-
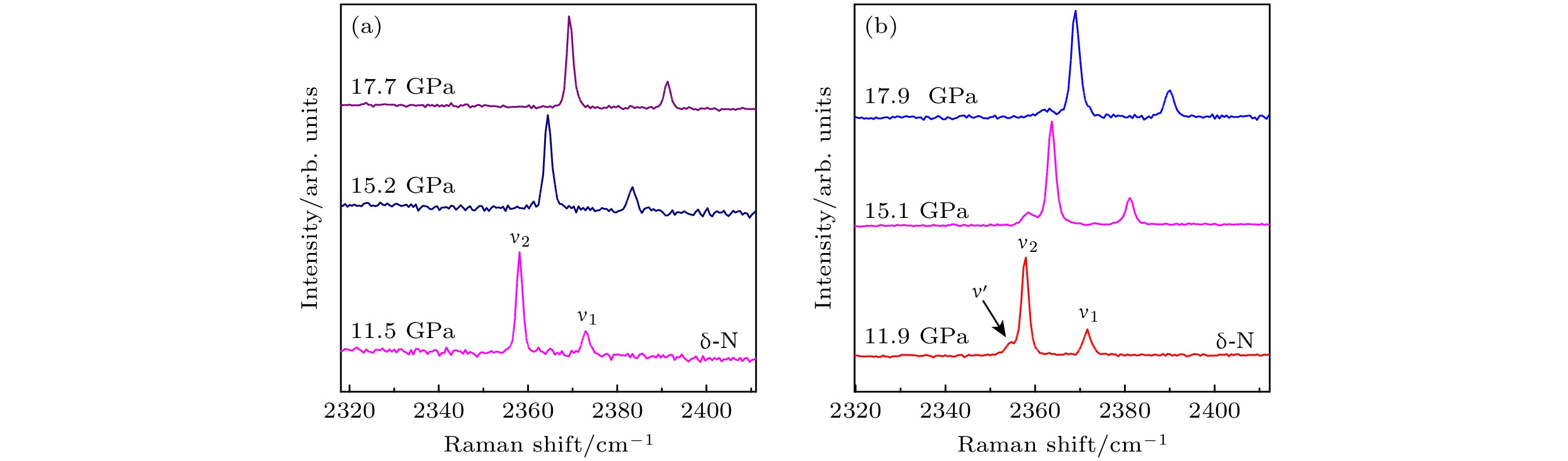
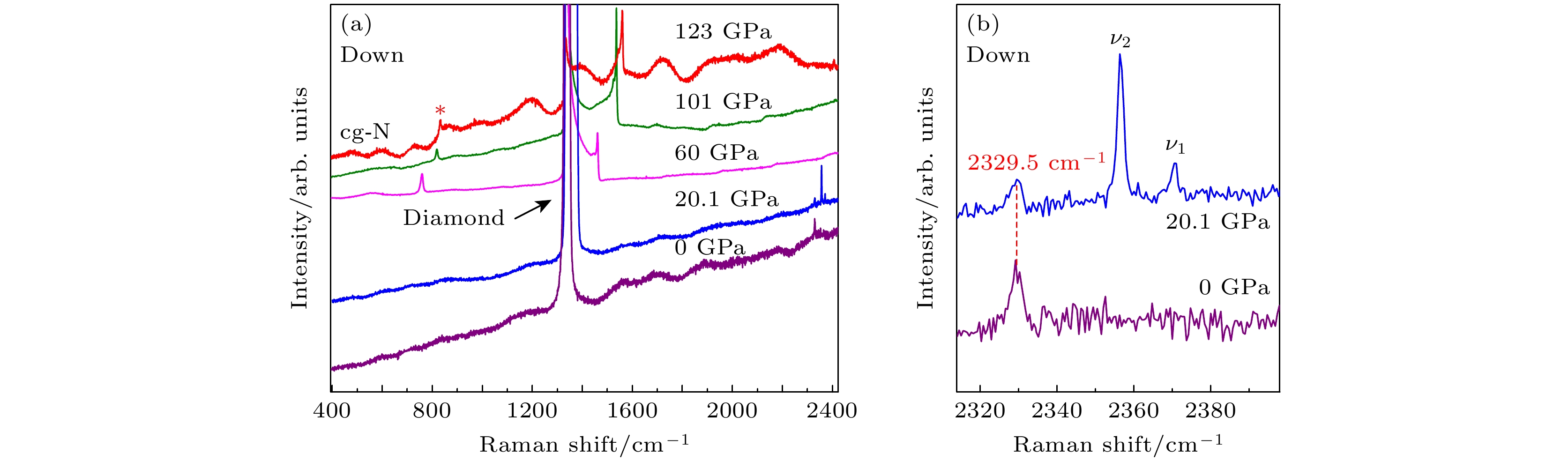
聚合氮被认为是一种极具潜力的新型高能量密度材料, 但是高温高压条件下合成的聚合氮结构往往具有较低热力学稳定性. 限域策略有助于聚合氮高压结构的稳定, 为氮聚合提供了新的调控途径. 本文在氮化硼纳米管中限域分子氮, 利用高压原位拉曼散射光谱表征技术研究不同含氮量限域体系的高压诱导氮聚合及聚合氮结构的卸压稳定性. 研究表明, 在高含氮量的体系中, 限域到氮化硼纳米管内的N2与非限域的N2的拉曼特征振动峰表现出不同的拉曼光谱压力响应行为. 在123 GPa压力下, 利用激光加热诱导氮分子间聚合, 生成cg-N聚合氮结构. 卸压过程中, 未被限域的cg-N在40 GPa左右发生爆炸性分解, 分解产生的能量影响了限域cg-N的稳定性, 使其同样发生分解. 环境压力下限域N2可能以液态形式稳定存在. 在低含氮量限域体系中, 高温高压下限域N2结晶生成了含有N=N双键的晶体结构, 其中的N=N双键有两种长度, 分别接近
${\mathrm{N}}_3^- $ 阴离子及${\mathrm{N}}_4^+ $ 团簇中N=N双键的键长. 在卸压过程中这种结构可以稳定至25 GPa.Polymeric nitrogen has been recognized to be a new type of high-energy density material (HEDM). However, the polymeric nitrogen structure formed under high-pressure and high-temperature conditions is usually in poor thermodynamic stability. Confinement strategy is conductive to the stabilization of the high-pressure phase of polymeric nitrogen structures, providing a new modulation approach for realizing the polymerization of nitrogen. In this work, nitrogen molecules are confined into the boron nitride nanotubes (N2@BNNTs) under high-pressure condition. The pressure-induced polymerization of nitrogen in N2@BNNT samples with varying nitrogen content and the stabilities of polymeric nitrogen structure are characterized by high-pressure in situ Raman spectroscopy method. In the N2@BNNT sample with higher nitrogen content, the N2 confined to boron nitride nanotubes exhibits different Raman spectral pressure response behaviors compared with that of non confined N2, but both of them are transformed into cg-N structure after laser heating at about 123 GPa. With pressure decreasing to 40 GPa, the unconfined cg-N decomposes and releases huge energy, which affects the stability and results in the decomposition of the confined cg-N. Under ambient conditions, the confined N2 is stabilized in the liquid phase. In the N2@BNNTs sample with lower nitrogen content, the confined N2 is transformed into new polymeric nitrogen structure, which possesses N=N double bonds with different bond lengths close to the those in the${\mathrm{N}}_3^- $ anion and${\mathrm{N}}_4^+ $ clusters, respectively, after laser-heating in the pressure range of 122–150 GPa. This polynitrogen structure is stable with pressure decreasing to 25 GPa. This work provides new insights into the synthesis and stabilization of polymeric nitrogen structures, opening up new avenues for developing these advanced structures.-
Keywords:
- confinement /
- polymerization /
- high temperature and high pressure
[1] Légaré M A, Rang M, Bélanger-Chabot G, Schweizer J I, Krummenacher I, Bertermann R, Arrowsmith M, Holthausen M C, Braunschweig H 2019 Science 363 1329
 Google Scholar
Google Scholar
[2] Qi C, Li S H, Li Y C, Wang Y, Chen X K, Pang S P 2011 J. Mater. Chem. 21 3221
 Google Scholar
Google Scholar
[3] Klapötke T M, Piercey D G 2011 Inorg. Chem. 50 2732
 Google Scholar
Google Scholar
[4] Li Y C, Qi C, Li S H, Zhang H J, Sun C H, Yu Y Z, Pang S P 2010 J. Am. Chem. Soc. 132 12172
 Google Scholar
Google Scholar
[5] Eremets M I, Gavriliuk A G, Trojan I A, Dzivenko D A, Boehler R 2004 Nat. Mater. 3 558
 Google Scholar
Google Scholar
[6] Tomasino D, Kim M, Smith J, Yoo C S 2014 Phys. Rev. Lett. 113 205502
 Google Scholar
Google Scholar
[7] Laniel D, Geneste G, Weck G, Mezouar M, Loubeyre P 2019 Phys. Rev. Lett. 122 066001
 Google Scholar
Google Scholar
[8] Ji C, Adeleke A A, Yang L X, Wan B, Gou H Y 1, Yao Y S, Li B1, Meng Y, Smith J S, Prakapenka V B, Liu W J, Shen G Y, Mao W L, Mao H K 2019 Nat. Commun. 10 4515
 Google Scholar
Google Scholar
[9] Abou-Rachid H, Hu A, Timoshevskii V, Song Y F, Lussier L S 2008 Phys. Rev. Lett. 100 196401
 Google Scholar
Google Scholar
[10] VTimoshevskii V, Ji W, Abou-Rachid H, Lussier L S, Guo H 2009 Phys. Rev. B 80 115409
 Google Scholar
Google Scholar
[11] Shi X H, Liu B, Liu S J, Niu S F, Liu S, Liu R, Liu B B 2018 Sci. Rep. 8 13758
 Google Scholar
Google Scholar
[12] Li S, Li H Y, Yao Z, Lu S C 2021 Mater. Today. Commun. 26 101670
 Google Scholar
Google Scholar
[13] Lv H, Yao M G, Li Q J, Liu R, Liu B, Yao Z, Liu D D, Liu Z D, Liu J, Chen Z Q, Zou B, Cui T, Liu B B 2015 Sci. Rep. 5 13234
 Google Scholar
Google Scholar
[14] Wu Z Y, Benchafia E M, Iqbal Z, Wang X Q 2014 Chem. Int. Ed. 53 12555
 Google Scholar
Google Scholar
[15] Zhang C, Sun C, Hu B C, Yu C M, Lu M 2017 Science 355 374
 Google Scholar
Google Scholar
[16] Zhang C, Yang C, Yu C M, Zheng Z S, Sun C G 2017 Angew. Chem. 56 4512
 Google Scholar
Google Scholar
[17] Schneider H, Hafner W, Wokaun A, Olijnyk H 1992 J. Chem. Phys. 96 8046
 Google Scholar
Google Scholar
[18] Schiferl D, Buchsbaum S, Mills R L 1985 J. Phys. Chem. 89 2324
 Google Scholar
Google Scholar
[19] Eremets M I, Popov Yu M, Trojan I A, Denisov V N, Boehle R R 2004 J. Chem. Phys. 120 10618
 Google Scholar
Google Scholar
[20] Medvedev S A, Trojan I A, Eremets M I, Palasyuk T, Klapotke T M, Evers J 2009 J. Phys. Condens. Matter. 21 195404
 Google Scholar
Google Scholar
[21] Steele B A, Stavrou E, Crowhurst J C, Zaug J M, Prakapenka V B, Oleynik I I 2017 Chem. Mater. 29 735
 Google Scholar
Google Scholar
[22] Bartlett R J, web site http://www.qtp.ufl.edu/; bartlett/ downloads/polynitrog-en.pdf
[23] Lauderdale W J, Stanton J F, Bartlett R J 1992 J. Phys. Chem. 96 1173
 Google Scholar
Google Scholar
[24] Fathalizadeh A, Pham T, Mickelson W, Zettl A 2014 Nano. Lett. 14 48
 Google Scholar
Google Scholar
[25] Lin J F, Santoro M, Struzhkin V V, Mao H K, Hemley R J, 2004 Rev. Sci. Instrum. 75 3302
 Google Scholar
Google Scholar
-
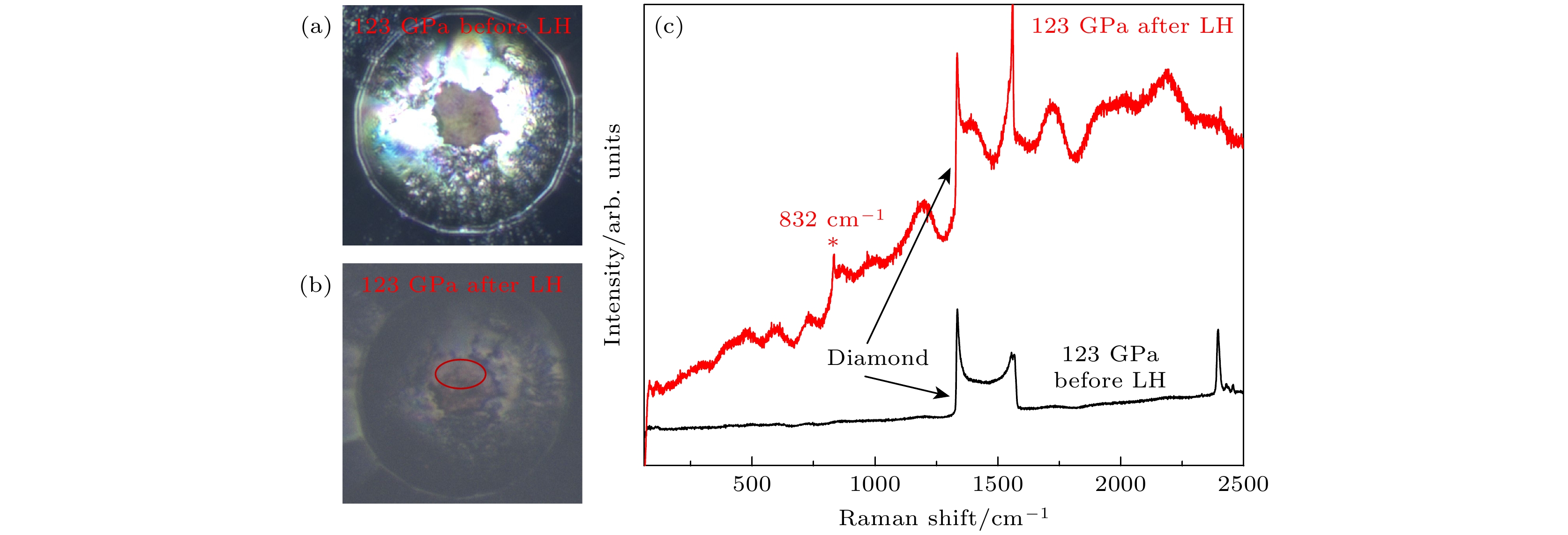
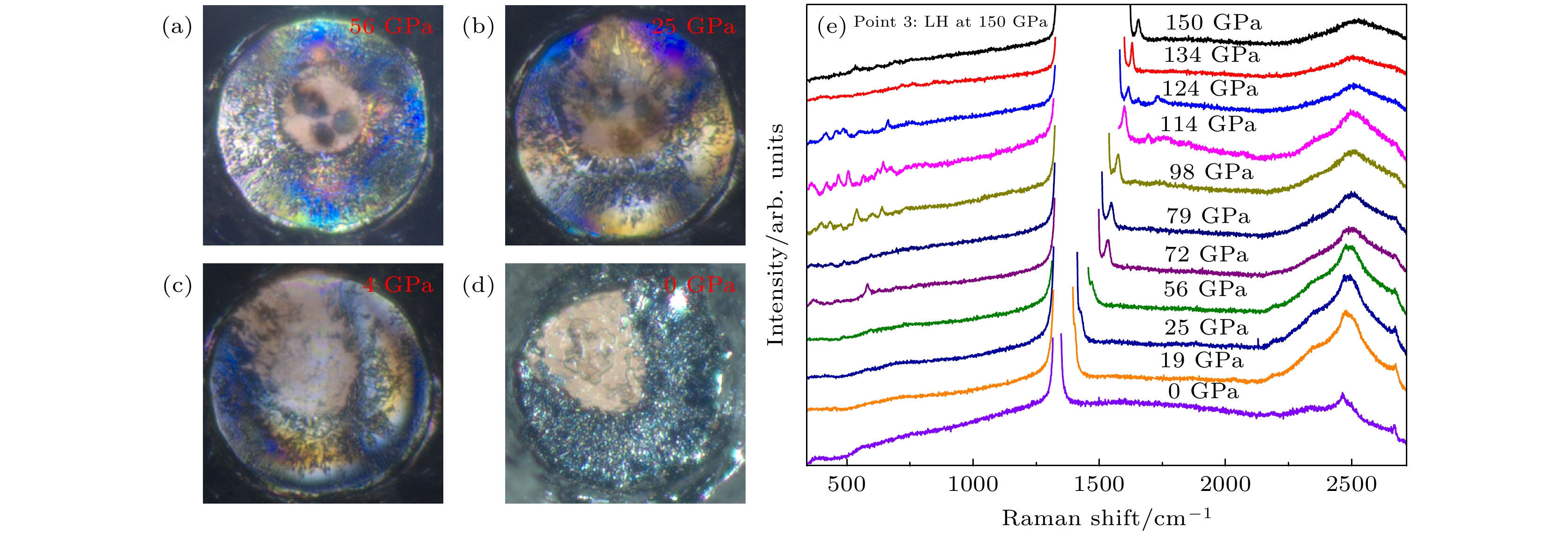
图 3 123 GPa压力下高含氮量N2@BNNTs样品(a)激光加热前及(b)激光热后样品腔的显微图像; 红色圆圈内为样品激光加热区域(c)高含氮量N2@BNNTs样品激光加热前后的拉曼光谱
Fig. 3. Microscopic images of sample cavity before (a) and after (b) laser heating at 123 GPa. Red circle shows an area where the sample was laser-heated; (c) the Raman spectra of high nitrogen content N2@BNNTs sample before and after laser heating at 123 GPa.
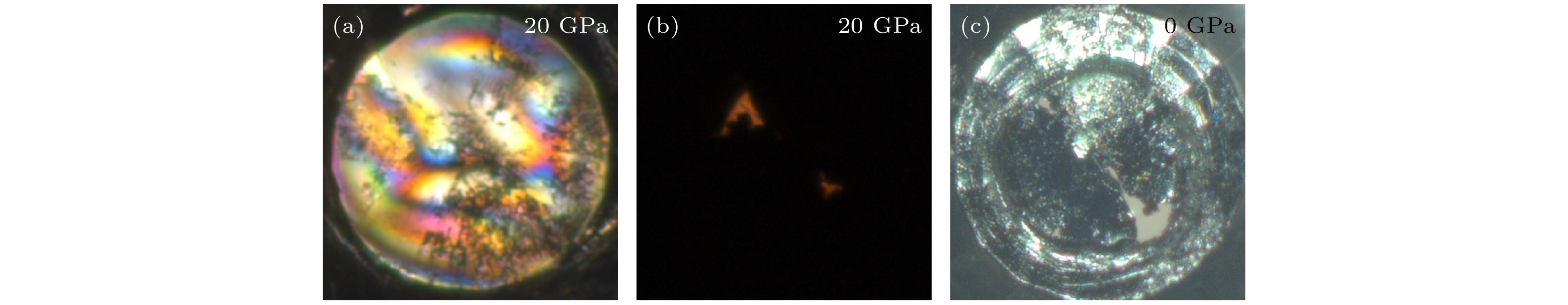
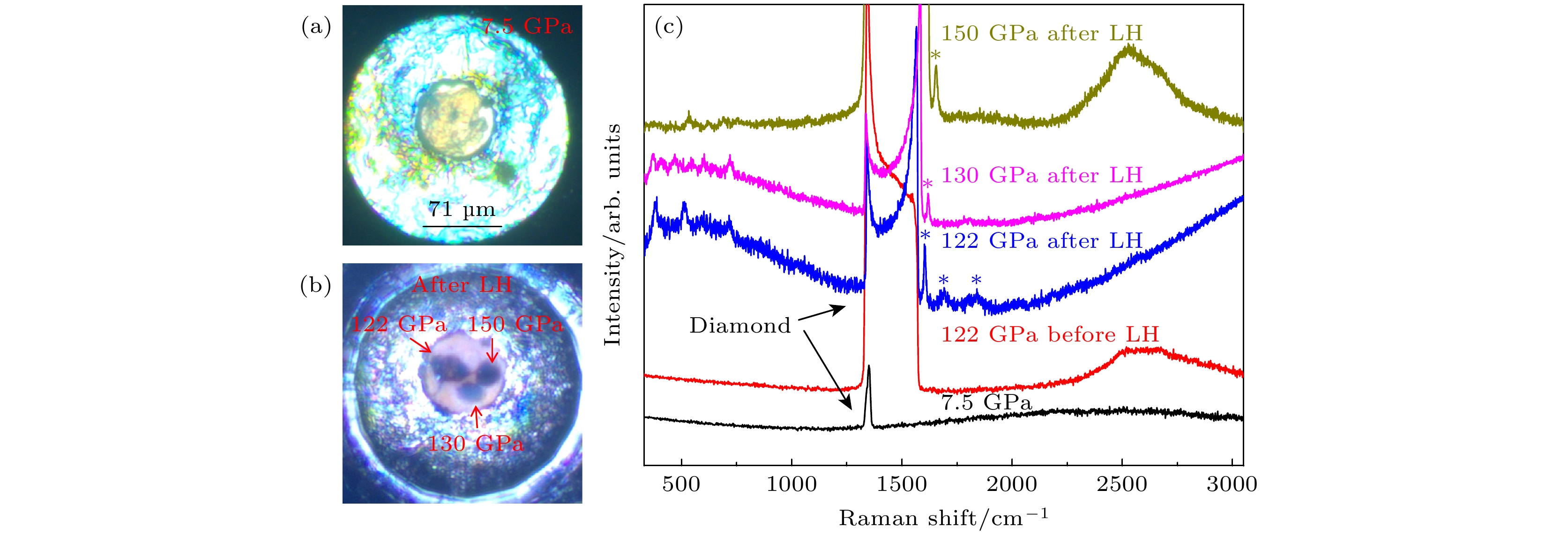
图 6 (a)封装液氮后样品腔的显微图像; (b)在122 GPa, 130 GPa以及150 GPa压力下分别激光加热后样品腔的显微图像; (c)低含氮量N2@BNNTs样品的部分升压拉曼光谱及在122 GPa, 130 GPa以及150 GPa压力下分别激光加热后的拉曼光谱
Fig. 6. (a) Microscopic image of the sample cavity after encapsulating liquid nitrogen; (b) microscopic images of sample cavities after laser heating at 122 GPa, 130 GPa, and 150 GPa, respectively; (c) the Raman spectra of low nitrogen content N2@BNNTs sample before and after laser heating at 122 GPa, 130 GPa and 150 GPa, respectively.
-
[1] Légaré M A, Rang M, Bélanger-Chabot G, Schweizer J I, Krummenacher I, Bertermann R, Arrowsmith M, Holthausen M C, Braunschweig H 2019 Science 363 1329
 Google Scholar
Google Scholar
[2] Qi C, Li S H, Li Y C, Wang Y, Chen X K, Pang S P 2011 J. Mater. Chem. 21 3221
 Google Scholar
Google Scholar
[3] Klapötke T M, Piercey D G 2011 Inorg. Chem. 50 2732
 Google Scholar
Google Scholar
[4] Li Y C, Qi C, Li S H, Zhang H J, Sun C H, Yu Y Z, Pang S P 2010 J. Am. Chem. Soc. 132 12172
 Google Scholar
Google Scholar
[5] Eremets M I, Gavriliuk A G, Trojan I A, Dzivenko D A, Boehler R 2004 Nat. Mater. 3 558
 Google Scholar
Google Scholar
[6] Tomasino D, Kim M, Smith J, Yoo C S 2014 Phys. Rev. Lett. 113 205502
 Google Scholar
Google Scholar
[7] Laniel D, Geneste G, Weck G, Mezouar M, Loubeyre P 2019 Phys. Rev. Lett. 122 066001
 Google Scholar
Google Scholar
[8] Ji C, Adeleke A A, Yang L X, Wan B, Gou H Y 1, Yao Y S, Li B1, Meng Y, Smith J S, Prakapenka V B, Liu W J, Shen G Y, Mao W L, Mao H K 2019 Nat. Commun. 10 4515
 Google Scholar
Google Scholar
[9] Abou-Rachid H, Hu A, Timoshevskii V, Song Y F, Lussier L S 2008 Phys. Rev. Lett. 100 196401
 Google Scholar
Google Scholar
[10] VTimoshevskii V, Ji W, Abou-Rachid H, Lussier L S, Guo H 2009 Phys. Rev. B 80 115409
 Google Scholar
Google Scholar
[11] Shi X H, Liu B, Liu S J, Niu S F, Liu S, Liu R, Liu B B 2018 Sci. Rep. 8 13758
 Google Scholar
Google Scholar
[12] Li S, Li H Y, Yao Z, Lu S C 2021 Mater. Today. Commun. 26 101670
 Google Scholar
Google Scholar
[13] Lv H, Yao M G, Li Q J, Liu R, Liu B, Yao Z, Liu D D, Liu Z D, Liu J, Chen Z Q, Zou B, Cui T, Liu B B 2015 Sci. Rep. 5 13234
 Google Scholar
Google Scholar
[14] Wu Z Y, Benchafia E M, Iqbal Z, Wang X Q 2014 Chem. Int. Ed. 53 12555
 Google Scholar
Google Scholar
[15] Zhang C, Sun C, Hu B C, Yu C M, Lu M 2017 Science 355 374
 Google Scholar
Google Scholar
[16] Zhang C, Yang C, Yu C M, Zheng Z S, Sun C G 2017 Angew. Chem. 56 4512
 Google Scholar
Google Scholar
[17] Schneider H, Hafner W, Wokaun A, Olijnyk H 1992 J. Chem. Phys. 96 8046
 Google Scholar
Google Scholar
[18] Schiferl D, Buchsbaum S, Mills R L 1985 J. Phys. Chem. 89 2324
 Google Scholar
Google Scholar
[19] Eremets M I, Popov Yu M, Trojan I A, Denisov V N, Boehle R R 2004 J. Chem. Phys. 120 10618
 Google Scholar
Google Scholar
[20] Medvedev S A, Trojan I A, Eremets M I, Palasyuk T, Klapotke T M, Evers J 2009 J. Phys. Condens. Matter. 21 195404
 Google Scholar
Google Scholar
[21] Steele B A, Stavrou E, Crowhurst J C, Zaug J M, Prakapenka V B, Oleynik I I 2017 Chem. Mater. 29 735
 Google Scholar
Google Scholar
[22] Bartlett R J, web site http://www.qtp.ufl.edu/; bartlett/ downloads/polynitrog-en.pdf
[23] Lauderdale W J, Stanton J F, Bartlett R J 1992 J. Phys. Chem. 96 1173
 Google Scholar
Google Scholar
[24] Fathalizadeh A, Pham T, Mickelson W, Zettl A 2014 Nano. Lett. 14 48
 Google Scholar
Google Scholar
[25] Lin J F, Santoro M, Struzhkin V V, Mao H K, Hemley R J, 2004 Rev. Sci. Instrum. 75 3302
 Google Scholar
Google Scholar
计量
- 文章访问数: 4534
- PDF下载量: 87
- 被引次数: 0

















 下载:
下载: