-
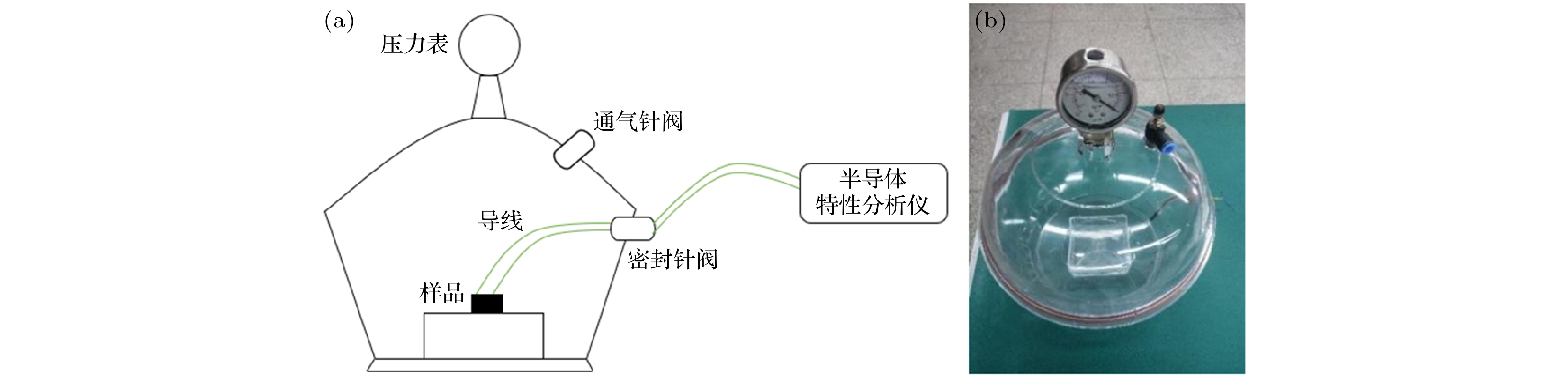
利用机械剥离和高温减薄方法制备了厚度约为5 nm的GeSe纳米片, 并通过设计实验装置测试了GeSe纳米片在不同浓度氧气(O2)和丁烷(C4H10)气体中的电导性能. 结果表明, 随着氧气浓度的增加, GeSe纳米片在相同电压下所测得的电流增大, 而在丁烷气体中所测得的电流减小. 通过第一性原理计算发现, O2分子从GeSe获得电子. 气体浓度越大, P型半导体GeSe主要载流子空穴的浓度也越大, 从而电导率增大. 当GeSe吸附丁烷气体时, 随着丁烷气体浓度的增加, 相同电压下电导率减小. 其原因可归结为GeSe薄膜器件在加工过程中从空气中吸附了O2分子, 由于薄膜中存在较高密度的Se空位, 导致O2的高密度吸附. 从而导致在吸附还原性气体时, 丁烷气体易失电子. 即电子从丁烷气体分子中转移到GeSe薄膜表面与空穴中和, 降低了GeSe薄膜中的载流子空穴浓度, 从而降低电导率. 本文的研究有助于GeSe纳米片在氧气和丁烷气中的光电器件应用.As a type of two-dimensional (2D) semiconductor material, 2D germanium selenide (GeSe) exhibits excellent optoelectronic properties, and has potential applications in optoelectronic devices. The GeSe is a layered material with weak van der Waals interaction. Because of the high brittleness of GeSe, it is not easy to obtain 2D GeSe samples only by mechanical peeling technique. In order to obtain a thinner GeSe sheet, we use heat treatment to thin the bulk GeSe at a high temperature in vacuum. The GeSe samples obtained by mechanical peeling are placed in a tubular furnace with a pressure of 5 × 10-4 Pa for high temperature heating and thinning. In order to explore the better thinning effect, we set four temperatures to be at 320, 330, 340 and 350 ℃, respectively. After high temperature thinning, the samples are characterized and observed by atomic force microscope (AFM), scanning electron microscope (SEM), Raman spectrometer and photoluminescence (PL) spectrometer. From the above experiments, the GeSe nanosheet with a thickness of about 5 nm is prepared by mechanical peeling and high temperature thinning technology. Then, the electrical conductivities of GeSe nanosheets in oxygen (O2) and butane (C4H10) with different concentrations are evaluated by our designed experimental device. The results show that with the increase of oxygen concentration, the electrical conductivity of GeSe nanosheets increases. When the GeSe nanosheet is in butane gas, its conductivity under the same voltage decreases with the increase of the concentration of butane gas. In order to further analyze the mechanism of gas adsorption on GeSe nanosheets, we carry out the first-principles calculations. Our calculation results show that the adsorption energy of GeSe nanosheets for oxygen and butane is –4.555 eV and –4.865 eV, respectively. It is shown that both adsorption systems have a certain stability. The adsorption energy of C4H10 is smaller than that of O2, which corresponds to the smaller layer spacing of C4H10 than that of O2 on GeSe surface. From Bader analysis, it is shown that 0.262e is transferred from the surface of GeSe nanosheet to O2 molecule, which is much larger than 0.022e transferred from GeSe to C4H10 molecule. It can be inferred that the bond formed between GeSe and O2 molecule is covalent bond, while GeSe adsorption C4H10 is very fragile hydrogen bond adsorption. In an ideal condition (single atomic GeSe layer, no Se vacancy, and the device preparation process is vacuum), our calculation results show that C4H10 still has a weak ability to obtain electrons from the GeSe nanosheet. However, the complex conditions such as the actual layer thickness, the appearance of Se vacancy and the adsorption of O2 molecules on the surface leads to the difference between the experimental results and the theoretical calculations, which can be attributed to the adsorption of O2 molecules on the GeSe surface from the air during the processing of GeSe thinning and device fabrication. Owing to the high density of Se vacancies in the thin film, the high density of O2 adsorption is caused. Thus, butane gas is easy to lose electrons on the GeSe surface due to the O2 adsorption. In other words, electrons are transferred from butane gas molecules to the surface of GeSe film and neutralized with holes, which reduces the concentration of carriers and the concentration of holes in GeSe film, thus reducing the conductivity. Our research will contribute to the application of GeSe nanosheets in optoelectronic devices at the atmosphere of oxygen and butane.
-
Keywords:
- GeSe nanosheet /
- electrical conductance /
- gas adsorption /
- first-principle
[1] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[2] Geim A K, Novoselov K S 2007 Nat. Mater. 6 183
 Google Scholar
Google Scholar
[3] Xia F, Wang H, Xiao D, Dubey M, Ramasubramaniam A 2014 Nat. Photonics 8 899
 Google Scholar
Google Scholar
[4] Jing Y, Zhang X, Zhou Z 2016 Wiley Interdiscip. Rev. Comput. Mol. Sci. 6 5
 Google Scholar
Google Scholar
[5] Mao Y L, Stocks G M, Zhong J X 2010 New J. Phys. 12 033046
 Google Scholar
Google Scholar
[6] Xu C S, Yuan J M, Wang D D, Mao Y L 2018 Mater. Res. Express 6 036305
 Google Scholar
Google Scholar
[7] Mao Y L, Zhong J X 2008 Nanotechnology 19 205708
 Google Scholar
Google Scholar
[8] Salvo P, Melai B, Calisi N, Paoletti C, Bellagambi F G, Kirchhain A, Trivella M G, Fuoco R, Francesco F D 2017 Sens. Actuators, B 256 976
[9] Chu K, Wang X H, Li Y B, Huang D J, Geng Z R, Zhao X L, Liu H, Zhuang H 2018 Mater. Des. 140 85
 Google Scholar
Google Scholar
[10] Prashantha K, Roger F 2017 J. Macromol. Sci. Part A Pure Appl. Chem. 54 24
 Google Scholar
Google Scholar
[11] Zhang C, Man B Y, Yang C, Jiang S Z, Liu M H, Chen C S, Xu S C, Sun Z C, Gao X G, Chen X F 2013 Nanotechnology 24 395603
 Google Scholar
Google Scholar
[12] Sun Z C, Yang C, Liu M, Chen C S, Xu S C, Zhang C, Man B Y 2014 Appl. Surf. Sci. 315 368
 Google Scholar
Google Scholar
[13] Stankovich S, Dikin D A, Piner R D, Kohlhaas K A, Kleinhammes A, Jia YY, Wu Y, Nguyen S T, Ruoff R S 2007 Carbon 45 1558
 Google Scholar
Google Scholar
[14] Foo M E, Gopinath S C B 2017 Biomed. Pharmacother. 94 354
 Google Scholar
Google Scholar
[15] Mao Y L, Mao X, Zhao H Q, Zhang N D, Shi X, Yuan J M 2018 Sci. Rep. 8 17671
 Google Scholar
Google Scholar
[16] Hu Y W, Long L B, Mao Y L, Zhong J X 2018 Appl. Surf. Sci. 442 390
 Google Scholar
Google Scholar
[17] Zhang S L, Xie M Q, Li F Y, Yan Z, Li Y F, Kan E, Liu W, Chen Z F, Zeng H B 2016 Angew. Chem. 55 1666
 Google Scholar
Google Scholar
[18] Dutta S N, Jeffrey G A 1965 Inorg. Chem. 4 1363
 Google Scholar
Google Scholar
[19] Mao Y L, Xu C S, Yuan J M, Zhao H Q 2018 Phys. Chem. Chem. Phys. 20 6929
 Google Scholar
Google Scholar
[20] Vaughn I D D, Patel R J, Hickner M A, Schaak R E 2010 J. Am. Chem. Soc. 132 15170
 Google Scholar
Google Scholar
[21] Mao Y L, Guo G, Yuan J M, Zhong J X 2019 Appl. Surf. Sci. 464 236
 Google Scholar
Google Scholar
[22] Zhao H Q, Mao Y L, Mao X, Shi X, X C S, Wang C X, Zhang S M, Zhou D H 2018 Adv. Funct. Mater. 28 1704855
 Google Scholar
Google Scholar
[23] Shi G S, Kioupakis E 2015 Nano Lett. 15 6926
 Google Scholar
Google Scholar
[24] Xue D J, Tan J H, Hu J S, Hu W P, Guo Y G, Wan L J 2012 Adv. Mater. 24 4528
 Google Scholar
Google Scholar
[25] Zhang S L, Liu S G, Huang S P, Cai B, Xie M Q, Qu L H, Zou Y S, Hu Z Y, Yu X C, Zeng H B 2015 Sci. China Mater. 58 929
[26] Liu L, Yang Q, Ye H Y, Chen X P, Zhang G Q 2017 International Conference on Thermal, Mechanical and Multi-Physics Simulation and Experiments in Microelectronics and Microsystems Dresden, Germany, May 2–5, 2017 p1
[27] Mao Y L, Long L B, Yuan J M, Zhong J X, Zhao H Q 2018 Chem. Phys. Lett. 706 501
 Google Scholar
Google Scholar
[28] Kresse G, Joubert D P 1999 Phys. Rev. B 59 1758
[29] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[30] Ceperley D M, Alder B J 1980 Phys. Rev. Lett. 45 566
 Google Scholar
Google Scholar
[31] Matthias E, Gustavo E S 1999 J. Chem. Phys. 110 5029
 Google Scholar
Google Scholar
[32] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[33] Fei R X, Li W B, Li J, Yang L 2015 Appl. Phys. Lett. 107 173104
 Google Scholar
Google Scholar
[34] Tkatchenko A, Scheffler M 2009 Phys. Rev. Lett. 102 073005
 Google Scholar
Google Scholar
[35] Savin A, Nesper R, Wengert S, Fassler T F 1997 Angew. Chem. Int. Ed. 36 1808
 Google Scholar
Google Scholar
-
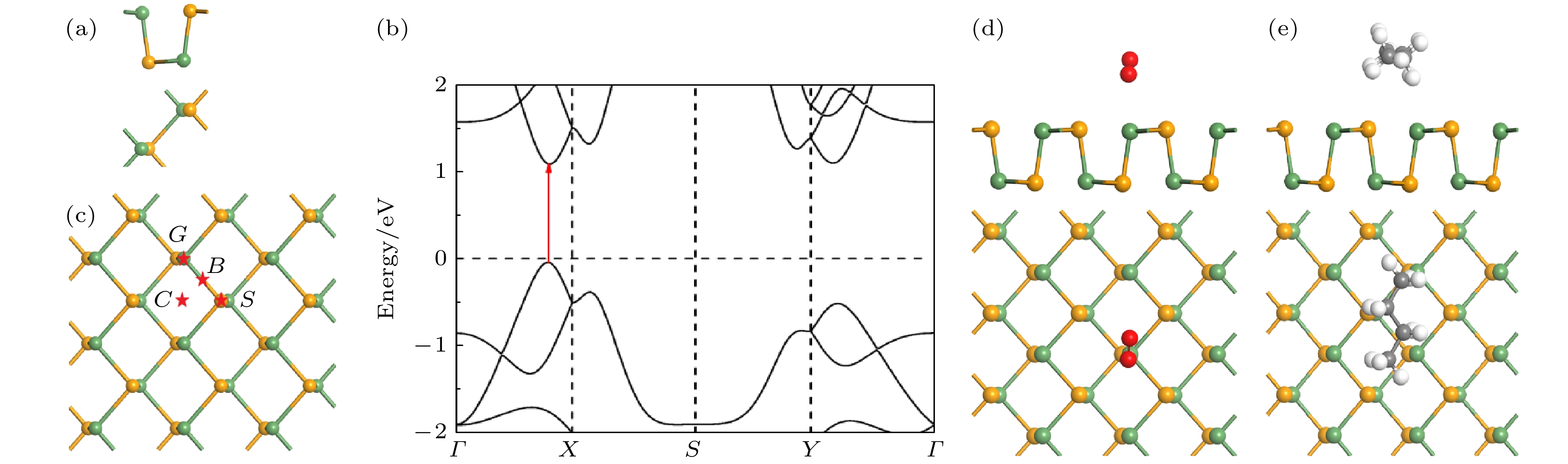
图 3 (a) GeSe单胞的结构; (b) GeSe单层的能带结构; (c) GeSe上吸附位点G点, S点, C点和B点的示意图; (d) GeSe吸附氧气最稳定的吸附结构示意图(侧视图与俯视图); (e) GeSe吸附丁烷最稳定的吸附结构示意图(侧视图与俯视图). 黄色, 绿色, 红色, 黑色和白色的球分别代表着Se, Ge, O, C和H原子
Fig. 3. (a) Optimized structure of GeSe monolayer; (b) band structures of GeSe monolayer; (c) considered positions for gas molecules adsorption: G site, S site, C site and B site on GeSe monolayer, respectively; (d) obtained stable adsorption configuration (side and top view) for O2 on GeSe monolayer; (e) obtained stable adsorption configuration (side and top view) for C4H10 on GeSe monolayer. The yellow, green, red, black and white balls denote Se, Ge, O, C and H atoms, respectively.
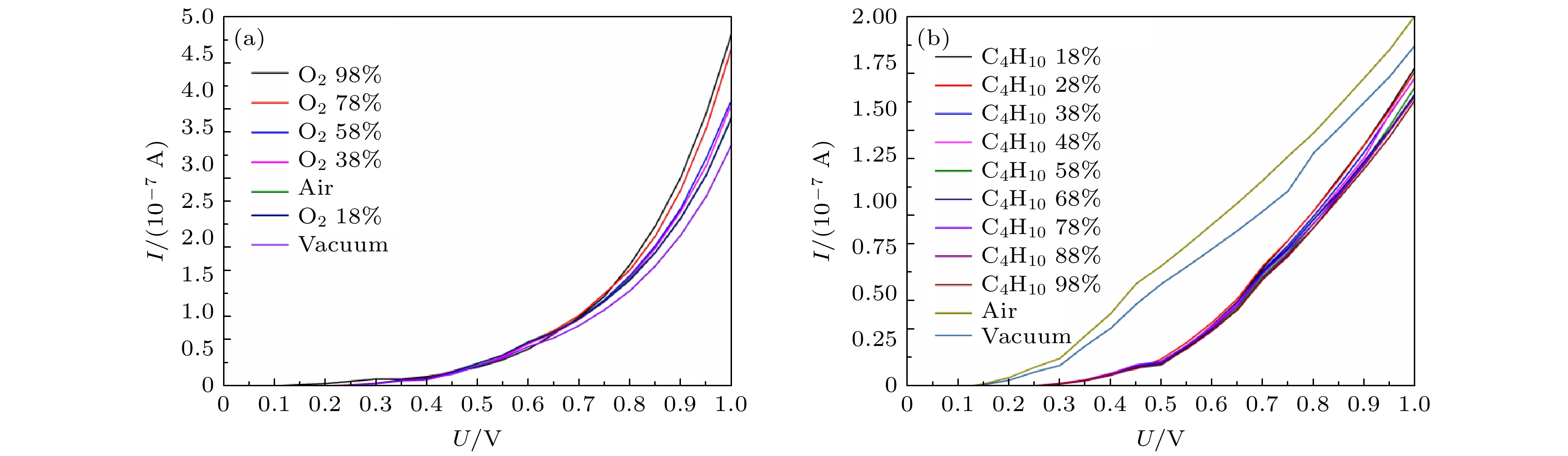
图 4 (a) GeSe纳米片吸附不同浓度的氧气时电压-电流特性曲线; (b) GeSe纳米片吸附不同浓度的丁烷气时电压-电流特性曲线
Fig. 4. (a) Voltage-current characteristic curve when germanium selenide device adsorbs different concentrations of oxygen; (b) voltage-current characteristic curve when germanium selenide device adsorbs different concentrations of butane gas.
图 5 GeSe吸附(a)氧气和(b)丁烷的差分电荷密度图, 等值面分别取0.01和0.0009e/A3. 其中蓝色原子为硒原子, 棕色原子为锗原子, 红色为氧原子, 黑色为碳原子, 粉色为氢原子; 黄色部分表示得到电子, 绿色部分表示失去电子. GeSe吸附(c)氧气和(d)丁烷的电子局域图, 左边是ELF (e/A3) 的参考值
Fig. 5. Charge density difference for the configurations of (a) O2 and (b) C4H10 on GeSe monolayer. The plotted isosurface is 0.01e/A3 and 0.0009e/A3, respectively. Blue atoms are selenium atoms, brown atoms are germanium atoms, red are oxygen atoms, black are carbon atoms, and pink are hydrogen atoms. In the differential charge density graph, the yellow part indicates the gain of electrons, and the green part indicates the loss of electrons. Electron localization function (ELF) for configurations of (c) O2 and (d) C4H10 adsorbed on GeSe monolayer, respectively. The ELF value is listed at the left side with a unit of e/A3.
表 1 吸附能(Ea), GeSe与气体分子之间的电荷转移量(ρ)以及它们之间的最近距离(d )
Table 1. Calculated adsorption energy (Ea), the charge transfer (ρ) between gas molecules and monolayer GeSe, and the nearest distance (d ) between them.
Gas molecule Ea/eV d /Å ρ/e style O2 –4.555 2.687 0.262 acceptor C4H10 –4.865 2.404 0.022 acceptor -
[1] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[2] Geim A K, Novoselov K S 2007 Nat. Mater. 6 183
 Google Scholar
Google Scholar
[3] Xia F, Wang H, Xiao D, Dubey M, Ramasubramaniam A 2014 Nat. Photonics 8 899
 Google Scholar
Google Scholar
[4] Jing Y, Zhang X, Zhou Z 2016 Wiley Interdiscip. Rev. Comput. Mol. Sci. 6 5
 Google Scholar
Google Scholar
[5] Mao Y L, Stocks G M, Zhong J X 2010 New J. Phys. 12 033046
 Google Scholar
Google Scholar
[6] Xu C S, Yuan J M, Wang D D, Mao Y L 2018 Mater. Res. Express 6 036305
 Google Scholar
Google Scholar
[7] Mao Y L, Zhong J X 2008 Nanotechnology 19 205708
 Google Scholar
Google Scholar
[8] Salvo P, Melai B, Calisi N, Paoletti C, Bellagambi F G, Kirchhain A, Trivella M G, Fuoco R, Francesco F D 2017 Sens. Actuators, B 256 976
[9] Chu K, Wang X H, Li Y B, Huang D J, Geng Z R, Zhao X L, Liu H, Zhuang H 2018 Mater. Des. 140 85
 Google Scholar
Google Scholar
[10] Prashantha K, Roger F 2017 J. Macromol. Sci. Part A Pure Appl. Chem. 54 24
 Google Scholar
Google Scholar
[11] Zhang C, Man B Y, Yang C, Jiang S Z, Liu M H, Chen C S, Xu S C, Sun Z C, Gao X G, Chen X F 2013 Nanotechnology 24 395603
 Google Scholar
Google Scholar
[12] Sun Z C, Yang C, Liu M, Chen C S, Xu S C, Zhang C, Man B Y 2014 Appl. Surf. Sci. 315 368
 Google Scholar
Google Scholar
[13] Stankovich S, Dikin D A, Piner R D, Kohlhaas K A, Kleinhammes A, Jia YY, Wu Y, Nguyen S T, Ruoff R S 2007 Carbon 45 1558
 Google Scholar
Google Scholar
[14] Foo M E, Gopinath S C B 2017 Biomed. Pharmacother. 94 354
 Google Scholar
Google Scholar
[15] Mao Y L, Mao X, Zhao H Q, Zhang N D, Shi X, Yuan J M 2018 Sci. Rep. 8 17671
 Google Scholar
Google Scholar
[16] Hu Y W, Long L B, Mao Y L, Zhong J X 2018 Appl. Surf. Sci. 442 390
 Google Scholar
Google Scholar
[17] Zhang S L, Xie M Q, Li F Y, Yan Z, Li Y F, Kan E, Liu W, Chen Z F, Zeng H B 2016 Angew. Chem. 55 1666
 Google Scholar
Google Scholar
[18] Dutta S N, Jeffrey G A 1965 Inorg. Chem. 4 1363
 Google Scholar
Google Scholar
[19] Mao Y L, Xu C S, Yuan J M, Zhao H Q 2018 Phys. Chem. Chem. Phys. 20 6929
 Google Scholar
Google Scholar
[20] Vaughn I D D, Patel R J, Hickner M A, Schaak R E 2010 J. Am. Chem. Soc. 132 15170
 Google Scholar
Google Scholar
[21] Mao Y L, Guo G, Yuan J M, Zhong J X 2019 Appl. Surf. Sci. 464 236
 Google Scholar
Google Scholar
[22] Zhao H Q, Mao Y L, Mao X, Shi X, X C S, Wang C X, Zhang S M, Zhou D H 2018 Adv. Funct. Mater. 28 1704855
 Google Scholar
Google Scholar
[23] Shi G S, Kioupakis E 2015 Nano Lett. 15 6926
 Google Scholar
Google Scholar
[24] Xue D J, Tan J H, Hu J S, Hu W P, Guo Y G, Wan L J 2012 Adv. Mater. 24 4528
 Google Scholar
Google Scholar
[25] Zhang S L, Liu S G, Huang S P, Cai B, Xie M Q, Qu L H, Zou Y S, Hu Z Y, Yu X C, Zeng H B 2015 Sci. China Mater. 58 929
[26] Liu L, Yang Q, Ye H Y, Chen X P, Zhang G Q 2017 International Conference on Thermal, Mechanical and Multi-Physics Simulation and Experiments in Microelectronics and Microsystems Dresden, Germany, May 2–5, 2017 p1
[27] Mao Y L, Long L B, Yuan J M, Zhong J X, Zhao H Q 2018 Chem. Phys. Lett. 706 501
 Google Scholar
Google Scholar
[28] Kresse G, Joubert D P 1999 Phys. Rev. B 59 1758
[29] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[30] Ceperley D M, Alder B J 1980 Phys. Rev. Lett. 45 566
 Google Scholar
Google Scholar
[31] Matthias E, Gustavo E S 1999 J. Chem. Phys. 110 5029
 Google Scholar
Google Scholar
[32] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[33] Fei R X, Li W B, Li J, Yang L 2015 Appl. Phys. Lett. 107 173104
 Google Scholar
Google Scholar
[34] Tkatchenko A, Scheffler M 2009 Phys. Rev. Lett. 102 073005
 Google Scholar
Google Scholar
[35] Savin A, Nesper R, Wengert S, Fassler T F 1997 Angew. Chem. Int. Ed. 36 1808
 Google Scholar
Google Scholar
计量
- 文章访问数: 5586
- PDF下载量: 89
- 被引次数: 0













 下载:
下载: