-
铀及铀合金贮存环境中的水分子与铀反应会产生氢气 (H2) , 进而对铀表面产生腐蚀作用. 基于密度泛函理论, 本文开展了H2在钼 (Mo) 涂层γ-U(100) 表面(U(100)/Mo) 吸附行为的第一性原理研究, 建立了γ-U(100)及U(100)/Mo表面模型, 计算了H2在不同吸附位点下的结构参数、吸附能、Bader电荷、表面功函数、电子态密度. 研究结果表明, H2在γ-U(100) 和U(100)/Mo表面的吸附主要为物理吸附, 在空位平行吸附构型下, H2完全解离成两个H原子, 化学吸附于基底表面. Bader电荷分布结果表明, 此时净电荷的变化量大于物理吸附时对应的净电荷变化量. H2在U(100)/Mo表面最稳定吸附构型下 (HMo-Hor) 的吸附能小于γ-U(100) 表面最稳定吸附构型 (HU-Hor) 的吸附能, 相比于H2在γ-U(100) 表面的吸附, H2在U(100)/Mo表面的吸附更稳定. 本文为铀合金及其Mo涂层表面氢化腐蚀研究提供了理论依据, 为未来开展铀合金表面抗腐蚀研究提供理论基础和实验技术支持.
Uranium (U) is one of the most natural radioactive elements widely used in the nuclear industry. In the civilian field, uranium is the most important nuclear fuel in nuclear reactors; militarily, uranium is an important raw material for nuclear weapons. In addition, uranium is also used for radiation shielding and ship ballast due to its high-density properties. Depending on the temperature, U has three kinds of allotrope phases: the orthogonal α phase at temperature below 940 K, the body-centered tetragonal (BCT) β phase at temperature ranging from 940 K to 1050 K, and the body-centered cubic (BCC) γ phase at temperature above 1050 K. Compared with the other two structures, the crystal structure of γ phase has good symmetry and excellent mechanical properties. However, γ-U is extremely unstable at low temperature. No matter what heat treatment method is adopted, γ-U will undergo phase transformation and become α phase. It is shown that adding certain alloying elements, such as Mo, Nb, Zr, Ti and Hf, into uranium can stabilize γ-U to room temperature and improve the mechanical properties of uranium greatly. As an important uranium alloy, γ-U by doping Mo atom has excellent mechanical properties, structural stability and thermal conductivity, and is an important nuclear reactor fuel. However, uranium has special physical and chemical properties due to its complex electronic structure and strong correlation of 5f electrons. Because of its special valence electron structure, it is highly susceptible to chemical and electrochemical reactions of environmental media. After the reaction between uranium and hydrogen, hydrogen embrittlement will occur, and even easily break into powder, which reduces the performance of uranium in service and brings hidden trouble to its storage. With the increase of service life, surface corrosion becomes more serious, and the safety and reliability of U alloys are seriously affected. The adsorption and dissociation of hydrogen on U alloy surface is the primary process of hydrogenation corrosion. Based on density functional theory, first-principles study of hydrogen adsorption and dissociation on γ-U(100) surface by Mo atoms coatings is carried out in this work. The model of γ-U(100) and Mo atoms coatings on γ-U(100) surface are established, and the structural parameters, adsorption energy, Bader charge, surface work function, and electron state density of H2 at highly symmetrical adsorption sites are calculated. The results show that H2 molecule occurs when physical dissociation adsorption takes place on γ-U(100) and U(100)/Mo surface. The electron state density shows that H2 does not bond to the surface atoms and no new hybridization peak appears. However, in the hollow parallel adsorption configuration, H2 is completely dissociated into two H atoms and occurs chemical adsorption and dissociation on γ-U(100) and U(100)/Mo surface. The H/1s orbital electrons are hybridized with the U/6p, U/6d, Mo/5s, Mo/4p, Mo/4d orbital electrons, and the H atom forms stable chemical bonds with the Mo atoms. Bader charge distribution results show that the change of chemical adsorption net charge of H2 on U(100)/Mo is more than that of physical adsorption. Because the adsorption energy of H2 in the most stable configuration (HMo-Hor) on U(100)/Mo is less than that of the most stable configuration (HU-Hor) on γ-U(100), the adsorption of H2 on U(100)/Mo is more stable than that of γ-U(100) surface. -
Keywords:
- U alloys /
- first principles /
- chemical adsorption /
- coating
[1] 伯格J J 著 (石琪 译) 1983 铀合金物理冶金 (北京: 原子能出版社) 第76—79页
Burke J J (translated by Shi Q)1983 Physical Metallurgy of Uranium Alloys (Beijing: Atomic Energy Press) pp76–79 (in Chinese)
[2] D. R. Lide, 2012 Handbook of Chemistry and Physics (Boca Raton: CRC) pp1–5
[3] Koelling D D, Freeman A J 1973 Phys. Rev. B 7 4454
 Google Scholar
Google Scholar
[4] David A Y, 1991 Phase Diagrams of the Elements (Berkeley: University of California Press) pp222–223
[5] Neogy, S, Laik A, Saify M. T, Jha S. K, Srivastava D, Dey G. K 2017 Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 6 2819
 Google Scholar
Google Scholar
[6] Kim-Ngan N, Tkach I, Mašková S, Gonçalves A, Havela L 2013 J. Alloys Compd. 580 223
 Google Scholar
Google Scholar
[7] Bajaj S, Landa A, Söderlind P, Turchi P E A, Arróyave R 2011 J. Nucl. Mater. 419 177
 Google Scholar
Google Scholar
[8] Yoo C S, Akella J, Moriarty J A 1993 Phys. Rev. B 48 15529
 Google Scholar
Google Scholar
[9] Shen Z Y, Kong Y, Du Y, Zhang S Y 2021 Calphad 72 102241
 Google Scholar
Google Scholar
[10] Söderlind P, Eriksson O, Johansson B, Wills J, Boring A 1995 Nature 374 524
 Google Scholar
Google Scholar
[11] Swissa W, Bloch J, Atzmony U, Mintz M H 1989 Surf. Sci. 214 323
 Google Scholar
Google Scholar
[12] McLean W, Colmenares C A, Smith R L, Somorjai G A 1982 Phys. Rev. B 25 8
 Google Scholar
Google Scholar
[13] Asada K, Ono K, Yamaguchi K, Yamamoto T, Maekawa A, Oe S, Yamawaki M 1995 J. Alloys Compd. 231 780
 Google Scholar
Google Scholar
[14] Banos A, Harker N J, Scott T B 2018 Corros. Sci. 136 129
 Google Scholar
Google Scholar
[15] Yang Y, Zhang P, Shi P, Wang X L 2011 J. Phys. Chem. C 115 23381
 Google Scholar
Google Scholar
[16] Chattaraj D, Parida S C, Majumder C 2011 Physica B 406 4317
 Google Scholar
Google Scholar
[17] Hasan, M Z, Hossain M M, Islam M S, Parvin F, Islam A K M A 2012 Comput. Mater. Sci. 63 256
 Google Scholar
Google Scholar
[18] 房彩红, 尚家香, 刘增辉 2012 61 047101
 Google Scholar
Google Scholar
Fang C H, Shang J X, Liu Z H 2012 Acta Phys. Sin. 61 047101
 Google Scholar
Google Scholar
[19] Liu G D, Liu Z X, Ao B Y, Hu W Y, Deng H Q 2018 Comput. Mater. Sci. 144 85
 Google Scholar
Google Scholar
[20] Cheng S, Li S, Liu J, Liu B, Zhang Z 2019 Nucl. Instrum. Meth. B 457 63
 Google Scholar
Google Scholar
[21] Tian X F, Wang Yu, Li L S, Wu M D, Yu Y 2020 Comput. Mater. Sci. 179 109633
 Google Scholar
Google Scholar
[22] Harris J, Andersson S 1985 Phys. Rev. Lett. 55 1583
 Google Scholar
Google Scholar
[23] Bloch J, Mintz M H 1996 J. Alloys Compd. 241 224
 Google Scholar
Google Scholar
[24] Bloch J, Mintz M H 1997 J. Alloys Compd. 253 529
 Google Scholar
Google Scholar
[25] Bingert J F, Hanrahan R J, Field R D 2004 J. Alloys Compd. 362 138
 Google Scholar
Google Scholar
[26] Greenbaum Y, Barlam D, Mintz M H, Shneck R Z 2008 J. Alloys Compd. 452 325
 Google Scholar
Google Scholar
[27] Harker R M 2006 J. Alloys Compd. 426 106
 Google Scholar
Google Scholar
[28] Teter D F, Hanrahan R J, Wetteland C J 2000 Uranium Hydride Initation Kinetics: Effect of Oxide Thickness (New Mexico: Los Alamos National Laboratory) pp1–8
[29] Teter D F, Hanrahan R J, Wetteland C J 2001 Uranium Hydride Nucleation Kinetics: Effects of Oxide Thickness and Vacuum Outgassing (New Mexico: Los alamos national laboratory) pp1–15
[30] Bazley S G, Petherbridge J R, Glascott J 2012 Solid State Ionics 211 1
 Google Scholar
Google Scholar
[31] Kim K H, Park J M, Kim C K, Hofman G L, Meyer M K 2002 Nucl. Eng. Des. 211 229
 Google Scholar
Google Scholar
[32] Park J M, Kim K H, Kim C K, Meyer M K, Hofman, G L, Strain R V 2001 Met. Mater. Int. 7 151
 Google Scholar
Google Scholar
[33] Hohenberg P, Kohn W 1964 Phys. Rev. 136 B864
 Google Scholar
Google Scholar
[34] Kohn W, Sham L J 1965 Phys. Rev. 140 A1133
 Google Scholar
Google Scholar
[35] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[36] Kresse G, Hafner J 1993 Phys. Rev. B Condens. Matter. 48 13115
 Google Scholar
Google Scholar
[37] Kresse G, Joubert D 1999 Phys. Rev. B Condens. Matter. 59 1758
 Google Scholar
Google Scholar
[38] Perdew J P, Chevary J A, Vosko S H, Jackson K A, Pederson M R, Singh D J, Fiolhais C 1993 Phys. Rev. B Condens. Matter. 46 6671
[39] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[40] Pack James D, Monkhorst H J 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[41] 蒙大桥, 罗文华, 李赣, 陈虎翅 2009 58 8224
 Google Scholar
Google Scholar
Meng D Q, Luo W H, Li G, Chen H C 2009 Acta Phys. Sin. 58 8224
 Google Scholar
Google Scholar
[42] Payne M C, Teter M P, Allan D C, Arias T A, Joannopoulos J D 1992 Rev. Modern phys. 64 1045
 Google Scholar
Google Scholar
[43] Xiang S K, Huang H C, Hsiung L M 2008 J. Nucl. Mater. 375 113
 Google Scholar
Google Scholar
[44] Chiotti P, Klepfer H H, White R W 1959 Trans. Am. Soc. Met. 51 772
[45] 李赣, 罗文华, 陈虎翅 2010 物理化学学报 22 1283
Li G, Luo W H, Chen H C 2010 Chem. Res. Appl. 22 1283
[46] Neugebauer J, Scheffler M 1992 Phys. Rev. B 46 16067
 Google Scholar
Google Scholar
[47] Henkelman G, Arnaldsson A, Jónsson H 2006 Comput. Mater. Sci. 36 354
 Google Scholar
Google Scholar
[48] Electronegativity of Chemical Elements, material-properties https://material-properties.org/electronegativity-of-chemical-elements/
[49] Hopkins B J, Sargood A J 1967 Properties of Vapor-Deposited Uranium Films in Ultrahigh Vacuum And In Hydrogen (Southampton: Southampton University) pp1–15
[50] Lea C, Mee C H B 1968 J. Appl. Phys. 39 5890
 Google Scholar
Google Scholar
[51] Hao Y G, Eriksson O, Fernando G W 1993 Phys. Rev. B Condens. Matter. 47 6680
 Google Scholar
Google Scholar
[52] BéNARD J, BERTHIER Y. 1983 Adsorption on Metal Surfaces: An Integrated Approach (New York: Elsevier Scientific Pub. Co.) pp151–165
[53] Soon A, Todorova M, Delley B, Stampfl C 2007 Phys. Rev. B 75 125420
 Google Scholar
Google Scholar
[54] Fu C F, Sun J Y, Luo Q Q, Li X X, Hu W, Yang J L 2018 Nano Lett. 18 6312
 Google Scholar
Google Scholar
[55] Yu S Q, Wei W, Li F P, Huang B B, Dai Y 2020 Phys. Chem. 22 25675
-
图 2 H2在γ-U (100) 表面吸附模型的俯视图和侧视图 (a) 顶位平行; (b) 顶位垂直; (c) 空位平行; (d) 空位垂直; (e) 桥位平行; (f) 桥位平行2; (g) 桥位垂直. H和U元素分别为红色和蓝色
Fig. 2. Top and side views of absorption models of H2 molecule on γ-U (100) surface: (a) Top parallel; (b) top vertical; (c) hollow parallel; (d) hollow vertical; (e) bridge parallel; (f) bridge parallel 2; (g) bridge vertical. Hydrogen and uranium elements are red and blue, respectively.
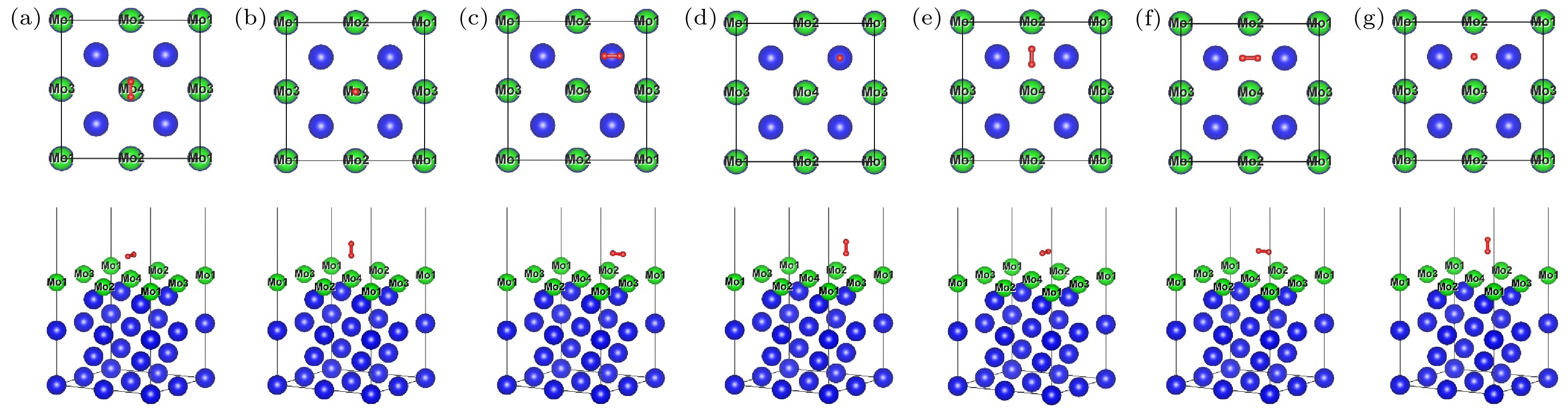
图 3 H2在U (100)/Mo表面吸附模型的俯视图和侧视图 (a) 顶位平行; (b) 顶位垂直; (c) 空位平行; (d) 空位垂直; (e) 桥位平行; (f) 桥位平行2; (g) 桥位垂直. H元素、U元素、Mo元素分别为红色、蓝色和绿色
Fig. 3. Top and side views of absorption models of H2 molecule on U (100)/Mo surface: (a) Top parallel; (b) top vertical; (c) hollow parallel; (d) hollow vertical; (e) bridge parallel; (f) bridge parallel 2; (g) bridge vertical. Hydrogen, uranium and molybdenum elements are red, blue and green, respectively.
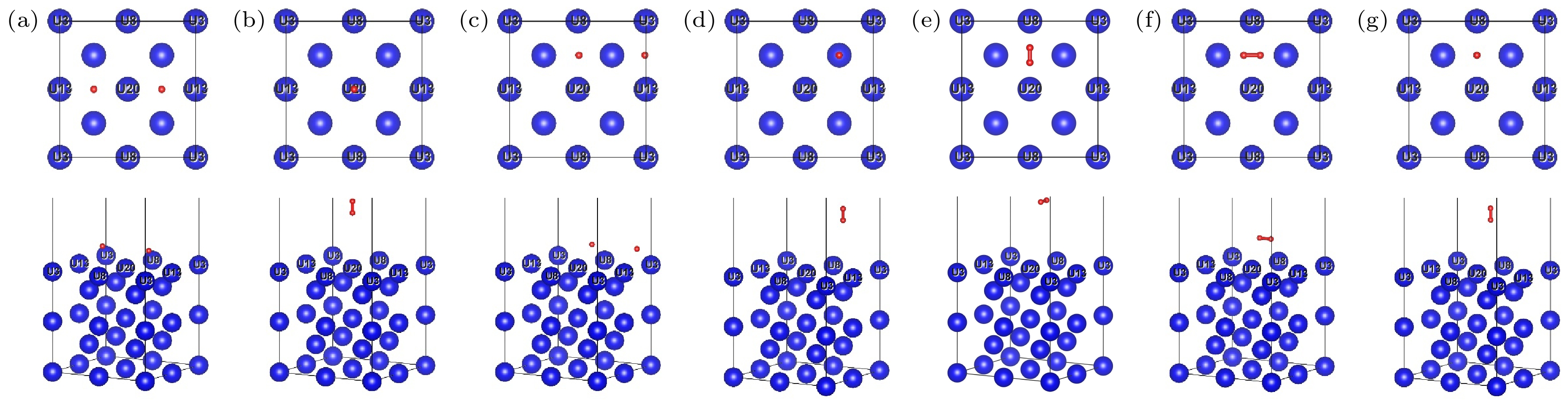
图 4 H2在γ-U (100) 表面吸附优化后的俯视图和侧视图 (a) TU-Hor; (b) TU-Ver; (c) HU-Hor; (d) HU-Ver; (e) BU-Hor; (f) BU-Hor2; (g) BU-Ver
Fig. 4. Top and side views of the optimization structures for H2 molecule absorption on γ-U (100) surface: (a) TU-Hor; (b) TU-Ver; (c) HU-Hor; (d) HU-Ver; (e) BU-Hor; (f) BU-Hor2; (g) BU-Ver.
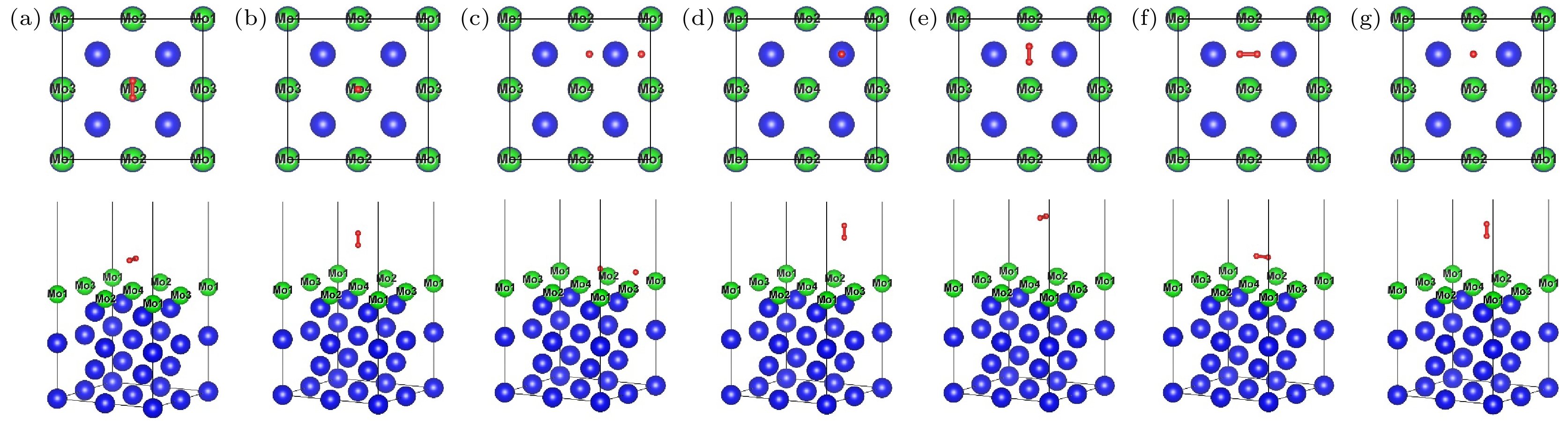
图 5 H2在U (100)/Mo表面吸附优化后的俯视图和侧视图 (a) TMo-Hor; (b) TMo-Ver; (c) HMo-Hor; (d) HMo-Ver; (e) BMo-Hor; (f) BMo-Hor2; (g) BMo-Ver
Fig. 5. Top and side views of the optimization structures for H2 molecule absorption on U (100)/Mo surface: (a) TMo-Hor; (b) TMo-Ver; (c) HMo-Hor; (d) HMo-Ver; (e) BMo-Hor; (f) BMo-Hor2; (g) BMo-Ver.
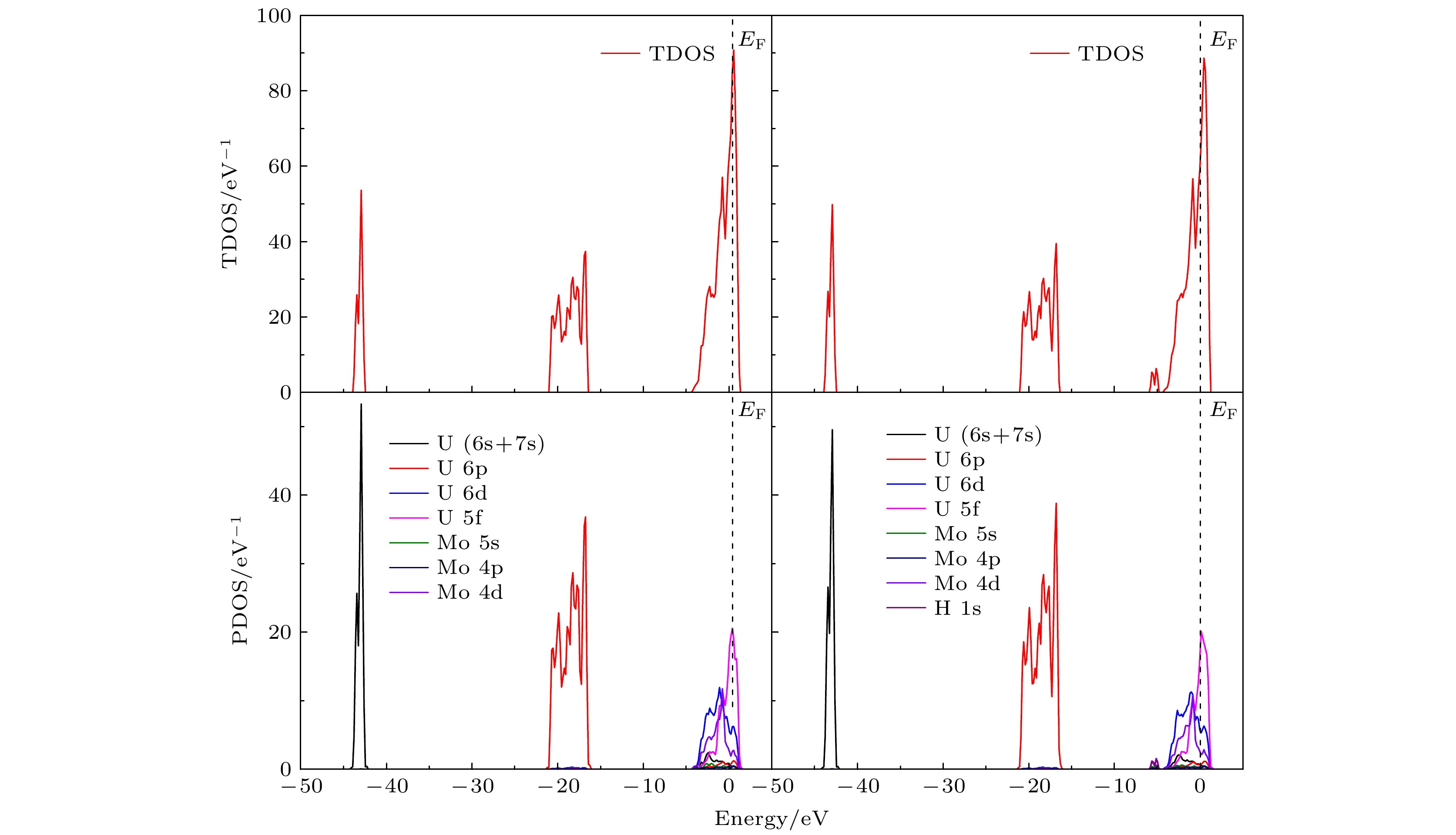
图 7 (a) U (100)/Mo表面的总态密度; (b) H2-U (100)/Mo吸附体系中最稳定吸附状态(HMo-Hor)总态密度; (c) U (100)/Mo表面的分态密度; (d) H2-U (100)/Mo吸附体系中最稳定吸附状态(HMo-Hor)分态密度
Fig. 7. (a) TDOS of the clean U (100)/Mo surface; (b) TDOS of the most stable configuration (HMo-Hor) for H2-U (100)/Mo adsorption system; (c) PDOS of the clean U (100)/Mo surface; (d) PDOS of the most stable configuration (HMo-Hor) for H2-U (100)/Mo adsorption system.
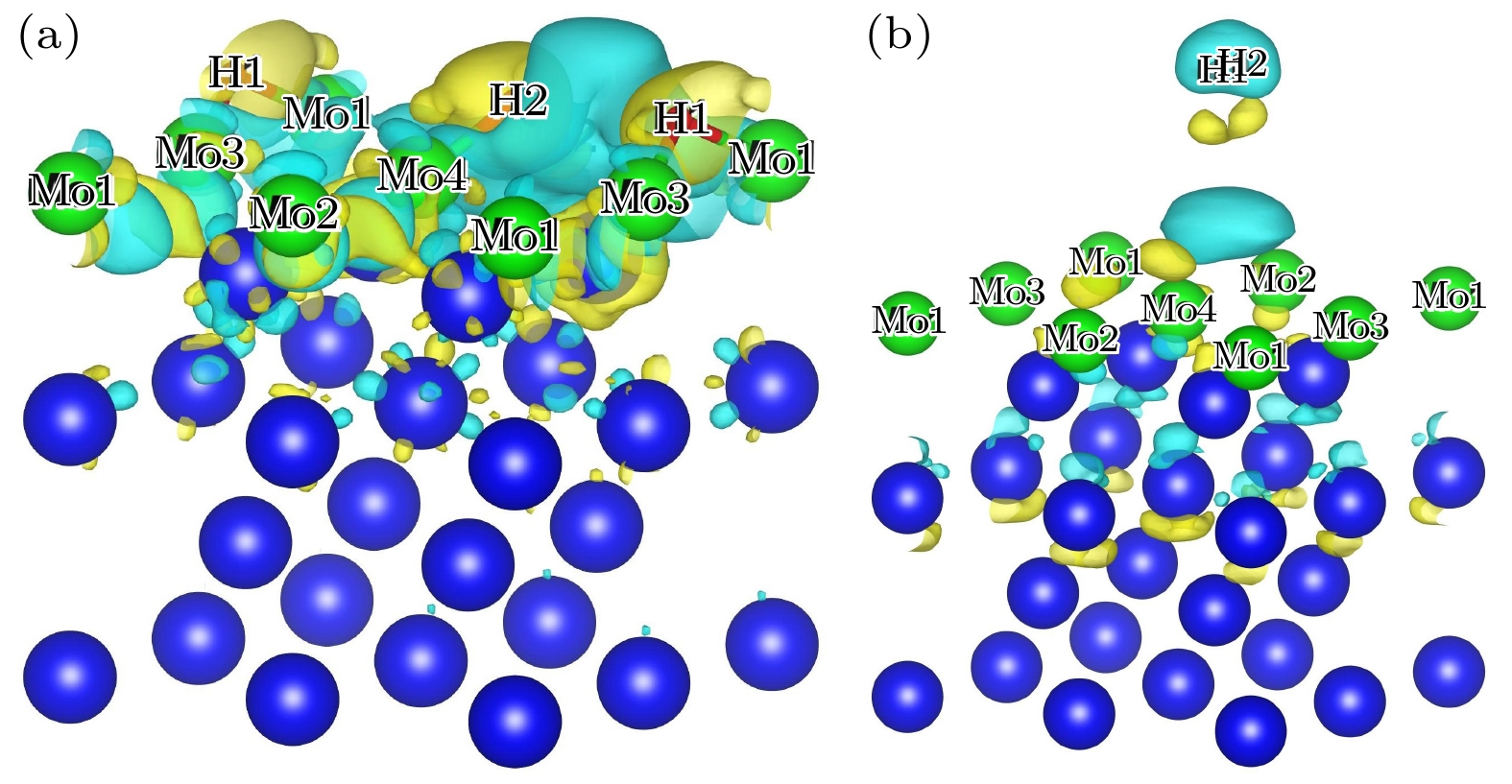
图 9 (a) HMo-Hor吸附构型差分电荷密度 (等值面: 0.0015 e/Å3); (b) BMo-Hor吸附构型差分电荷密度(等值面: 4×10–5 e/Å3), 黄色表示电荷密度增大, 蓝色表示电荷密度减小
Fig. 9. (a) Isosurfaces of differential charge density for the HMo-Hor configuration (Isosurfaces level: 0.0015 e/Å3); (b) isosurfaces of differential charge density for the BMo-Hor configuration (Isosurfaces level: 4×10–5 e/Å3), yellow means an increase in charge density and blue means a decrease in charge density.
表 1 氢气吸附在γ-U(100) 表面的吸附能和几何结构参数
Table 1. Absorption energy and geometrical parameters of H2 adsorption on the γ-U(100) surface.
Configuration Eads/eV hH1-Surf/Å hH2-Surf/Å dH1-U/Å dH2-U/Å dH1-H2/Å TU–Hor –0.451 1.296 1.296 2.150 2.150 3.430 TU–Ver –0.020 3.636 4.389 3.636 4.389 0.753 HU–Hor –0.454 1.301 1.301 2.155 2.155 3.330 HU–Ver –0.028 3.358 4.112 4.143 4.775 0.755 BU–Hor –0.014 4.183 4.183 4.393 4.393 0.750 BU–Hor2 0.030 1.767 1.767 2.498 2.498 0.829 BU–Ver –0.021 3.258 4.014 3.683 4.365 0.756 表 2 氢气吸附在U(100)/Mo表面的吸附能和几何结构参数
Table 2. Absorption energy and geometrical parameters of H2 adsorption on the U(100)/Mo surface.
Configuration Eads/eV hH1-Surf/Å hH2-Surf/Å dH1-U/Å dH2-U/Å dH1-Mo/Å dH2-Mo/Å dH1-H2/Å TMo–Hor –0.331 1.978 1.978 3.849 3.849 2.019 2.019 0.807 TMo–Ver –0.026 2.635 3.390 3.390 3.390 2.635 2.635 0.755 HMo–Hor –0.746 0.783 0.783 1.939 1.939 2.381 2.381 2.540 HMo–Ver –0.029 3.756 3.003 4.962 4.209 4.472 3.861 0.753 BMo–Hor –0.015 4.016 4.016 5.509 5.509 4.234 4.234 0.751 BMo–Hor2 0.118 1.599 1.599 3.095 3.095 2.381 2.381 0.819 BMo–Ver –0.029 3.715 2.960 5.211 4.506 4.092 3.422 0.754 表 3 H2-U(100)/Mo体系的Bader电荷布局数, qH1和qH2为第一个和第二个氢原子的Bader电荷, qtotal为两个氢原子上的总Bader电荷数, q1st, q2nd, q3rd, q4th和q5th分别表示U(100)/Mo表面第1层到第5层的Bader电荷数
Table 3. Bader charge distribution number of H2-U(100)/Mo system. qH1 and qH2 are the Bader charge number of the H1 and H2 atom, qtotal is the total Bader charge number of the H1 and H2 atoms, q1st, q2nd, q3rd, q4th and q5th represent the total Bader charge number of first to fifth layers on the U(100)/Mo surface, respectively.
Configuration qH1/e qH2/e qtotal/e q1 st/e q2 nd/e q3 rd/e q4 th/e q5 th/e Atom 0.0616 –0.0616 0 — — — — — free surface — — — 1.0016 –0.5646 –0.6812 0.7102 –0.5094 TMo-Hor –0.0297 0.0883 0.0586 0.9551 –0.6080 –0.6148 0.6679 –0.5000 TMo-Ver –0.0619 0.0812 0.0193 1.0149 –0.5955 –0.6572 0.6838 –0.5089 HMo-Hor 0.3806 0.3806 0.7612 0.4796 –0.8028 –0.6881 0.6848 –0.4759 HMo-Ver –0.0362 0.0504 0.0142 0.9853 –0.5404 –0.7052 0.7109 –0.5094 BMo-Hor –0.0665 0.0700 0.0035 1.0006 –0.5761 –0.6594 0.6830 –0.5085 BMo-Hor2 0.1220 0.0058 0.1278 0.9261 –0.6205 –0.6598 0.6697 –0.4848 BMo-Ver 0.0317 –0.0162 0.0155 1.0037 –0.5741 –0.6773 0.6963 –0.5087 表 A1 γ-U (100) , U (100)/Mo表面弛豫度, Δdij表示第i层和第j层原子间的平均距离, d0表示γ-U晶胞优化后的晶格常数
Table A1. The relative surface relaxation for the γ-U (100) and U (100)/Mo, Δdij represents the average distance between the i-th and j-th atomic layer of these surfaces. d0 represents the lattice constant of γ-U unit cell after optimization.
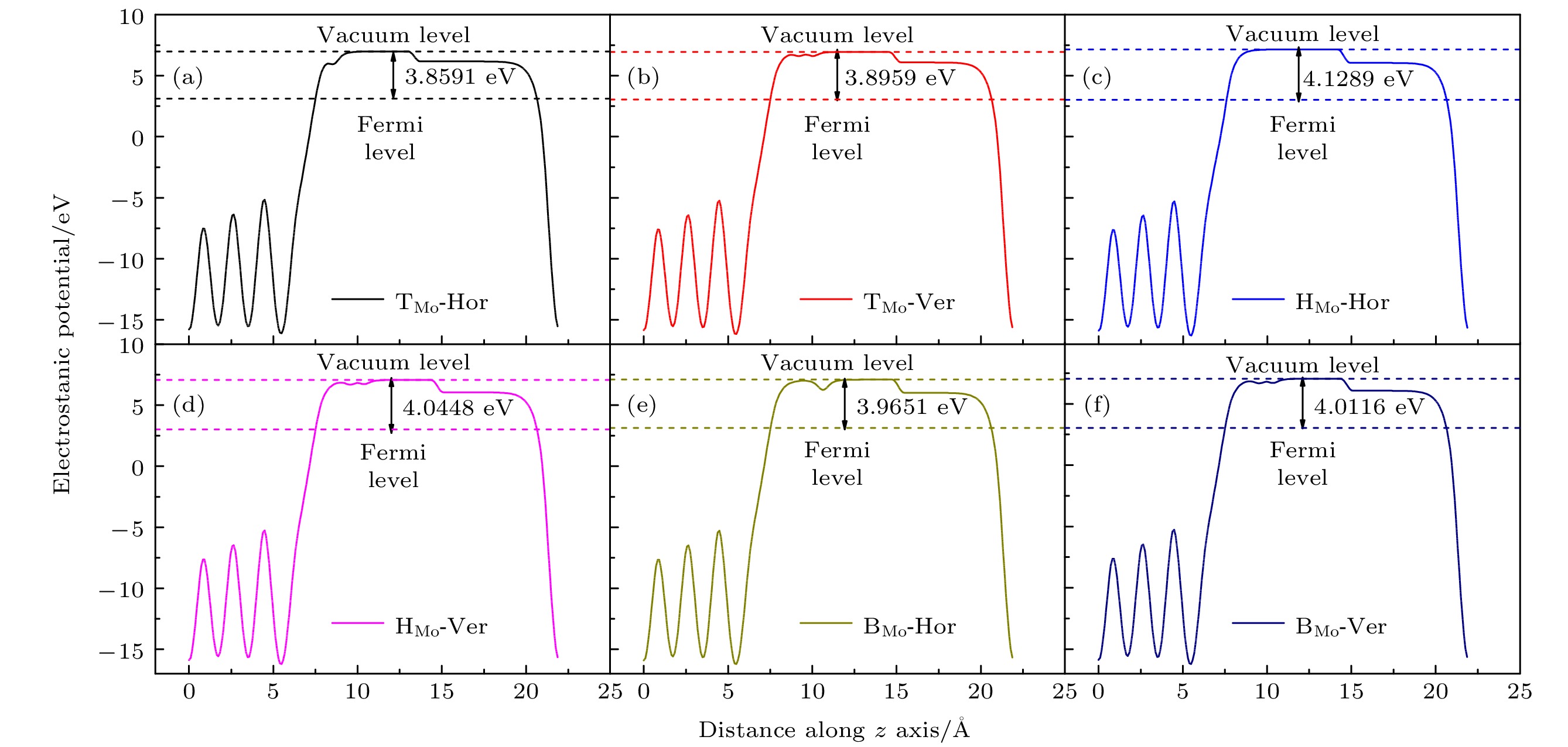
Slap γ-U(100) 文献[15] U(100)/Mo Δd12/d0 –25.041% –26.4% –29.875% Δd23/d0 14.239% 15.6% 8.773% Δd34/d0 –8.289% — 4.246% 表 A2 H2分子在U(100)/Mo表面不同吸附点位表面功函数变化, ΔΦ为功函数的变化
Table A2. Surface work function changes of H2 molecule at different adsorption sites on U(100)/Mo Surface, ΔΦ is the change of the work function.
Configuration Evacuum/eV EFermi/eV Φ/eV ΔΦ/eV Free surface 7.1244 3.0700 4.0544 — TMo-Hor 6.9897 3.1306 3.8591 –0.1953 TMo-Ver 6.9469 3.0510 3.8959 –0.1585 HMo-Hor 7.1502 3.0213 4.1289 0.0745 HMo-Ver 7.0689 3.0241 4.0448 –0.0096 BMo-Hor 7.1045 3.1394 3.9651 –0.0893 BMo-Ver 7.0430 3.0314 4.0116 –0.0428 -
[1] 伯格J J 著 (石琪 译) 1983 铀合金物理冶金 (北京: 原子能出版社) 第76—79页
Burke J J (translated by Shi Q)1983 Physical Metallurgy of Uranium Alloys (Beijing: Atomic Energy Press) pp76–79 (in Chinese)
[2] D. R. Lide, 2012 Handbook of Chemistry and Physics (Boca Raton: CRC) pp1–5
[3] Koelling D D, Freeman A J 1973 Phys. Rev. B 7 4454
 Google Scholar
Google Scholar
[4] David A Y, 1991 Phase Diagrams of the Elements (Berkeley: University of California Press) pp222–223
[5] Neogy, S, Laik A, Saify M. T, Jha S. K, Srivastava D, Dey G. K 2017 Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 6 2819
 Google Scholar
Google Scholar
[6] Kim-Ngan N, Tkach I, Mašková S, Gonçalves A, Havela L 2013 J. Alloys Compd. 580 223
 Google Scholar
Google Scholar
[7] Bajaj S, Landa A, Söderlind P, Turchi P E A, Arróyave R 2011 J. Nucl. Mater. 419 177
 Google Scholar
Google Scholar
[8] Yoo C S, Akella J, Moriarty J A 1993 Phys. Rev. B 48 15529
 Google Scholar
Google Scholar
[9] Shen Z Y, Kong Y, Du Y, Zhang S Y 2021 Calphad 72 102241
 Google Scholar
Google Scholar
[10] Söderlind P, Eriksson O, Johansson B, Wills J, Boring A 1995 Nature 374 524
 Google Scholar
Google Scholar
[11] Swissa W, Bloch J, Atzmony U, Mintz M H 1989 Surf. Sci. 214 323
 Google Scholar
Google Scholar
[12] McLean W, Colmenares C A, Smith R L, Somorjai G A 1982 Phys. Rev. B 25 8
 Google Scholar
Google Scholar
[13] Asada K, Ono K, Yamaguchi K, Yamamoto T, Maekawa A, Oe S, Yamawaki M 1995 J. Alloys Compd. 231 780
 Google Scholar
Google Scholar
[14] Banos A, Harker N J, Scott T B 2018 Corros. Sci. 136 129
 Google Scholar
Google Scholar
[15] Yang Y, Zhang P, Shi P, Wang X L 2011 J. Phys. Chem. C 115 23381
 Google Scholar
Google Scholar
[16] Chattaraj D, Parida S C, Majumder C 2011 Physica B 406 4317
 Google Scholar
Google Scholar
[17] Hasan, M Z, Hossain M M, Islam M S, Parvin F, Islam A K M A 2012 Comput. Mater. Sci. 63 256
 Google Scholar
Google Scholar
[18] 房彩红, 尚家香, 刘增辉 2012 61 047101
 Google Scholar
Google Scholar
Fang C H, Shang J X, Liu Z H 2012 Acta Phys. Sin. 61 047101
 Google Scholar
Google Scholar
[19] Liu G D, Liu Z X, Ao B Y, Hu W Y, Deng H Q 2018 Comput. Mater. Sci. 144 85
 Google Scholar
Google Scholar
[20] Cheng S, Li S, Liu J, Liu B, Zhang Z 2019 Nucl. Instrum. Meth. B 457 63
 Google Scholar
Google Scholar
[21] Tian X F, Wang Yu, Li L S, Wu M D, Yu Y 2020 Comput. Mater. Sci. 179 109633
 Google Scholar
Google Scholar
[22] Harris J, Andersson S 1985 Phys. Rev. Lett. 55 1583
 Google Scholar
Google Scholar
[23] Bloch J, Mintz M H 1996 J. Alloys Compd. 241 224
 Google Scholar
Google Scholar
[24] Bloch J, Mintz M H 1997 J. Alloys Compd. 253 529
 Google Scholar
Google Scholar
[25] Bingert J F, Hanrahan R J, Field R D 2004 J. Alloys Compd. 362 138
 Google Scholar
Google Scholar
[26] Greenbaum Y, Barlam D, Mintz M H, Shneck R Z 2008 J. Alloys Compd. 452 325
 Google Scholar
Google Scholar
[27] Harker R M 2006 J. Alloys Compd. 426 106
 Google Scholar
Google Scholar
[28] Teter D F, Hanrahan R J, Wetteland C J 2000 Uranium Hydride Initation Kinetics: Effect of Oxide Thickness (New Mexico: Los Alamos National Laboratory) pp1–8
[29] Teter D F, Hanrahan R J, Wetteland C J 2001 Uranium Hydride Nucleation Kinetics: Effects of Oxide Thickness and Vacuum Outgassing (New Mexico: Los alamos national laboratory) pp1–15
[30] Bazley S G, Petherbridge J R, Glascott J 2012 Solid State Ionics 211 1
 Google Scholar
Google Scholar
[31] Kim K H, Park J M, Kim C K, Hofman G L, Meyer M K 2002 Nucl. Eng. Des. 211 229
 Google Scholar
Google Scholar
[32] Park J M, Kim K H, Kim C K, Meyer M K, Hofman, G L, Strain R V 2001 Met. Mater. Int. 7 151
 Google Scholar
Google Scholar
[33] Hohenberg P, Kohn W 1964 Phys. Rev. 136 B864
 Google Scholar
Google Scholar
[34] Kohn W, Sham L J 1965 Phys. Rev. 140 A1133
 Google Scholar
Google Scholar
[35] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[36] Kresse G, Hafner J 1993 Phys. Rev. B Condens. Matter. 48 13115
 Google Scholar
Google Scholar
[37] Kresse G, Joubert D 1999 Phys. Rev. B Condens. Matter. 59 1758
 Google Scholar
Google Scholar
[38] Perdew J P, Chevary J A, Vosko S H, Jackson K A, Pederson M R, Singh D J, Fiolhais C 1993 Phys. Rev. B Condens. Matter. 46 6671
[39] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[40] Pack James D, Monkhorst H J 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[41] 蒙大桥, 罗文华, 李赣, 陈虎翅 2009 58 8224
 Google Scholar
Google Scholar
Meng D Q, Luo W H, Li G, Chen H C 2009 Acta Phys. Sin. 58 8224
 Google Scholar
Google Scholar
[42] Payne M C, Teter M P, Allan D C, Arias T A, Joannopoulos J D 1992 Rev. Modern phys. 64 1045
 Google Scholar
Google Scholar
[43] Xiang S K, Huang H C, Hsiung L M 2008 J. Nucl. Mater. 375 113
 Google Scholar
Google Scholar
[44] Chiotti P, Klepfer H H, White R W 1959 Trans. Am. Soc. Met. 51 772
[45] 李赣, 罗文华, 陈虎翅 2010 物理化学学报 22 1283
Li G, Luo W H, Chen H C 2010 Chem. Res. Appl. 22 1283
[46] Neugebauer J, Scheffler M 1992 Phys. Rev. B 46 16067
 Google Scholar
Google Scholar
[47] Henkelman G, Arnaldsson A, Jónsson H 2006 Comput. Mater. Sci. 36 354
 Google Scholar
Google Scholar
[48] Electronegativity of Chemical Elements, material-properties https://material-properties.org/electronegativity-of-chemical-elements/
[49] Hopkins B J, Sargood A J 1967 Properties of Vapor-Deposited Uranium Films in Ultrahigh Vacuum And In Hydrogen (Southampton: Southampton University) pp1–15
[50] Lea C, Mee C H B 1968 J. Appl. Phys. 39 5890
 Google Scholar
Google Scholar
[51] Hao Y G, Eriksson O, Fernando G W 1993 Phys. Rev. B Condens. Matter. 47 6680
 Google Scholar
Google Scholar
[52] BéNARD J, BERTHIER Y. 1983 Adsorption on Metal Surfaces: An Integrated Approach (New York: Elsevier Scientific Pub. Co.) pp151–165
[53] Soon A, Todorova M, Delley B, Stampfl C 2007 Phys. Rev. B 75 125420
 Google Scholar
Google Scholar
[54] Fu C F, Sun J Y, Luo Q Q, Li X X, Hu W, Yang J L 2018 Nano Lett. 18 6312
 Google Scholar
Google Scholar
[55] Yu S Q, Wei W, Li F P, Huang B B, Dai Y 2020 Phys. Chem. 22 25675
计量
- 文章访问数: 8407
- PDF下载量: 155
- 被引次数: 0














 下载:
下载: