-
甲烷水合物具有分布范围广、资源储量大、能量密度高、清洁无污染等特点, 因此一经发现便得到人们的广泛关注. 虽然如此, 甲烷水合物的开采存在较多的困难, 主要涉及笼型甲烷水合物的分解过程, 因此本文通过分子动力学模拟, 探索太赫兹电磁波对该水合物分解的特异性影响. 通过分析甲烷水合物中水分子的振动谱, 发现其区别于体相水在低温下的特异性振动峰. 通过引入频率与该振动峰中心频率一致的太赫兹波发现, 该电磁场会破坏甲烷水合物原有的氢键网络, 降低甲烷周围水分子的配位数, 从而使甲烷分子从水笼中脱离. 进一步地, 对序参数F4的分析也证明, 在太赫兹电磁刺激下, 甲烷壳层水由原来的晶体水变成了液态水. 同时证明了该频率具有相比于其他频率的绝对优势, 因此具有其特异性. 本文的发现有望通过非热效应来实现甲烷水合物的分解, 高效、低能耗地提高开采效率, 推动未来新能源的发展.Methane hydrate (so-called flammable ice) has many advantages such as wide distribution, large resource reserves, high energy density, being clean and pollution-free, etc. Thus, it has attracted much attention since it was discovered. Unfortunately, its exploration encounters many difficulties, which involve mainly with the dissolution process of caged methane hydrate. Therefore, in this work the specific effect of THz electromagnetic wave on decomposition of the hydrate is explored through molecular dynamics simulations. Analyzing the vibrational spectrum of the hydrogen-bond network in methane hydrate, no specific absorption peak is found in the bulk water. Applying a THz wave at this specific frequency to the methane hydrate, the original hydrogen-bond network is broken, the coordinate number of water molecules for the methane decreases, and ultimately the methane frees from the water cage. The F4 ordered parameters further validate the phase change from the crystal water to liquid water under the same THz field irritation. It is also proved that this peak absorption frequency has a remarkable superiority over other frequencies in decomposing the methane hydrate, hence it has specificity. Our findings support the feasibility of non-thermally dissolving methane hydrate, which promises to promote the exploitation efficiency and development of new energy sources in the future.
-
Keywords:
- terahertz /
- methane /
- hydrogen bond /
- decomposition /
- molecular dynamics
[1] Jeppesen E, Beklioğlu M, Özkan K, Akyürek Z 2020 The Innovation 1 100030
 Google Scholar
Google Scholar
[2] 朱金龙, 赵予生, 靳常青 2019 68 018203
 Google Scholar
Google Scholar
Zhu J L, Zhao Y S, Jin C Q 2019 Acta Phys. Sin. 68 018203
 Google Scholar
Google Scholar
[3] Alavi S, Ripmeester J 2010 J. Chem. Phys. 132 144703
 Google Scholar
Google Scholar
[4] 颜克凤, 李小森, 陈朝阳, 李刚, 唐良广, 樊栓狮 2007 56 4994
 Google Scholar
Google Scholar
Yan K F, Li X S, Chen C Y, Li G, Tang L G, Fan S S, 2007 Acta Phys. Sin. 56 4994
 Google Scholar
Google Scholar
[5] Yan K F, Li X S, Chen Z Y, Li B, Xu C G 2013 Mol. Simul. 39 251
 Google Scholar
Google Scholar
[6] Ding L Y, Geng C Y, Zhao Y H, Wen H 2007 Mol. Simul. 33 1005
 Google Scholar
Google Scholar
[7] Ding L Y, Geng C Y, Zhao Y H, He X F, Wen H 2008 Sci. China, Ser. B Chem. 51 651
 Google Scholar
Google Scholar
[8] Yagasaki T, Matsumoto M, Tanaka H 2015 Phys. Chem. Chem. Phys. 17 32347
 Google Scholar
Google Scholar
[9] Myshakin E M, Jiang H, Warzinski R P, Jordan K D 2009 J. Phys. Chem. A 113 1913
 Google Scholar
Google Scholar
[10] Bai D S, Zhang X R, Chen G J, Wang W C 2012 Energy Environ. Sci. 5 7033
 Google Scholar
Google Scholar
[11] Smirnov K S 2017 Phys. Chem. Chem. Phys. 19 23095
 Google Scholar
Google Scholar
[12] Luis D, Herrera-Hernández E, Saint-Martin H 2015 J. Chem. Phys. 143 204503
 Google Scholar
Google Scholar
[13] Xu T T, Lang X M, Fan S S, Wang Y H, Chen J B 2019 Comput. Theor. Chem. 1149 57
 Google Scholar
Google Scholar
[14] Zhu Z, Chang C, Shu Y S, Song B 2019 J. Phys. Chem. Lett. 11 256
 Google Scholar
Google Scholar
[15] Zhu Z, Chen C, Chang C, Song B 2020 ACS Photonics 8 781
 Google Scholar
Google Scholar
[16] Li Y M, Chang C, Zhu Z, Sun L, Fan C H 2021 J. Am. Chem. Soc. 143 4311
 Google Scholar
Google Scholar
[17] Liu X, Qiao Z, Chai Y M, Zhu Z, Wu K J, Ji W L, Li D G, Xiao Y J, Mao L Q, Chang C 2021 Proc. Natl. Acad. Sci. U.S.A. 118 2015685118
 Google Scholar
Google Scholar
[18] Zhang J X, He Y, Liang S S, Liao X, Li T, Qiao Z, Chang C, Jia H B, Chen X W 2021 Nat. Commun. 12 1
 Google Scholar
Google Scholar
[19] Wu K J, Qi C H, Zhu Z, Wang C L, Song B, Chang C 2020 J. Phys. Chem. Lett. 11 7002
 Google Scholar
Google Scholar
[20] Liu G Z, Chang C, Qiao Z, Wu K J, Zhu Z, Cui G Q, Peng W Y, Tang Y Z, Li J, Fan C H 2019 Adv. Funct. Mater. 29 1807862
 Google Scholar
Google Scholar
[21] Wang K C, Yang L X, Wang S M, Guo L H, Ma J L, Tang J C, Bo W F, Wu Z, Zeng B Q, Gong Y B 2020 Phys. Chem. Chem. Phys. 22 9316
 Google Scholar
Google Scholar
[22] Li N, Peng D L, Zhang X J, Shu Y S, Zhang F, Jiang L, Song B 2021 Nano Res. 14 40
 Google Scholar
Google Scholar
[23] Martínez L, Andrade R, Birgin E G, Martínez J M 2009 J. Comput. Chem. 30 2157
 Google Scholar
Google Scholar
[24] Martínez J M, Martínez L 2003 J. Comput. Chem. 24 819
 Google Scholar
Google Scholar
[25] Hess B, Kutzner C, Van Der Spoel D, Lindahl E 2008 J. Chem. Theory Comput. 4 435
 Google Scholar
Google Scholar
[26] Abascal J, Sanz E, García Fernández R, Vega C 2005 J. Chem. Phys. 122 234511
 Google Scholar
Google Scholar
[27] Nosé S 1984 J. Chem. Phys. 81 511
 Google Scholar
Google Scholar
[28] Hoover W G 1985 Phys. Rev. A 31 1695
 Google Scholar
Google Scholar
[29] Yagasaki T, Matsumoto M, Andoh Y, Okazaki S, Tanaka H 2014 J. Phys. Chem. B 118 1900
 Google Scholar
Google Scholar
[30] Wu J Y, Chen L J, Chen Y P, Lin S T 2016 Phys. Chem. Chem. Phys. 18 9935
 Google Scholar
Google Scholar
[31] Choudhary N, Chakrabarty S, Roy S, Kumar R 2019 Chem. Phys. 516 6
 Google Scholar
Google Scholar
[32] Rodger P, Forester T, Smith W 1996 Fluid Phase Equilib. 116 326
 Google Scholar
Google Scholar
[33] Walsh M R, Beckham G T, Koh C A, Sloan E D, Wu D T, Sum A K 2011 J. Phys. Chem. C 115 21241
 Google Scholar
Google Scholar
[34] Zhang Z C, Liu C J, Walsh M R, Guo G J 2016 Phys. Chem. Chem. Phys. 18 15602
 Google Scholar
Google Scholar
[35] Lauricella M, Meloni S, English N J, Peters B, Ciccotti G 2014 J. Phys. Chem. C 118 22847
 Google Scholar
Google Scholar
[36] Zhang Z C, Guo G J 2017 Phys. Chem. Chem. Phys. 19 19496
 Google Scholar
Google Scholar
[37] Yang D X, Zhu Q G, Han B X 2020 The Innovation 1 100016
 Google Scholar
Google Scholar
-
图 1 (a) 太赫兹加速分解甲烷水合物的概念图, 其中蓝色基底为甲烷水合物晶体, 红色和白色小球为甲烷分子中的碳和氢原子, 笼状物为包裹甲烷的水分子, 其中黄色小球为水中的氧原子. (b) 上图为模拟的初始构型, 其中绿线左侧为笼型甲烷水合物, 右侧为高温融解后的甲烷和水, 聚集的蓝色部分为甲烷气体, 周围红色为水分子; 中图为260 K温度下NVT平衡200 ns后甲烷水合物的状态, 其中交界部分已经出现成核的现象, 同时聚集的甲烷分子散开, 有继续成核的趋势; 下图为260 K温度下施加特定频率的太赫兹电磁刺激后, 甲烷水合物的状态, 大部分原有的甲烷水合物已经分解, 并且有进一步分解的趋势
Fig. 1. (a) Conceptual graph describing terahertz wave accelerated decomposition of methane hydrate, where the blue substrate is methane hydrate crystals, the red and white balls are the carbon and hydrogen atoms, and the clathrate is the water molecules enveloping the methane. (b) Above: Initial simulated configuration. The left side of the green line is caged methane hydrate, while the right side is methane and water mixture after high temperature melting. The blue cluster therein is the methane gas, surrounded by water molecules (in red). Middle: State of methane hydrate after the NVT equilibration for 200 ns at a temperature of 260 K. The nucleation has occurred in the interface, and the initially gathered methane molecules have partly diffused and are expected to form more nucleation. Bottom: State of methane hydrate after a specific terahertz electromagnetic (THz-EM) stimulation at 260 K. Most of the original methane hydrate has been decomposed and developed into a methane cluster.
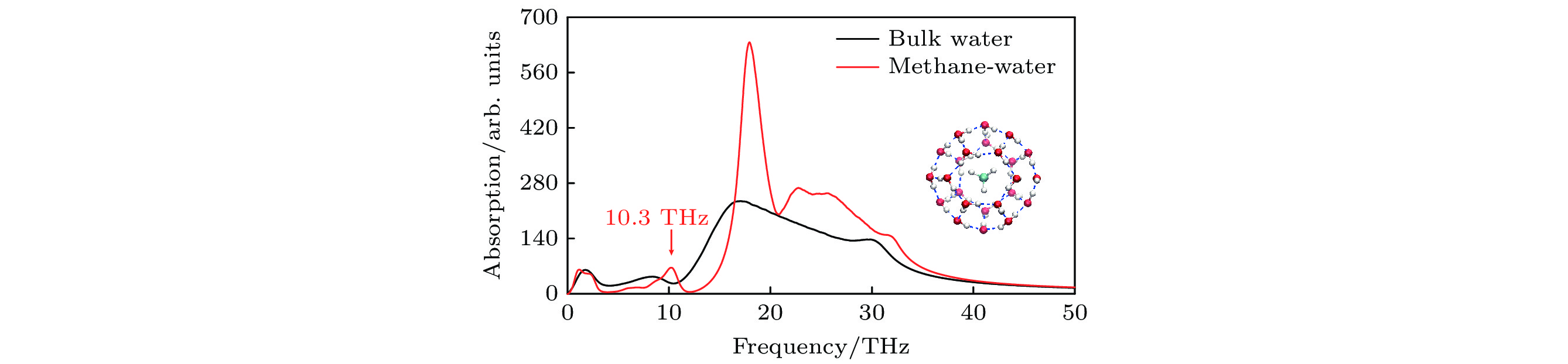
图 2 水在260 K温度下的振动吸收谱, 其中红线为笼状结构甲烷水合物中水的振动吸收谱, 黑线为体相水的振动吸收谱, 可见甲烷水合物中存在10.3 THz的吸收峰, 而体相水对该频率下的太赫兹电磁刺激只有弱吸收, 使用该频率的电磁刺激能够特异性影响甲烷水合物的氢键网络. 内插图为甲烷水合物的笼状结构, 中心蓝白色球棍结构代表甲烷分子, 其外圈包围的为水分子
Fig. 2. Vibrational absorption spectra of water at a temperature of 260 K. The red line corresponds to the spectrum of water in the caged methane hydrate, while the black one denotes the spectrum of bulk water. There exists an absorption peak at 10.3 THz in methane hydrate but an absorption valley in bulk water. Hence, an EM stimulus at this specific frequency could alter the hydrogen-bond network of methane hydrate. Inset: caged methane hydrate. The inner blue-white ball-stick structure denotes methane molecule, surrounded by water molecules in the outer.
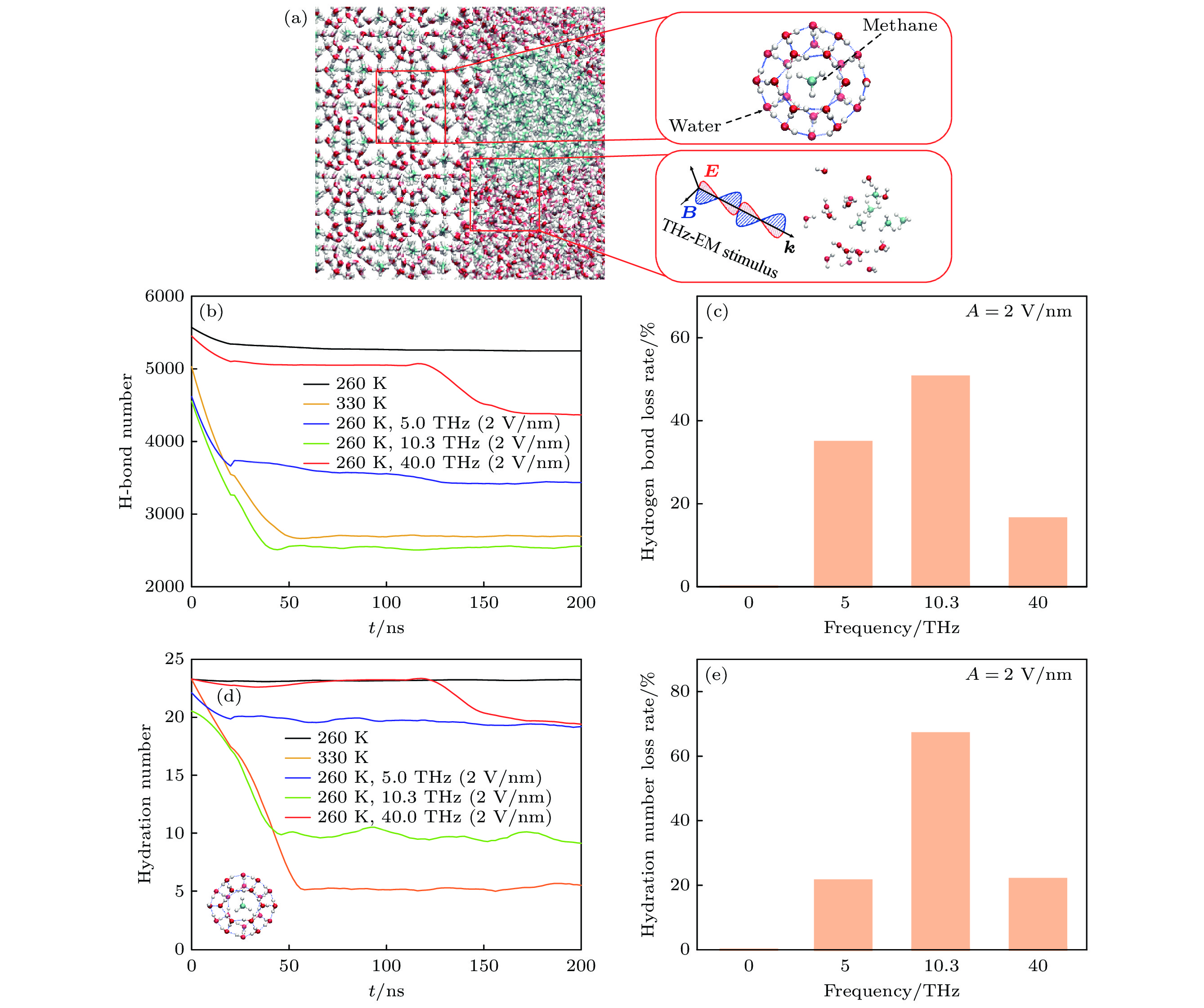
图 3 (a) 甲烷水合物在结晶态(右上插图)以及在太赫兹场刺激下分解后(右下插图)甲烷分子和水的空间分布形态; (b) 模拟体系总的氢键数目随时间的变化; (c) 引入电场强度为2 V/nm情况下, 体系氢键损失率与频率的关系; (d) 水合物中甲烷周围水分子的配位数, 这个配位数是在0.57 nm的壳层半径内计算的, 该壳层半径对应于1个稳定的包合物的C—O分布函数中的第1个最小值, 内插图为单个甲烷分子被水包围的示意图; (e) 引入电场强度为2 V/nm时, 水分子配位数降低率与频率的关系
Fig. 3. (a) Spatial distribution of water and methane molecules in the caged methane hydrate (up-right inset) and decomposed mixture (down-right inset) after THz-EM stimulus. (b) Change of the total number of hydrogen bonds in simulated system with time. (c) Relationship between the hydrogen bond loss rate of system and the external electric field frequency at an intensity of 2 V/nm. (d) Coordination number of water molecules for the methane in hydrate. It is calculated within a shell radius of 0.57 nm, which corresponds to the first minimum value in the C—O distribution function of a stable clathrate. The inset describes a single methane molecule surrounded by water. (e) Relationship between the reduction rate of the coordination number and the introduced field frequency at an intensity of 2 V/nm.
图 4 (a) 260 K温度下, 不同频率的太赫兹电磁刺激对水分子中O原子相对于甲烷分子中C原子的径向分布函数的影响; (b) 260 K温度下, 不同频率的太赫兹电磁刺激对甲烷分子中C原子相对周围甲烷分子中的C原子的径向分布函数的影响
Fig. 4. (a) Effect of THz-EM stimulation at different frequencies on the radial distribution function (RDF) of O atoms in water molecules w.r.t. the C atom in a methane molecule at 260 K; (b) effect of the stimulations on the RDF of C atoms in surrounding methane molecules w.r.t. the C atom in a methane molecule at 260 K.
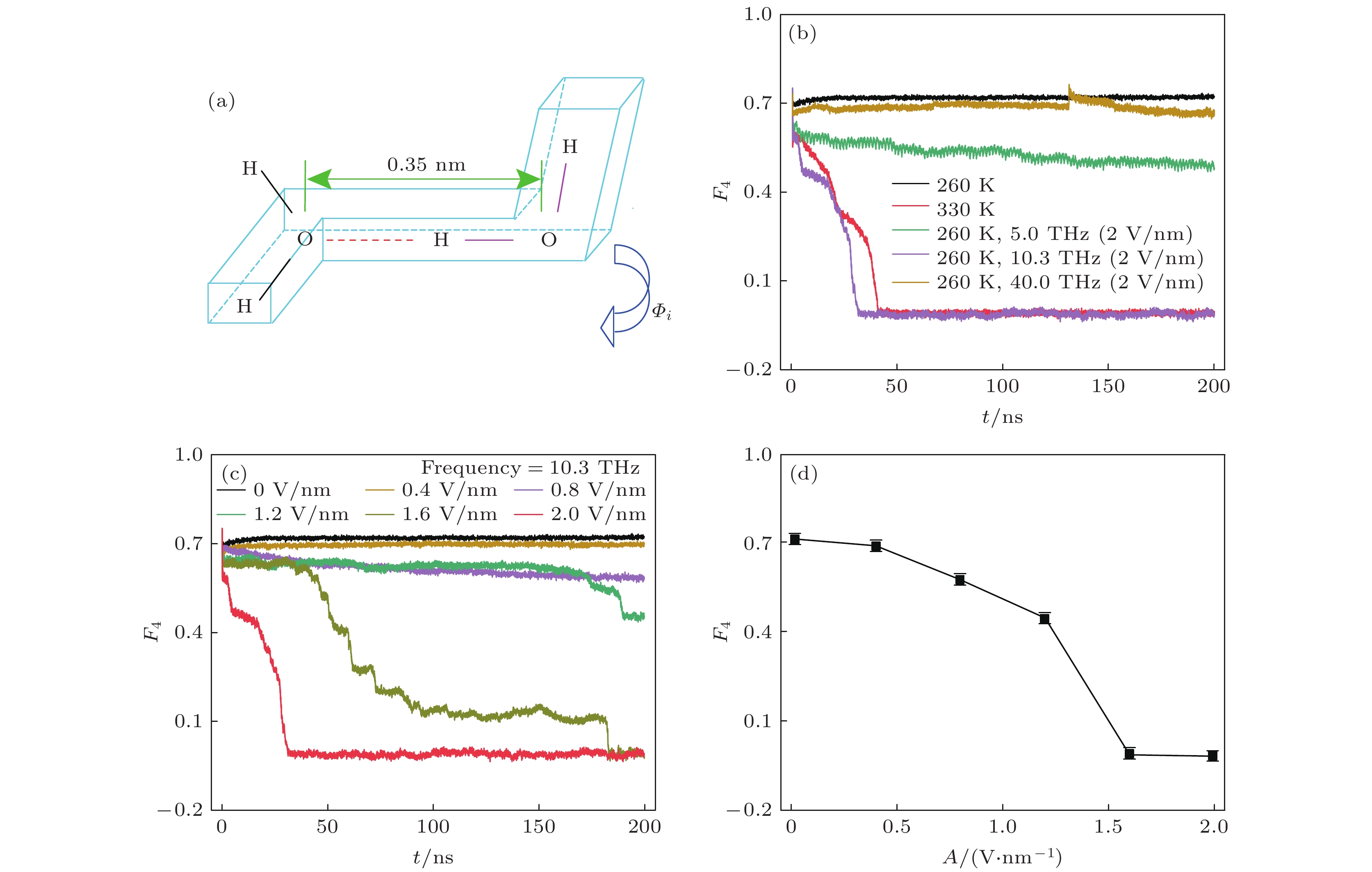
图 5 (a) F4值参数的示意图, 红色虚线为氢键, 字母H, O表示两个水分子的氢和氧原子位置, 水分子中氧原子间的距离在0.35 nm内, 多面体内两端的H—O···O—H为距离最远的一对H,
$ {\varPhi }_{i} $ 为扭转角; (b) 不同条件下F4值随时间的变化量; (c) 频率为10.3 THz的电磁波, 不同强度下F4值随时间的变化量; (d) 频率为10.3 THz, 强度不同的电磁波作用下的F4值Fig. 5. (a) Schematic diagram of the F4 value parameter. The red dashed line denotes the hydrogen bond, and the H and O letters locates the hydrogen and oxygen atoms in two water molecules. The distance between two oxygen atoms is within 0.35 nm. The H pair at both ends in the H—O···O—H polyhedron accounts for the largest distance.
$ {{\varPhi } }_{i} $ is the torsion angle. (b) Change of the F4 value with time under different conditions. (c) Variations of the F4 value with time under different filed intensities but the same frequency of 10.3 THz. (d) Relation between the F4 value and the EM field intensity at the same frequency of 10.3 THz. -
[1] Jeppesen E, Beklioğlu M, Özkan K, Akyürek Z 2020 The Innovation 1 100030
 Google Scholar
Google Scholar
[2] 朱金龙, 赵予生, 靳常青 2019 68 018203
 Google Scholar
Google Scholar
Zhu J L, Zhao Y S, Jin C Q 2019 Acta Phys. Sin. 68 018203
 Google Scholar
Google Scholar
[3] Alavi S, Ripmeester J 2010 J. Chem. Phys. 132 144703
 Google Scholar
Google Scholar
[4] 颜克凤, 李小森, 陈朝阳, 李刚, 唐良广, 樊栓狮 2007 56 4994
 Google Scholar
Google Scholar
Yan K F, Li X S, Chen C Y, Li G, Tang L G, Fan S S, 2007 Acta Phys. Sin. 56 4994
 Google Scholar
Google Scholar
[5] Yan K F, Li X S, Chen Z Y, Li B, Xu C G 2013 Mol. Simul. 39 251
 Google Scholar
Google Scholar
[6] Ding L Y, Geng C Y, Zhao Y H, Wen H 2007 Mol. Simul. 33 1005
 Google Scholar
Google Scholar
[7] Ding L Y, Geng C Y, Zhao Y H, He X F, Wen H 2008 Sci. China, Ser. B Chem. 51 651
 Google Scholar
Google Scholar
[8] Yagasaki T, Matsumoto M, Tanaka H 2015 Phys. Chem. Chem. Phys. 17 32347
 Google Scholar
Google Scholar
[9] Myshakin E M, Jiang H, Warzinski R P, Jordan K D 2009 J. Phys. Chem. A 113 1913
 Google Scholar
Google Scholar
[10] Bai D S, Zhang X R, Chen G J, Wang W C 2012 Energy Environ. Sci. 5 7033
 Google Scholar
Google Scholar
[11] Smirnov K S 2017 Phys. Chem. Chem. Phys. 19 23095
 Google Scholar
Google Scholar
[12] Luis D, Herrera-Hernández E, Saint-Martin H 2015 J. Chem. Phys. 143 204503
 Google Scholar
Google Scholar
[13] Xu T T, Lang X M, Fan S S, Wang Y H, Chen J B 2019 Comput. Theor. Chem. 1149 57
 Google Scholar
Google Scholar
[14] Zhu Z, Chang C, Shu Y S, Song B 2019 J. Phys. Chem. Lett. 11 256
 Google Scholar
Google Scholar
[15] Zhu Z, Chen C, Chang C, Song B 2020 ACS Photonics 8 781
 Google Scholar
Google Scholar
[16] Li Y M, Chang C, Zhu Z, Sun L, Fan C H 2021 J. Am. Chem. Soc. 143 4311
 Google Scholar
Google Scholar
[17] Liu X, Qiao Z, Chai Y M, Zhu Z, Wu K J, Ji W L, Li D G, Xiao Y J, Mao L Q, Chang C 2021 Proc. Natl. Acad. Sci. U.S.A. 118 2015685118
 Google Scholar
Google Scholar
[18] Zhang J X, He Y, Liang S S, Liao X, Li T, Qiao Z, Chang C, Jia H B, Chen X W 2021 Nat. Commun. 12 1
 Google Scholar
Google Scholar
[19] Wu K J, Qi C H, Zhu Z, Wang C L, Song B, Chang C 2020 J. Phys. Chem. Lett. 11 7002
 Google Scholar
Google Scholar
[20] Liu G Z, Chang C, Qiao Z, Wu K J, Zhu Z, Cui G Q, Peng W Y, Tang Y Z, Li J, Fan C H 2019 Adv. Funct. Mater. 29 1807862
 Google Scholar
Google Scholar
[21] Wang K C, Yang L X, Wang S M, Guo L H, Ma J L, Tang J C, Bo W F, Wu Z, Zeng B Q, Gong Y B 2020 Phys. Chem. Chem. Phys. 22 9316
 Google Scholar
Google Scholar
[22] Li N, Peng D L, Zhang X J, Shu Y S, Zhang F, Jiang L, Song B 2021 Nano Res. 14 40
 Google Scholar
Google Scholar
[23] Martínez L, Andrade R, Birgin E G, Martínez J M 2009 J. Comput. Chem. 30 2157
 Google Scholar
Google Scholar
[24] Martínez J M, Martínez L 2003 J. Comput. Chem. 24 819
 Google Scholar
Google Scholar
[25] Hess B, Kutzner C, Van Der Spoel D, Lindahl E 2008 J. Chem. Theory Comput. 4 435
 Google Scholar
Google Scholar
[26] Abascal J, Sanz E, García Fernández R, Vega C 2005 J. Chem. Phys. 122 234511
 Google Scholar
Google Scholar
[27] Nosé S 1984 J. Chem. Phys. 81 511
 Google Scholar
Google Scholar
[28] Hoover W G 1985 Phys. Rev. A 31 1695
 Google Scholar
Google Scholar
[29] Yagasaki T, Matsumoto M, Andoh Y, Okazaki S, Tanaka H 2014 J. Phys. Chem. B 118 1900
 Google Scholar
Google Scholar
[30] Wu J Y, Chen L J, Chen Y P, Lin S T 2016 Phys. Chem. Chem. Phys. 18 9935
 Google Scholar
Google Scholar
[31] Choudhary N, Chakrabarty S, Roy S, Kumar R 2019 Chem. Phys. 516 6
 Google Scholar
Google Scholar
[32] Rodger P, Forester T, Smith W 1996 Fluid Phase Equilib. 116 326
 Google Scholar
Google Scholar
[33] Walsh M R, Beckham G T, Koh C A, Sloan E D, Wu D T, Sum A K 2011 J. Phys. Chem. C 115 21241
 Google Scholar
Google Scholar
[34] Zhang Z C, Liu C J, Walsh M R, Guo G J 2016 Phys. Chem. Chem. Phys. 18 15602
 Google Scholar
Google Scholar
[35] Lauricella M, Meloni S, English N J, Peters B, Ciccotti G 2014 J. Phys. Chem. C 118 22847
 Google Scholar
Google Scholar
[36] Zhang Z C, Guo G J 2017 Phys. Chem. Chem. Phys. 19 19496
 Google Scholar
Google Scholar
[37] Yang D X, Zhu Q G, Han B X 2020 The Innovation 1 100016
 Google Scholar
Google Scholar
计量
- 文章访问数: 7725
- PDF下载量: 172
- 被引次数: 0














 下载:
下载: