-
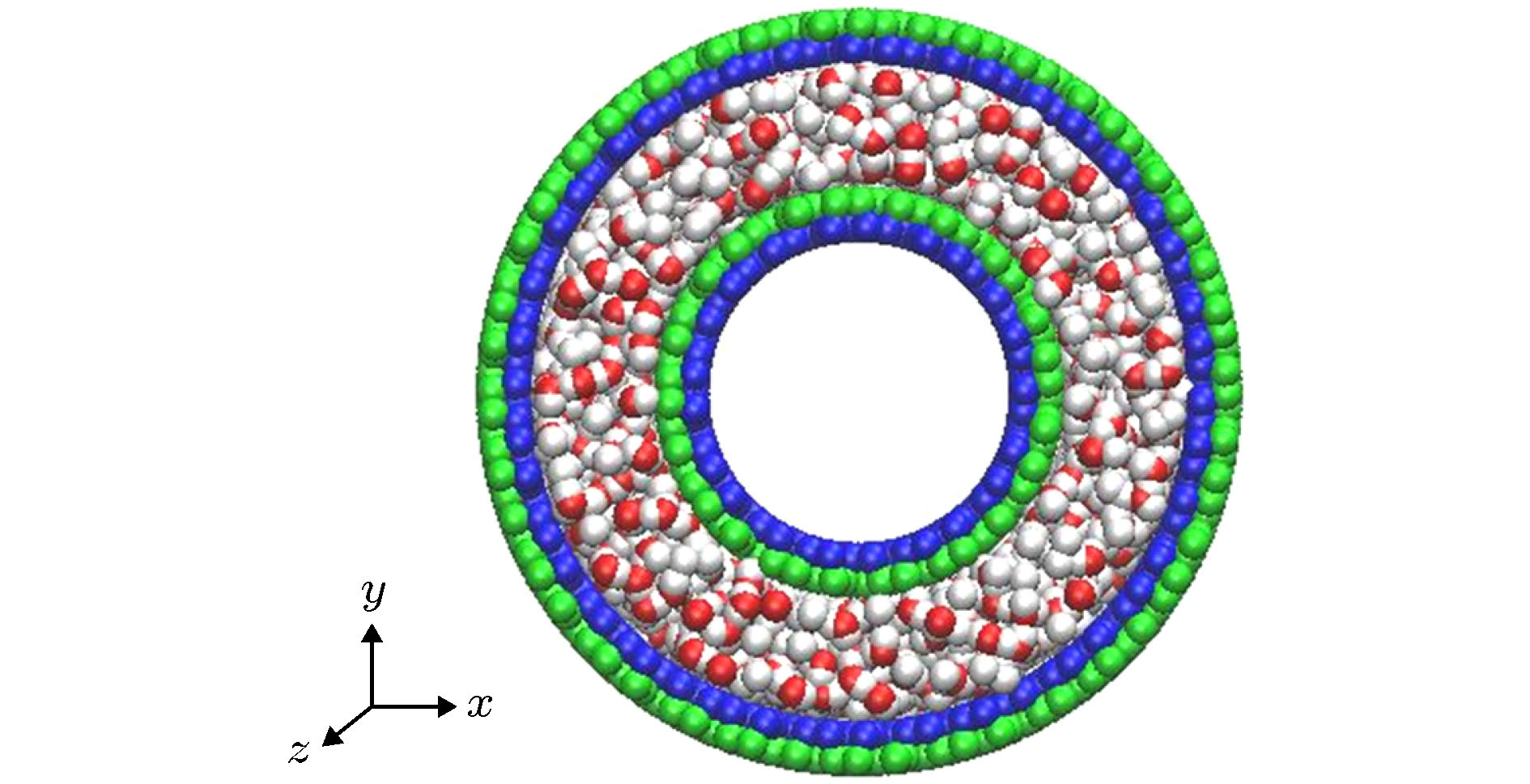
Since the advent of two-dimensional materials, the micro/nano technology has been greatly developed, and the design of micro/nano fluid devices has become an important research area. As a new two-dimensional material, the black phosphorus (BP) has attracted wide attention because of its excellent properties such as anisotropy, and it has been applied to many areas. In this paper, the axial motion properties of water molecules in the rotating black phosphorus nanotube (BPNT) are studied by the molecular dynamics method. The results show that water molecules in the rotating chiral BPNT can move along the axis, and the moving direction of water molecules is determined by the rotating direction of the nanotube. The velocity of water molecules and the resultant force of water molecules received from the nanotube in the axial direction increase with the angular velocity increasing. The friction coefficient and slip characteristics of the water-BP interface are calculated by using the Couette flow model, and it is clarified that the natural anisotropic microstructure on the surface of BP is the essential reason for the axial motion of water molecules in the rotating BPNT. Besides, we construct a model of filling water molecules between two BPNTs. It is found that the axial movement of water molecules between two nanotubes will be enhanced when the internal and external tube rotate simultaneously. The radius of the nanotubes will also affect the directional motion of the water molecules. Specifically, at the same angular velocity of BPNTs, with the increase of the radius, the axial motion velocity of water molecules in the BPNT will decrease, while the force received from the BPNT will increase. The axial motion of water molecules in the double-walled BPNT is little different from that in the single-walled BPNT, which proves that the number of layers has no significant influence on the driving effect of water molecules. The influence of temperature on the motion properties of water molecules depends on the coupling effect of pressure and temperature in the tube on the convection-solid interface friction coefficient. When the temperature is lower than the normal temperature, the axial velocity of water molecules and the force exerted by the BPNT will increase with the increase of temperature, and when the temperature reaches the normal temperature, it will become stable. The results will provide a theoretical basis for the study of the flow characteristics of the fluid in BPNTs and the application of the fluid drive devices based on BPNTs.
-
Keywords:
- black phosphorus nanotubes /
- rotation /
- directional motion /
- molecular dynamics
[1] Novoselov K S, Fal'ko V I, Colombo L, Gellert P R, Schwab M G, Kim K 2012 Nature 490 192
 Google Scholar
Google Scholar
[2] Naumis G G, Barraza-Lopez S, Oliva-Leyva M, Terrones H 2017 Rep. Prog. Phys. 80 096501
 Google Scholar
Google Scholar
[3] Stampfer C, Jungen A, Linderman R, Obergfell D, Roth S, Hierold C 2006 Nano Lett. 6 1449
 Google Scholar
Google Scholar
[4] So H M, Sim J W, Kwon J, Yun J, Baik S, Chang W S 2013 Mater. Res. Bull. 48 5036
 Google Scholar
Google Scholar
[5] Cagatay E, Kohler P, Lugli P, Abdellah A 2015 IEEE Sens. J. 15 3225
 Google Scholar
Google Scholar
[6] Turlo V, Politano O, Baras F 2015 Acta Materialia. 99 363
 Google Scholar
Google Scholar
[7] Thomas J A, McGaughey A J H 2008 Nano Lett. 8 2788
 Google Scholar
Google Scholar
[8] Longhurst M J, Quirke N 2007 Nano Lett. 7 3324
 Google Scholar
Google Scholar
[9] Yang X P, Yang X N, Liu S Y 2015 Chinese. J. Chem. Eng. 23 1587
 Google Scholar
Google Scholar
[10] Zhang Z Q, Ye H F, Liu Z, Ding J N, Cheng G G, Ling Z Y, Zheng Y G, Wang L, Wang J B 2012 J. Appl. Phys. 111 114304
 Google Scholar
Google Scholar
[11] Wang L Y, Wu H A, Wang F C 2017 Sci. Rep. 7 41717
 Google Scholar
Google Scholar
[12] Lu W L, Nan H Y, Hong J H, Chen Y M, Zhu C, Liang Z, Ma X Y, Ni Z H, Jin C H, Zhang, Z 2014 Nano Res. 7 853
 Google Scholar
Google Scholar
[13] Pang J B, Bachmatiuk A, Yin Y, Trzebicka B, Zhao L, Fu L, Mendes R G, Gemming T, Liu Z F, Rummeli M H 2018 Adv. Energy Mater. 8 1702093
 Google Scholar
Google Scholar
[14] Qiao J S, Kong X H, Hu Z X, Yang F, Ji W 2014 Nat. Commun. 5 4475
 Google Scholar
Google Scholar
[15] Hu T, Han Y, Dong J M 2014 Nanotechnology 25 455703
 Google Scholar
Google Scholar
[16] Yang Z Y, Zhao J H, Wei N 2015 Appl. Phys. Lett. 107 023107
 Google Scholar
Google Scholar
[17] Zhao J L, Zhu J J, Cao R, Wang H D, Guo Z N, Sang D K, Tang J N, Fan D Y, Li J Q, Zhang H 2019 Nat. Commun. 10 4062
 Google Scholar
Google Scholar
[18] Hyun C, Kin J H, Lee J Y, Lee G H, Kim K S 2020 RSC Adv. 10 350
 Google Scholar
Google Scholar
[19] Zhang Z Q, Liu H L, Liu Z, Zhang Z, Cheng G G, Wang X D, Ding J N 2019 Appl. Surf. Sci. 475 857
 Google Scholar
Google Scholar
[20] 张忠强, 刘汉伦, 范晋伟, 丁建宁, 程广贵 2019 68 170202
 Google Scholar
Google Scholar
Zhang Z Q, Liu H L, Fan J W, Ding J N, Cheng G G 2019 Acta Phys. Sin. 68 170202
 Google Scholar
Google Scholar
[21] Cai K, Wan J, Wei N, Qin Q H 2016 Nanotechnology 27 275701
 Google Scholar
Google Scholar
[22] Hao F, Liao X B, Xiao H, Chen X 2016 Nanotechnology 27 155703
 Google Scholar
Google Scholar
[23] Fernández-Escamilla H N, Quijano-Briones J J, Tlahuice-Flores A 2016 Phys. Chem. Chem. Phys. 18 12414
 Google Scholar
Google Scholar
[24] Horn H W, Swope W C, Pitera J W, Madura J D, Dick T J, Hura G L, Head-Gordon T 2004 J. Chem. Phys. 120 9665
 Google Scholar
Google Scholar
[25] Zhang H W, Ye H F, Zheng Y G, Zhang Z Q 2011 Microfluid. Nanofluid. 10 403
 Google Scholar
Google Scholar
[26] Cai K, Liu L, Shi J, Qin Q H 2017 Mater. Des. 121 406
 Google Scholar
Google Scholar
[27] Ryckaert J P, Ciccotti G, Berendsen H J C 1977 J. Comput. Phys. 23 327
 Google Scholar
Google Scholar
[28] Hou Q W, Cao B Y, Guo Z Y 2009 Nanotechnology 20 495503
 Google Scholar
Google Scholar
-
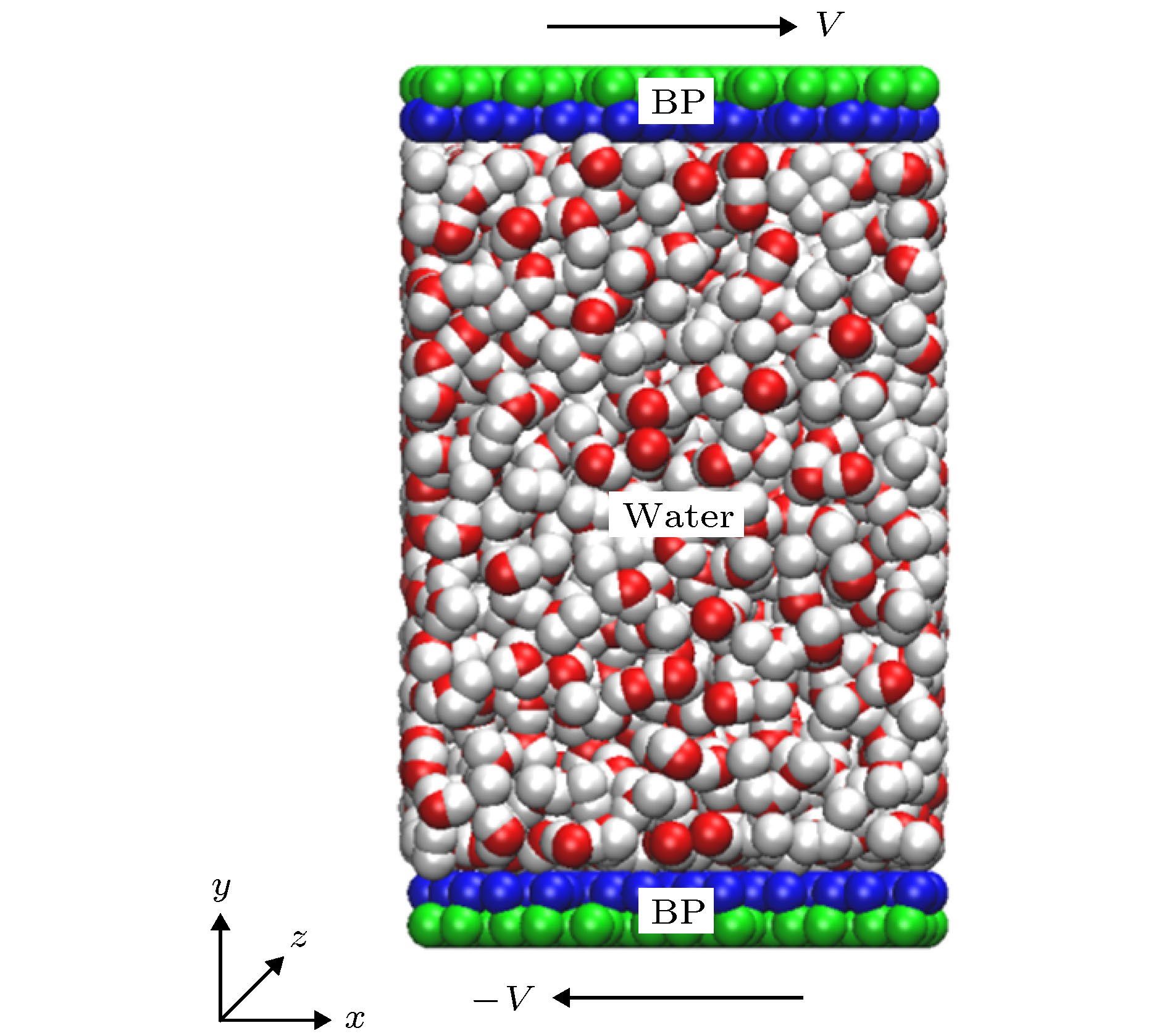
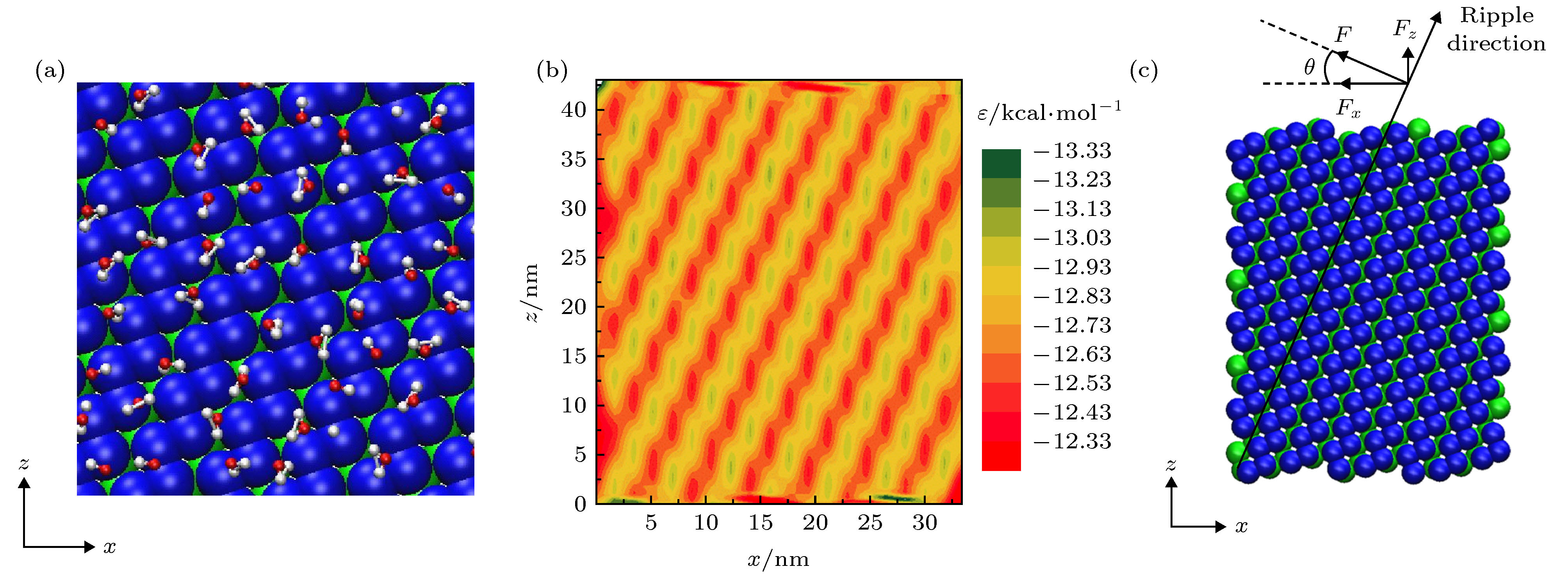
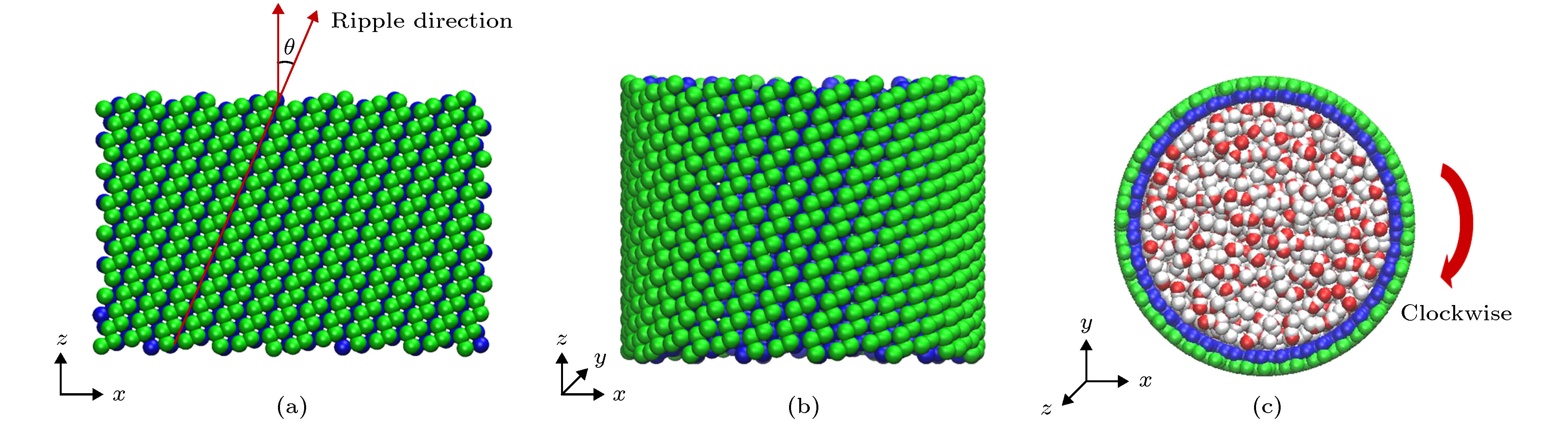
图 1 (a)单层黑磷模型, 其中手性角度θ指黑磷褶皱方向与z轴方向(纳米管轴向)的夹角; (b)手性角度为23.4°的黑磷纳米管; (c)填充水分子的黑磷纳米管旋转模型图
Figure 1. (a) Monolayer black phosphorus model, chiral angle θ is the intersection angle between the ripple direction of BP monolayer and z direction (the axial direction of the BPNT); (b) BPNT with a chiral angle of 23.4°; (c) model of the rotating BPNT filled with water molecules.
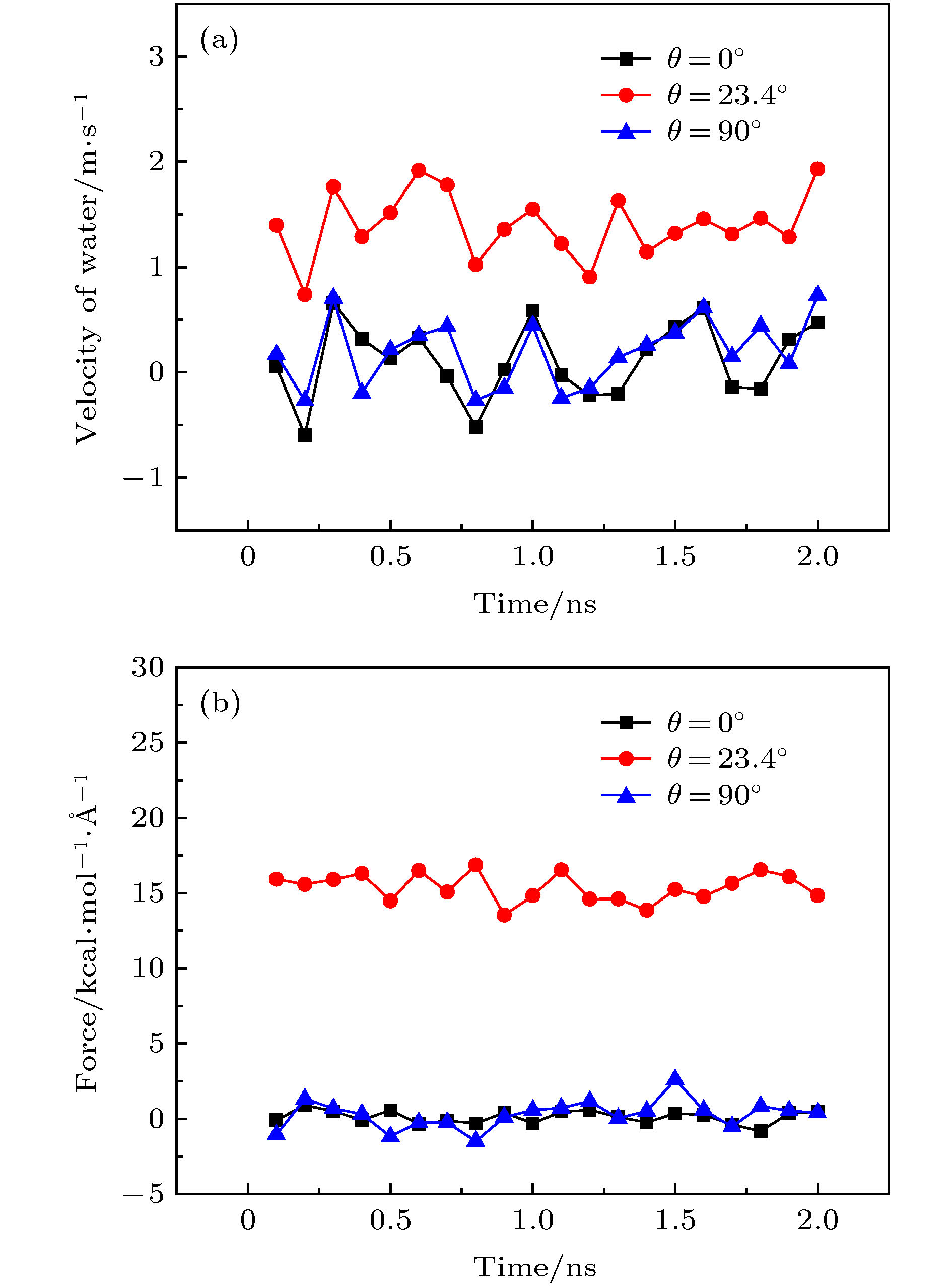
图 2 不同手性角度的黑磷纳米管以50 rad/ns的转速顺时针旋转时管内水分子沿轴线方向的(a)速度和(b)受力随时间的变化关系
Figure 2. For the angular velocity of the BPNT being 50 rad/ns, (a) the velocity in the axial direction of water molecules in BPNTs and (b) the resultant force in the axial direction of water molecules received from BPNTs with different chiral angles as a function of time.
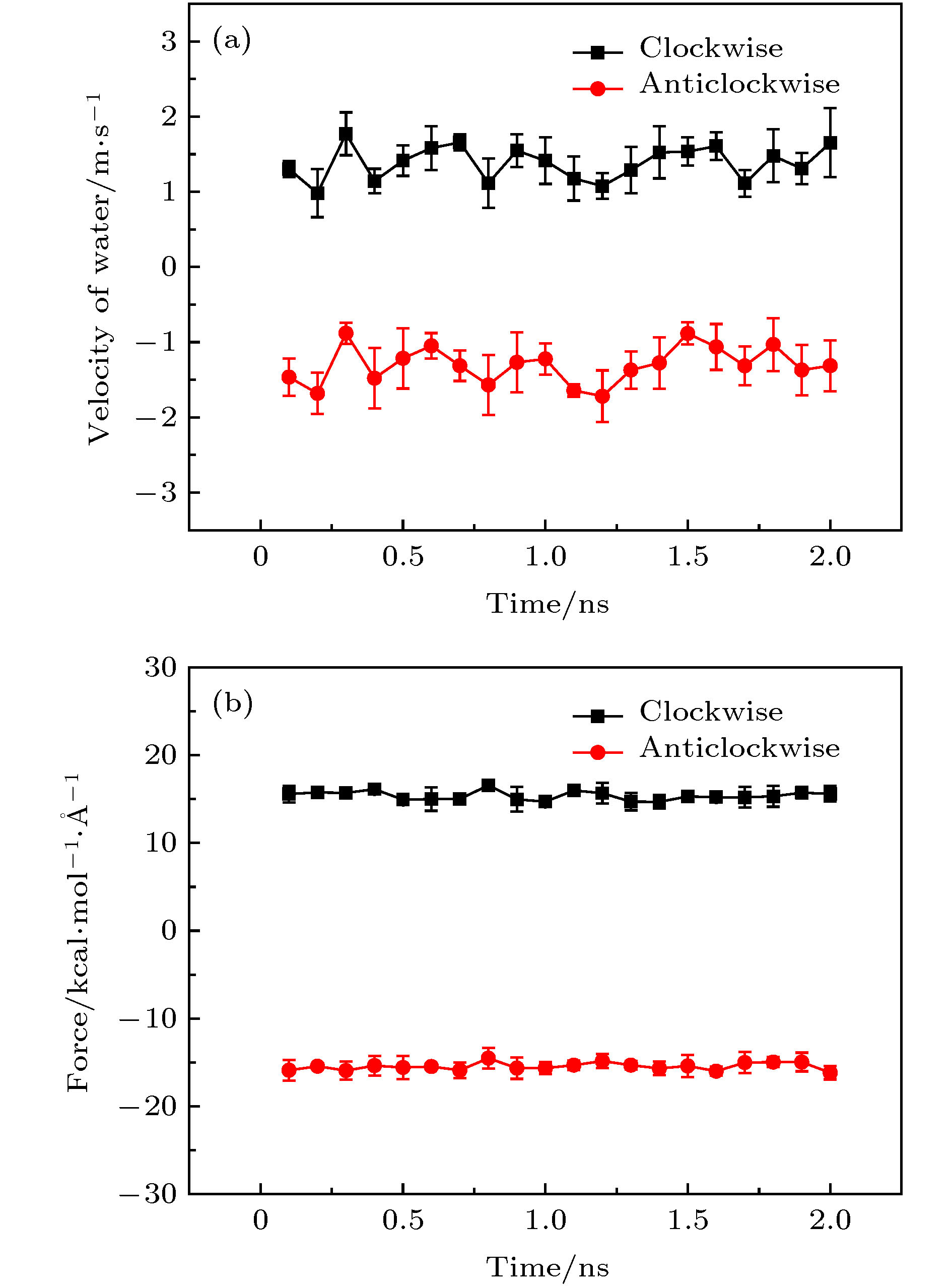
图 3 手性角度为23.4°的黑磷纳米管以50 rad/ns的转速沿不同方向旋转时管内水分子沿轴向的(a)速度和(b)受力随时间的变化关系
Figure 3. For the angular velocity of the BPNT being 50 rad/ns in different directions of rotation, (a) the velocity in the axial direction of water molecules in the BPNT and (b) the resultant force in the axial direction of water molecules received from the BPNT as a function of time when the chiral angle is 23.4°.
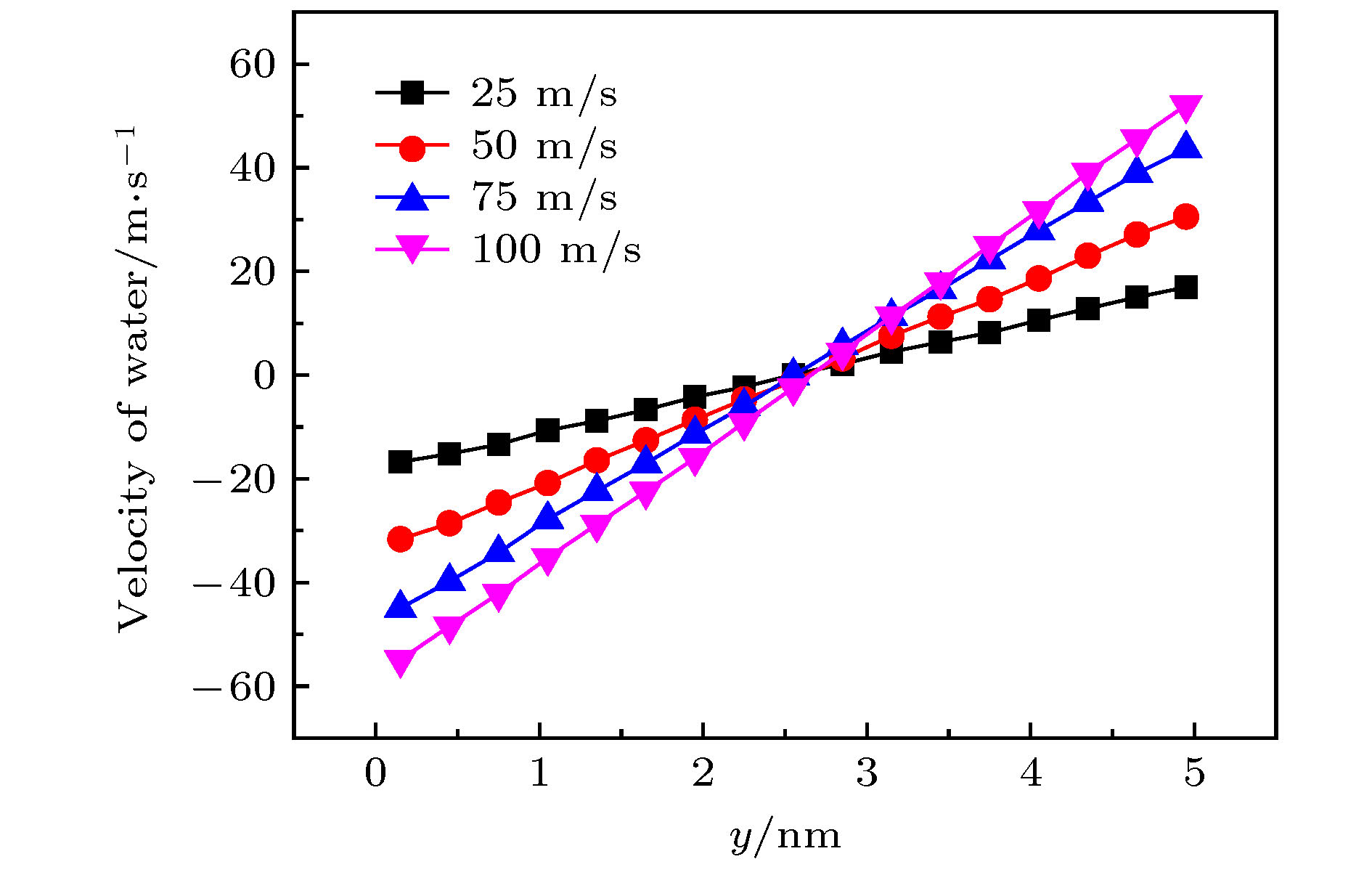
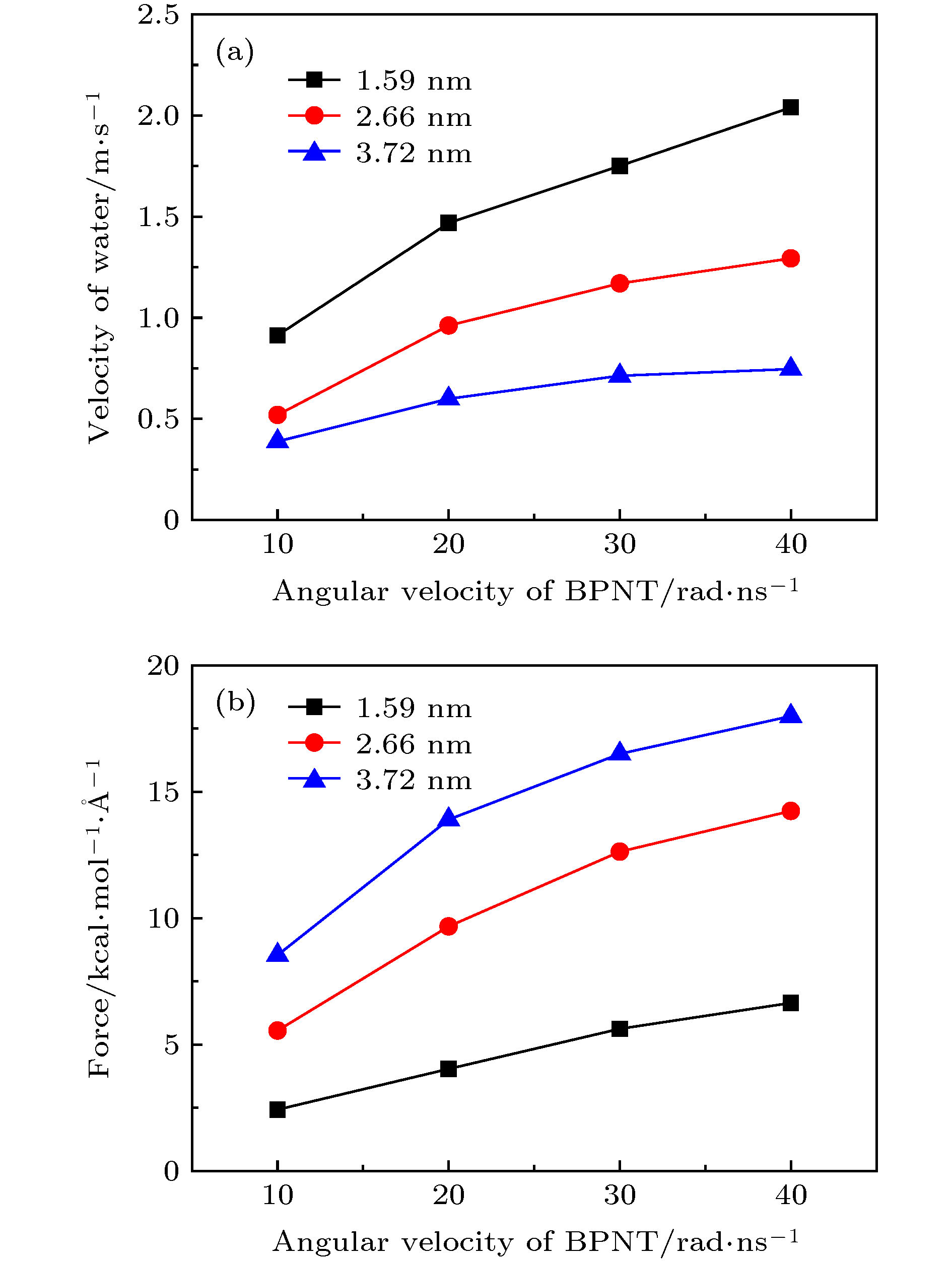
图 11 手性角度为23.4°时, 不同半径的黑磷纳米管内水分子沿轴线方向的(a)速度和(b)受力随黑磷纳米管转速的变化关系
Figure 11. For different radius, (a) the velocity in the axial direction of water molecules in BPNTs and (b) the resultant force in the axial direction of water molecules received from BPNTs as a function of the angular velocity of BPNTs when the chiral angle is 23.4°.
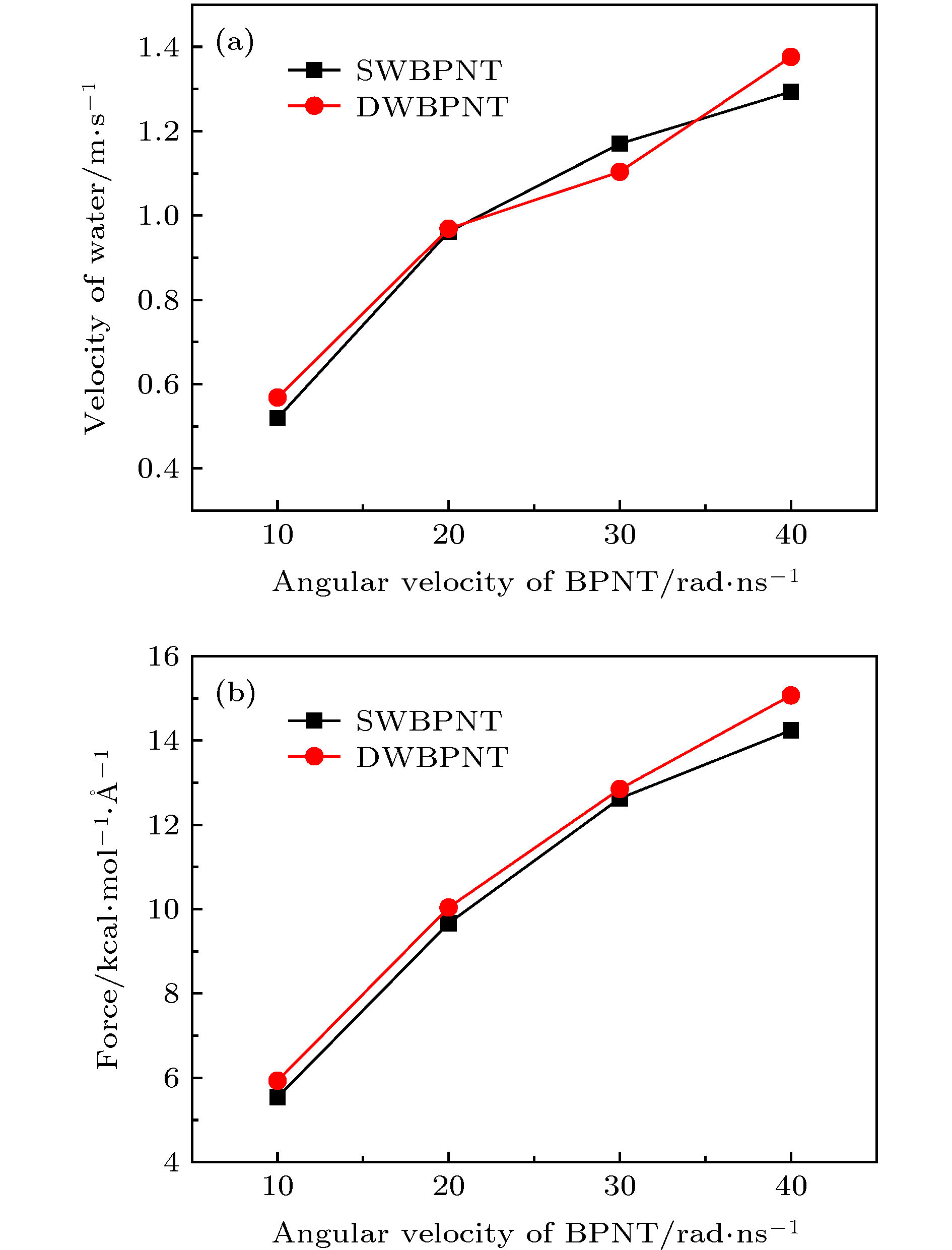
图 12 手性角度为23.4°时, 不同层数黑磷纳米管内水分子沿轴线方向的(a)速度和(b)受力随黑磷纳米管转速的变化关系
Figure 12. For different layers, (a) the velocity in the axial direction of water molecules in BPNTs and (b) the resultant force in the axial direction of water molecules received from BPNTs as a function of the angular velocity of BPNTs when the chiral angle is 23.4°.
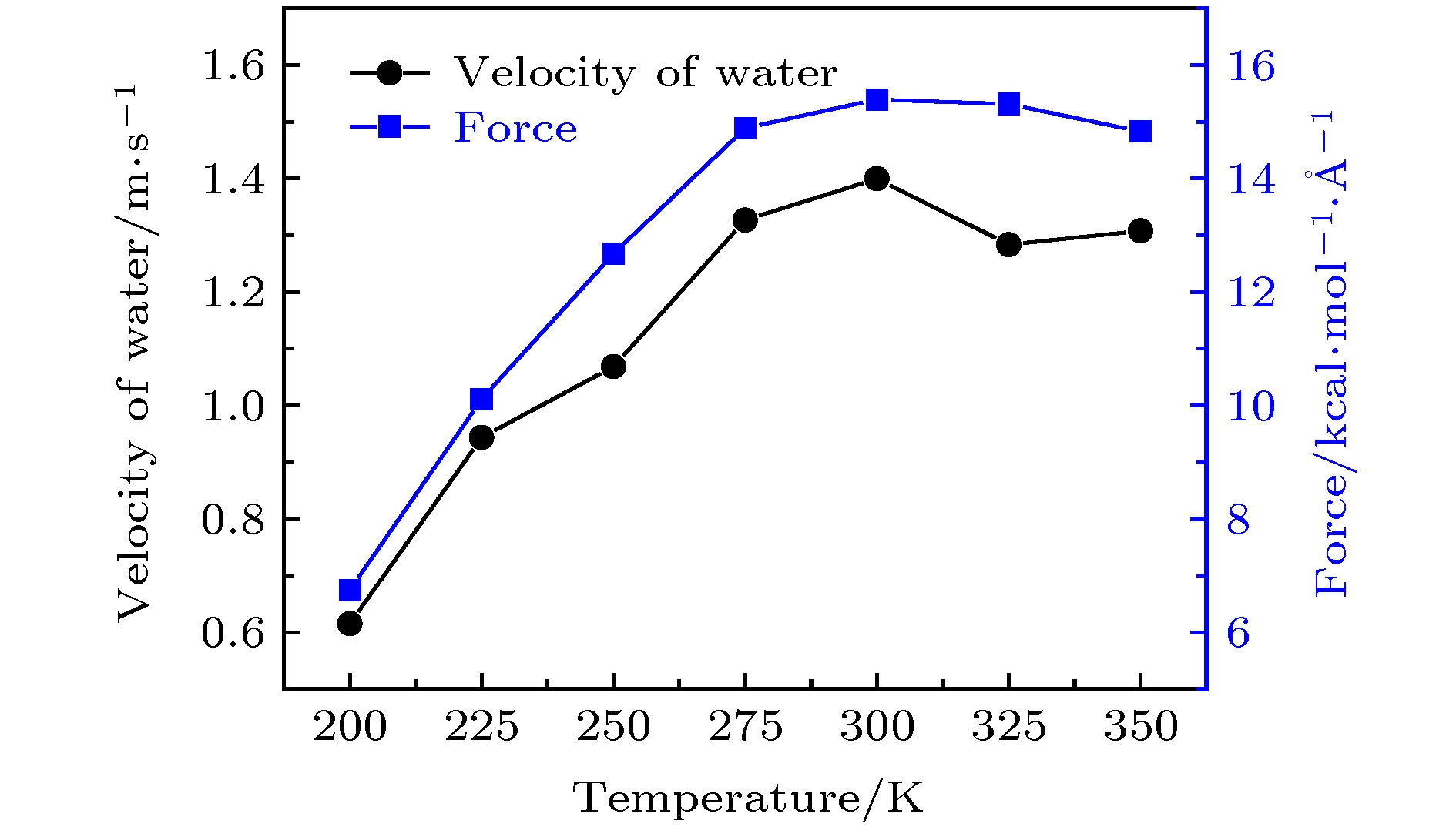
图 13 转速为50 rad/ns时, 手性角度为23.4°的黑磷纳米管内水分子的轴向速度与受力随温度的变化关系
Figure 13. For the angular velocity of the BPNT being 50 rad/ns, the velocity in the axial direction of water molecules in the BPNT and the resultant force in the axial direction of water molecules received from the BPNT as a function of the temperature when the chiral angle is 23.4°.
表 1 LJ势能函数的参数值
Table 1. Parameter values of LJ potential function
Atoms ε/kcal·mol–1 σ/Å P—P 0.36760 3.4380 O—O 0.16275 3.16435 P—O 0.24460 3.30120 -
[1] Novoselov K S, Fal'ko V I, Colombo L, Gellert P R, Schwab M G, Kim K 2012 Nature 490 192
 Google Scholar
Google Scholar
[2] Naumis G G, Barraza-Lopez S, Oliva-Leyva M, Terrones H 2017 Rep. Prog. Phys. 80 096501
 Google Scholar
Google Scholar
[3] Stampfer C, Jungen A, Linderman R, Obergfell D, Roth S, Hierold C 2006 Nano Lett. 6 1449
 Google Scholar
Google Scholar
[4] So H M, Sim J W, Kwon J, Yun J, Baik S, Chang W S 2013 Mater. Res. Bull. 48 5036
 Google Scholar
Google Scholar
[5] Cagatay E, Kohler P, Lugli P, Abdellah A 2015 IEEE Sens. J. 15 3225
 Google Scholar
Google Scholar
[6] Turlo V, Politano O, Baras F 2015 Acta Materialia. 99 363
 Google Scholar
Google Scholar
[7] Thomas J A, McGaughey A J H 2008 Nano Lett. 8 2788
 Google Scholar
Google Scholar
[8] Longhurst M J, Quirke N 2007 Nano Lett. 7 3324
 Google Scholar
Google Scholar
[9] Yang X P, Yang X N, Liu S Y 2015 Chinese. J. Chem. Eng. 23 1587
 Google Scholar
Google Scholar
[10] Zhang Z Q, Ye H F, Liu Z, Ding J N, Cheng G G, Ling Z Y, Zheng Y G, Wang L, Wang J B 2012 J. Appl. Phys. 111 114304
 Google Scholar
Google Scholar
[11] Wang L Y, Wu H A, Wang F C 2017 Sci. Rep. 7 41717
 Google Scholar
Google Scholar
[12] Lu W L, Nan H Y, Hong J H, Chen Y M, Zhu C, Liang Z, Ma X Y, Ni Z H, Jin C H, Zhang, Z 2014 Nano Res. 7 853
 Google Scholar
Google Scholar
[13] Pang J B, Bachmatiuk A, Yin Y, Trzebicka B, Zhao L, Fu L, Mendes R G, Gemming T, Liu Z F, Rummeli M H 2018 Adv. Energy Mater. 8 1702093
 Google Scholar
Google Scholar
[14] Qiao J S, Kong X H, Hu Z X, Yang F, Ji W 2014 Nat. Commun. 5 4475
 Google Scholar
Google Scholar
[15] Hu T, Han Y, Dong J M 2014 Nanotechnology 25 455703
 Google Scholar
Google Scholar
[16] Yang Z Y, Zhao J H, Wei N 2015 Appl. Phys. Lett. 107 023107
 Google Scholar
Google Scholar
[17] Zhao J L, Zhu J J, Cao R, Wang H D, Guo Z N, Sang D K, Tang J N, Fan D Y, Li J Q, Zhang H 2019 Nat. Commun. 10 4062
 Google Scholar
Google Scholar
[18] Hyun C, Kin J H, Lee J Y, Lee G H, Kim K S 2020 RSC Adv. 10 350
 Google Scholar
Google Scholar
[19] Zhang Z Q, Liu H L, Liu Z, Zhang Z, Cheng G G, Wang X D, Ding J N 2019 Appl. Surf. Sci. 475 857
 Google Scholar
Google Scholar
[20] 张忠强, 刘汉伦, 范晋伟, 丁建宁, 程广贵 2019 68 170202
 Google Scholar
Google Scholar
Zhang Z Q, Liu H L, Fan J W, Ding J N, Cheng G G 2019 Acta Phys. Sin. 68 170202
 Google Scholar
Google Scholar
[21] Cai K, Wan J, Wei N, Qin Q H 2016 Nanotechnology 27 275701
 Google Scholar
Google Scholar
[22] Hao F, Liao X B, Xiao H, Chen X 2016 Nanotechnology 27 155703
 Google Scholar
Google Scholar
[23] Fernández-Escamilla H N, Quijano-Briones J J, Tlahuice-Flores A 2016 Phys. Chem. Chem. Phys. 18 12414
 Google Scholar
Google Scholar
[24] Horn H W, Swope W C, Pitera J W, Madura J D, Dick T J, Hura G L, Head-Gordon T 2004 J. Chem. Phys. 120 9665
 Google Scholar
Google Scholar
[25] Zhang H W, Ye H F, Zheng Y G, Zhang Z Q 2011 Microfluid. Nanofluid. 10 403
 Google Scholar
Google Scholar
[26] Cai K, Liu L, Shi J, Qin Q H 2017 Mater. Des. 121 406
 Google Scholar
Google Scholar
[27] Ryckaert J P, Ciccotti G, Berendsen H J C 1977 J. Comput. Phys. 23 327
 Google Scholar
Google Scholar
[28] Hou Q W, Cao B Y, Guo Z Y 2009 Nanotechnology 20 495503
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 13099
- PDF Downloads: 101
- Cited By: 0














 DownLoad:
DownLoad: