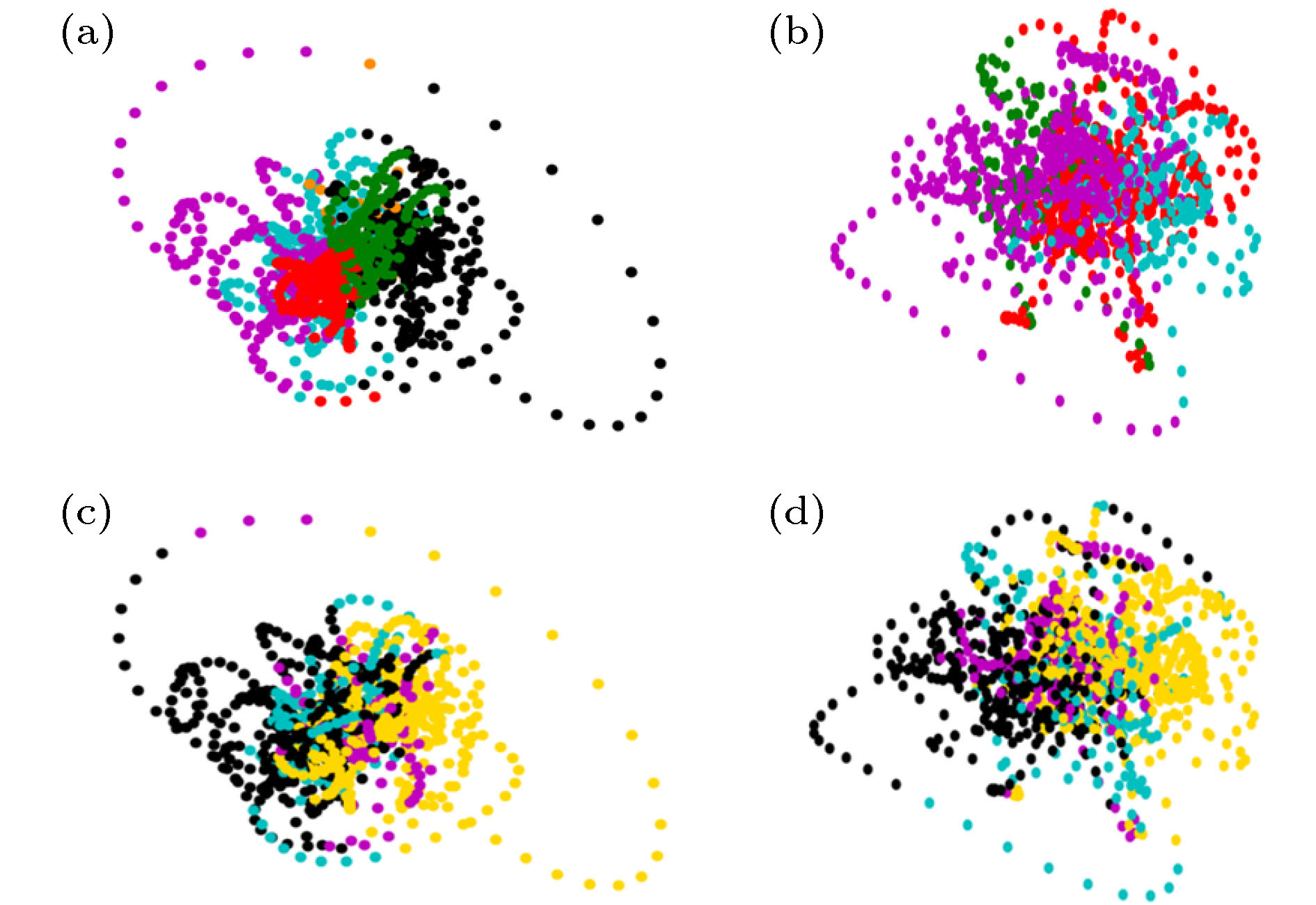
图 5 DMN状态与视听觉皮层状态的对应现象 (a), (b)根据吸引子的吸引域标签着色的DMN状态轨道; (c), (d) 根据(hV, Aud)标签序列着色的DMN状态轨道; (a), (c)被试1的结果; (b)(d)被试2的结果
Figure 5. Correspondence between the states of DMN and (visual, auditory) cortexes: (a), (b) DMN state orbits colored according to the labels of attraction basin; (c), (d) DMN state orbits colored according to the labels of (hV, Aud) states. (a), (c) the results of subject 1; (b)(d) the results of subject 2.
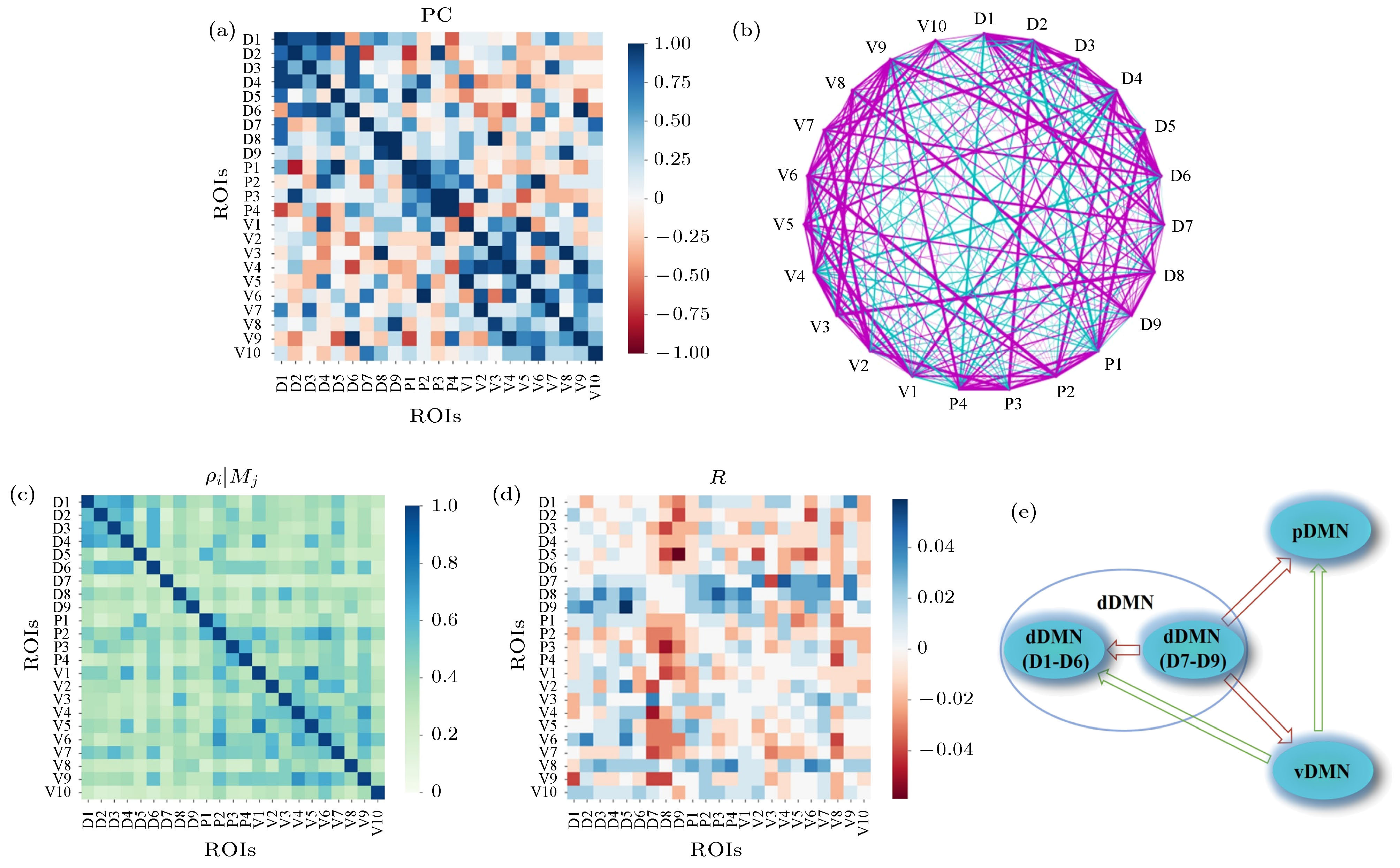
图 6 DMN内部23个ROI间关系分析 (a), (b)基于压缩感知的偏相关矩阵及网络结构, 网络中紫色线表示正相关, 青色线表示负相关, 线的粗细表示相关性强弱; (c) CCM方法中得到的ROI之间的单向影响程度
${\rho _{i|{M_j}}}$ , 延迟坐标嵌入维数为2, 延迟为1, 下同; (d) CCM方法得到的ROI间动力学因果性矩阵; (e)对应的ROI间动力学影响的粗粒化结果Figure 6. Relationships of the 23 ROIs within DMN: (a) Partial correlation matrix calculated based on compressive sensing; (b) the corresponding network structure, which includes the positive (magenta links) and negative (cyan links) correlations. The width of the line indicates the correlation strength; (c) the directed dynamical influence from ROI i to j,
${\rho _{i|{M_j}}}$ , obtained in the CCM method. The delay coordinate embedding dimension is 2 and the delay is 1; (d) the causality matrix obtained by CCM; (e) the coarse-grained result of the corresponding influence among ROIs within DMN.表 1 默认模式网络ROI的名称、标签、Brodmann分区(BA)编号及位置坐标信息
Table 1. Name, label, Brodmann area (BA) number, and location information of ROIs belonging to the default mode network
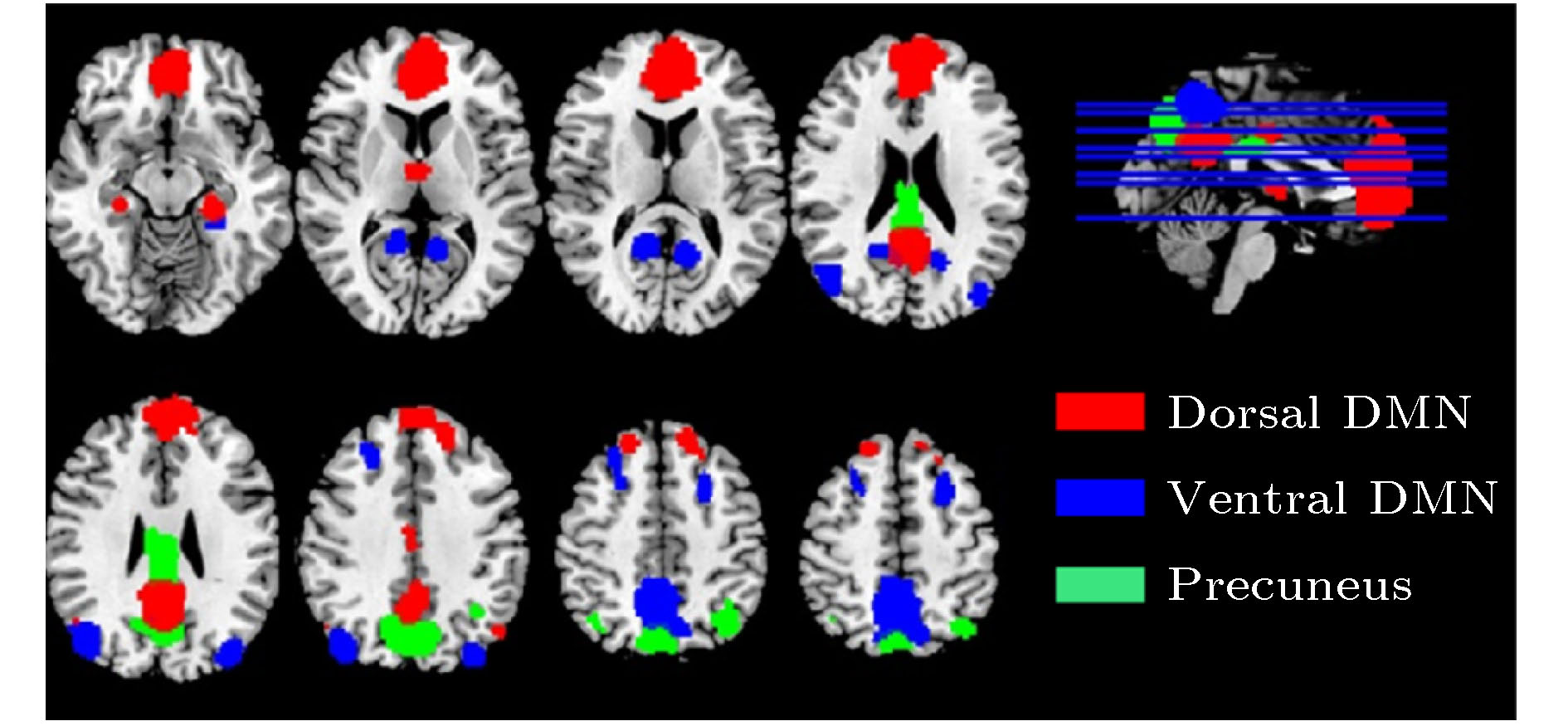
ROI Label L/R BA X Y Z Posterior Cingulate vDMN_1 L 31 –12 –62 10 Middle Frontal Gyrus vDMN_2 L 10 –27 –6 59 Culmen vDMN_3 L 37 –30 –39 –20 Superior Occipital Gyrus vDMN_4 L 19 –36 –88 28 Posterior Cingulate Gyrus vDMN_5 R 31 15 –56 13 Precuneus vDMN_6 7 –6 –61 56 Middle Frontal Gyrus vDMN_7 R 10 24 26 47 Culmen vDMN_8 R 37 27 –33 –23 Angular Gyrus vDMN_9 R 39 43 –79 28 Cerebellum vDMN_10 R 12 –47 –63 Ventral Posterior Cingulate Gyrus pDMN_1 23 0 –35 28 Precuneus pDMN_2 7 0 –76 38 Inferior Parietal Lobule pDMN_3 L 40 –39 –64 46 Inferior Parietal Lobule pDMN_4 R 40 39 –64 46 Middle Frontal Gyrus dDMN_1 9 0 49 12 Angular Gyrus dDMN_2 L 39 –48 –73 32 Superior Frontal Gyrus dDMN_3 R 6 18 38 51 Dorsal Posterior Cingulate Gyrus dDMN_4 31 0 –57 30 Ventral Anterior Cingulate Gyrus dDMN_5 24 0 –17 35 Angular Gyrus dDMN_6 R 39 48 –66 29 Thalamus dDMN_7 –6 –6 3 Parahippocampal Gyrus dDMN_8 L 36 –24 –37 –9 Parahippocampal Gyrus dDMN_9 R 36 24 –21 –23 -
[1] Sarraf S, Sun J 2016 arXiv: 1602.02225 [physics.med-ph]
[2] Liu C, Zhou C, Wang J, Loparo K 2018 IEEE Trans. Neural Syst. Rehabil. Eng. 26 1649
 Google Scholar
Google Scholar
[3] Lei Y, Song B, Chen L, Su J, Zhang X, Ni W, Yu Y, Xu B, Yu L, Gu Y, Mao Y 2018 Brain Imaging Behav. 11682
 Google Scholar
Google Scholar
[4] Chen J E, Glover G H, Greicius M D, Chang C 2017 Hum. Brain. Mapp. 38 2454
 Google Scholar
Google Scholar
[5] Chen B, Li X 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC) Banff, Canada, October 1–4, 2017 p2820
[6] Roberto T, Fox P Tomás P J 2008 Cereb. Cortex 18 2553
 Google Scholar
Google Scholar
[7] Raichle M E, Macleod A M, Snyder A Z, Powers W J, Gusnard D A, d Shulman G L 2001 Proc. Natl. Acad. Sci. U.S.A. 98 676
 Google Scholar
Google Scholar
[8] Greicius M D, Ben K, Reiss A L, Vinod M 2003 Proc. Natl. Acad. Sci. U.S.A. 100 253
 Google Scholar
Google Scholar
[9] Greicius M D, Kaustubh S, Vinod M, Dougherty R F 2009 Cereb. Cortex 19 72
 Google Scholar
Google Scholar
[10] Lin P, Yang Y, Jovicich J, Pisapia N D, Wang X, Zuo C S, Levitt J J 2016 Brain Imaging Behav. 10 212
 Google Scholar
Google Scholar
[11] Gusnard D A, Raichle M E 2001 Nat. Rev. Neurosci. 2 685
 Google Scholar
Google Scholar
[12] Li Y, Yao H, Lin P, Zheng L, Li C, Zhou B, Wang P, Zhang Z, Wang L, An N 2017 Front. Ag. Neurosci. 9 259
 Google Scholar
Google Scholar
[13] Anticevic A, Cole M W, Murray J D, Corlett P R, Wang X J, Krystal J H 2012 Trends Cogn. Sci. 16 584
 Google Scholar
Google Scholar
[14] Wang J, Wang Y, Wu X, Huang H, Jia Y, Zhong S, Wu X, Zhao L, He Y, Huang L, Huang R 2020 Brain Imaging Behav. 14 186
 Google Scholar
Google Scholar
[15] Zhao Q, Swati Z N, Metmer H, Sang X, Lu J 2019 Neurosci. Lett. 701 154
 Google Scholar
Google Scholar
[16] Greicius M D, Srivastava G, Reiss A L, Menon V 2004 Proc. Natl. Acad. Sci. U.S.A. 101 4637
 Google Scholar
Google Scholar
[17] Fassbender C, Zhang H, Buzy W M, Cortes C R, Mizuiri D, Beckett L, Schweitzer J B 2009 Brain Res. 1273 114
 Google Scholar
Google Scholar
[18] Uddin L Q, Kelly A M, Biswal B B, Margulies D S, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler L A, Castellanos F X, Milhama M P 2008 J. Neurosci. Methods 169 249
 Google Scholar
Google Scholar
[19] Manoliu A, Riedl V, Zherdin A, Mühlau M, Schwerthöffer D, Scherr M, Peters H, Zimmer C, Förstl H, Bäuml J, Wohlschläger A M, Sorg C 2014 Schizophrenia Bull. 40 428
 Google Scholar
Google Scholar
[20] Supekar K, Cai W, Krishnadas R, Palaniyappan L, Menon V 2019 Biol. Psychiatry 85 60
 Google Scholar
Google Scholar
[21] Bonnelle V, Ham T E, Leech R, Kinnunen K M, Mehta M A, Greenwood R J, Sharp D J 2012 Proc. Natl. Acad. Sci. U.S.A. 109 4690
 Google Scholar
Google Scholar
[22] Cui Y, Yu S, Zhang T, Zhang Y, Xia Y, Yao D, Guo D 2018 Brain Res. 1696 71
 Google Scholar
Google Scholar
[23] Wang S J, Ouyang G, Guang J, Zhang M, Wong K M, Zhou C 2016 Phys. Rev. Lett. 116 018101
 Google Scholar
Google Scholar
[24] Guo D, Guo F, Zhang Y, Li F, Xia Y, Xu P, Yao D 2018 Front. Comput. Neurosci. 12 21
 Google Scholar
Google Scholar
[25] Watanabe T, Rees G 2017 Nat. Commun. 8 1
 Google Scholar
Google Scholar
[26] Ashourvan A, Gu S, Mattar M G, Vettel J M, Bassett D S 2017 Neuroimage 157 364
 Google Scholar
Google Scholar
[27] Lee H, Lee D S, Kang H, Kim B N, Chung M K 2011 IEEE Trans. Med. Imaging 30 1154
 Google Scholar
Google Scholar
[28] Sugihara G, May R, Ye H, Hsieh C H, Deyle E, Fogarty M, Munch S 2012 Science 338 496
 Google Scholar
Google Scholar
[29] Finn E S, Shen X, Scheinost D, Rosenberg M D, Huang J, Chun M M, Papademetris X, Constable R T 2015 Nat. Neurosci. 18 1664
 Google Scholar
Google Scholar
[30] Qian J, Diez I, Ortiz-Terán L, Bonadio C, Liddell T, Goñi J, Sepulcre J 2018 Front. Neurosci. 12 38
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 18235
- PDF Downloads: 355
- Cited By: 0














 DownLoad:
DownLoad: