-
肌酐是肾脏疾病诊断和监测的关键生物标志物, 因此, 快速、灵敏的肌酐检测非常重要. 本文提供了一种通过提高低温下的光子诱导电荷转移效率来促进表面增强拉曼散射光谱(SERS)活性的有效策略. 采用种子生长法获得纳米金二十面体(Au20), 以此作为SERS活性基底. 采用极低温(98 K)SERS检测技术实现对染料分子结晶紫(CV)和生理盐水中的肌酐含量的快速、灵敏检测. 实验结果表明: 常温296 K下, Au20基底对CV分子的检测限(LOD)低至10–12 mol/L, 并且信号均匀; 低温98 K时, CV分子的LOD可达10–14 mol/L, 比296 K时降低2个数量级. 最后, 使用Au20基底对生理盐水中的肌酐进行无标记检测. 结果表明, 常温296 K 时该SERS基底对肌酐的LOD为10–6 mol/L, 1619 cm–1峰的线性相关系数为0.9839. 低温98 K时, 肌酐的浓度探测极限低至10–8 mol/L, 1619 cm–1峰的线性相关系数变为0.9973. 可知, 低温使肌酐浓度检测限和特征峰线性度都进一步提高. 本文的工作为生物医药领域肌酐浓度的精确检测提供了新的思路.Creatinine is a key biomarker for diagnosing and monitoring kidney disease, so rapid and sensitive testing is very important. Raman spectroscopy is particularly suitable for quantitatively detecting the creatinine in the human environment because it is sensitive to subtle changes in the concentration of the analyte. In this work an effective strategy is provided to promote the activity of surface-enhanced Raman scattering spectroscopy by enhancing the photon-induced charge transfer efficiency at low temperature. The nano-gold icosahedron (Au20) is obtained by the seed-growing method, which is used as an active substrate for SERS. The ultra-low temperature (98 K) SERS detection technology is used to realize the rapid and sensitive detection of the dye molecule crystal violet (CV) and creatinine in normal saline. The experimental results show that at room temperature of 296 K, the detection limit of Au20 substrate for CV molecules is as low as 10–12 mol/L, and the signals are uniform; at a low temperature of 98 K, the detection limit of CV molecules can reach 10–14 mol/L, which is two orders of magnitude lower than that at 296 K. As a result, the adopted cryogenic temperature can effectively weaken the lattice thermal vibration and reduce the release of phonons, then suppress phonon-assisted non-radiative recombination. So, it will increase the number of photo-induced electrons to participate in the photo-induced charge transfer efficiency. Finally, we perform the label-free detection of creatinine in saline by using an Au20 substrate. The results show that the detection limit of the SERS substrate for creatinine is 10–6 mol/L at 296 K, and the linear correlation coefficient of the 1619 cm–1 peak is 0.9839. At a low temperature of 98 K, the detection limit of creatinine concentration is as low as 10–8 mol/L, and the linear correlation coefficient of the 1619 cm–1 peak becomes 0.9973. It can be seen that low temperature may further improve the detection limit of creatinine concentration and the linearity of characteristic peak. In summary, the current work provides a new idea for accurately detecting the creatinine concentration in the field of biomedicine.
-
Keywords:
- low temperature /
- surface-enhanced Raman scattering /
- creatinine /
- ultra-sensitive detection
[1] Noorul A, Mahmood R T, Asad M J, Zafar M, Mehmood R A 2014 J. Cardiovasc. Dis. 2 2330
[2] Theadom A, Rodrigues M, Roxburgh R, Balalla S, Higgins C, Bhattacharjee R, Jones K, Krishnamurthi R, Feigin V 2014 Neuroepidemiology 43 259
 Google Scholar
Google Scholar
[3] Bikbov B, Purcell C A, Levey A S, Smith M, Abdoli A, Abebe M, Adebayo O M, Afarideh M, Agarwal S K, AgudeloBotero M 2020 Lancet 395 709
 Google Scholar
Google Scholar
[4] Du H, Chen R, Du J, Fan J, Peng X 2016 Ind. Eng. Chem. Res. 55 12334
 Google Scholar
Google Scholar
[5] Erenas M M, Ortiz-Gómez I, De Orbe-Payá I, Hernández Alonso D, Ballester P, Blondeau P, Andrade F J, Salinas-Castillo A, Capitán-Vallvey L F 2019 ACS Sensors 4 421
 Google Scholar
Google Scholar
[6] Sierra A F, Hernández-Alonso D, Romero M A, González Delgado J A, Pischel U, Ballester P 2020 J. Am. Chem. Soc. 142 4276
 Google Scholar
Google Scholar
[7] Titilope J J, Ma J, Thitima R 2021 J. Environ. Chem. Eng. 9 105770
 Google Scholar
Google Scholar
[8] Cialla-May D, Zheng X S, Weber K, Popp J 2017 Chem. Soc. Rev. 46 3945
 Google Scholar
Google Scholar
[9] Pang S, Yang T, He L 2016 Trends Analyt. Chem. 85 73
 Google Scholar
Google Scholar
[10] Zhang R, Xu B B, Liu X Q, Zhang Y L, Xu Y, Chen Q D, Sun H B 2012 Chem. Commun. 48 5913
 Google Scholar
Google Scholar
[11] Jensen L, Aikens C M, Schatz G C 2008 Chem. Soc. Rev. 37 1061
 Google Scholar
Google Scholar
[12] Wu D Y, Li J F, Ren B, Tian Z Q 2008 Chem. Soc. Rev. 37 1025
 Google Scholar
Google Scholar
[13] Morton S M, Jensen L 2009 J. Am. Chem. Soc. 131 4090
 Google Scholar
Google Scholar
[14] Cai Q, Gan W, Falin A, Kenji W, Takashi T, Zhuang J C, Hao W C, Huang S M, Tao T, Cheng Y, Li L H 2020 ACS Appl. Mater. Interfaces 12 21985
 Google Scholar
Google Scholar
[15] Li G H, Gong W B, Qiu T L, Cong S, Zhao Z G, Ma R Z, Yuichi M, Takayoshi S, Geng F X 2020 ACS Appl. Mater. Interfaces 12 23523
 Google Scholar
Google Scholar
[16] Lin J, Yu J, Akakuru O U, Wang X, Yuan B, Chen T, Guo L, Wu A 2020 Chem. Sci. 11 9414
 Google Scholar
Google Scholar
[17] Lohar A A, Shinde A, Gahlaut R, Sagdeo A, Mahamuni S 2018 J. Phys. Chem. C 122 25014
 Google Scholar
Google Scholar
[18] Milot R L, Klug M T, Davies C L, Wang Z, Kraus H, Snaith H J, Johnston M B, Herz L M 2018 Adv. Mater. 30 1804506
 Google Scholar
Google Scholar
[19] Wen P, Yang F, Ge C, Li S B, Xu Y, Chen L 2021 Nanotechnology 32 395502
 Google Scholar
Google Scholar
[20] Zhu W, Wen B Y, Jie L J, Tian X D, Yang Z L, Petar M R, Luo S Y, Tian Z Q, Li J F 2020 Biosens. Bioelectron. 154 112067
 Google Scholar
Google Scholar
[21] Kullavadee K O, Aroonsri N 2021 Appl. Surf. Sci. 546 149092
 Google Scholar
Google Scholar
[22] Nabadweep C, Ankita S, Aneesh M J, Pabitra N 2019 Sens. Actuators, B 285 108
 Google Scholar
Google Scholar
[23] Jiang Y N, Cai Y Z, Hu S, Guo X Y, Ying Y, Wen Y, Wu Y P, Yang H F 2021 J. Innovative Opt. Heal. Sci. 14 2141003
 Google Scholar
Google Scholar
[24] Moskovits M 1985 Rev. Mod. Phys. 57 783
 Google Scholar
Google Scholar
[25] Aravind P, Nitzan A, Metiu H 1981 Surf. Sci. 110 189
 Google Scholar
Google Scholar
[26] 刘小红, 姜珊, 常林, 张炜 2020 69 190701
 Google Scholar
Google Scholar
Liu X H, Jiang S, Chang L, Zhang W 2020 Acta Phys. Sin. 69 190701
 Google Scholar
Google Scholar
[27] Tian Y, Zhang H, Xu L, Chen M, Chen F 2018 Opt. Lett. 43 635
 Google Scholar
Google Scholar
[28] Xu L, Zhang H, Tian Y, Jiao A X, Chen F, Chen M 2019 Talanta 194 680
 Google Scholar
Google Scholar
[29] Tian Y, Li G H, Zhang H, Xu L, Jiao A X, Chen F, Chen M 2018 Opt. Express 26 23347
 Google Scholar
Google Scholar
[30] Zhang H, Xu L, Tian Y, Chen M, Liu X D, Chen F 2017 Opt. Express 25 29389
 Google Scholar
Google Scholar
[31] Liu Z, Yang Z B, Peng B, Cao C, Zhang C, You H J, Xiong Q H, Li Z Y, Fang J X 2014 Adv. Mater. 26 2431
 Google Scholar
Google Scholar
[32] Lin J, Shang Y, Li X, Yu J, Wang X T, Guo L 2017 Adv. Mater. 29 1604797
 Google Scholar
Google Scholar
[33] Ma C, Gao Q, Hong W, Fan J, Fang J X 2017 Adv. Funct. Mater. 27 1603233
 Google Scholar
Google Scholar
[34] Tamarat P, Bodnarchuk M I, rebbia J, Erni R, Kovalenko M V, Even J, Lounis B 2019 Nat. Mater. 18 717
 Google Scholar
Google Scholar
[35] Sultan B J, William J P, Raul Q C, Emiliano C, Carlos S V, Nadia A K, Stefan A M, Ivan P P 2016 Nat. Commun. 7 12189
 Google Scholar
Google Scholar
[36] Nicola´s G T, Emiliano C, Alexander D H N, Pilar C, Gonzalo U, Carlos A B, Marı´a E V, Roberto C S, Alejandro F 2011 ACS Nano 5 5433
 Google Scholar
Google Scholar
[37] Wang X, Huang S C, Hu S, Yan S, Ren B 2020 Nat. Rev. Phys. 2 253
 Google Scholar
Google Scholar
[38] Gao J, Hu Y J, Li S X, Zhang Y J, Chen X 2013 Chem. Phys. 410 81
 Google Scholar
Google Scholar
-
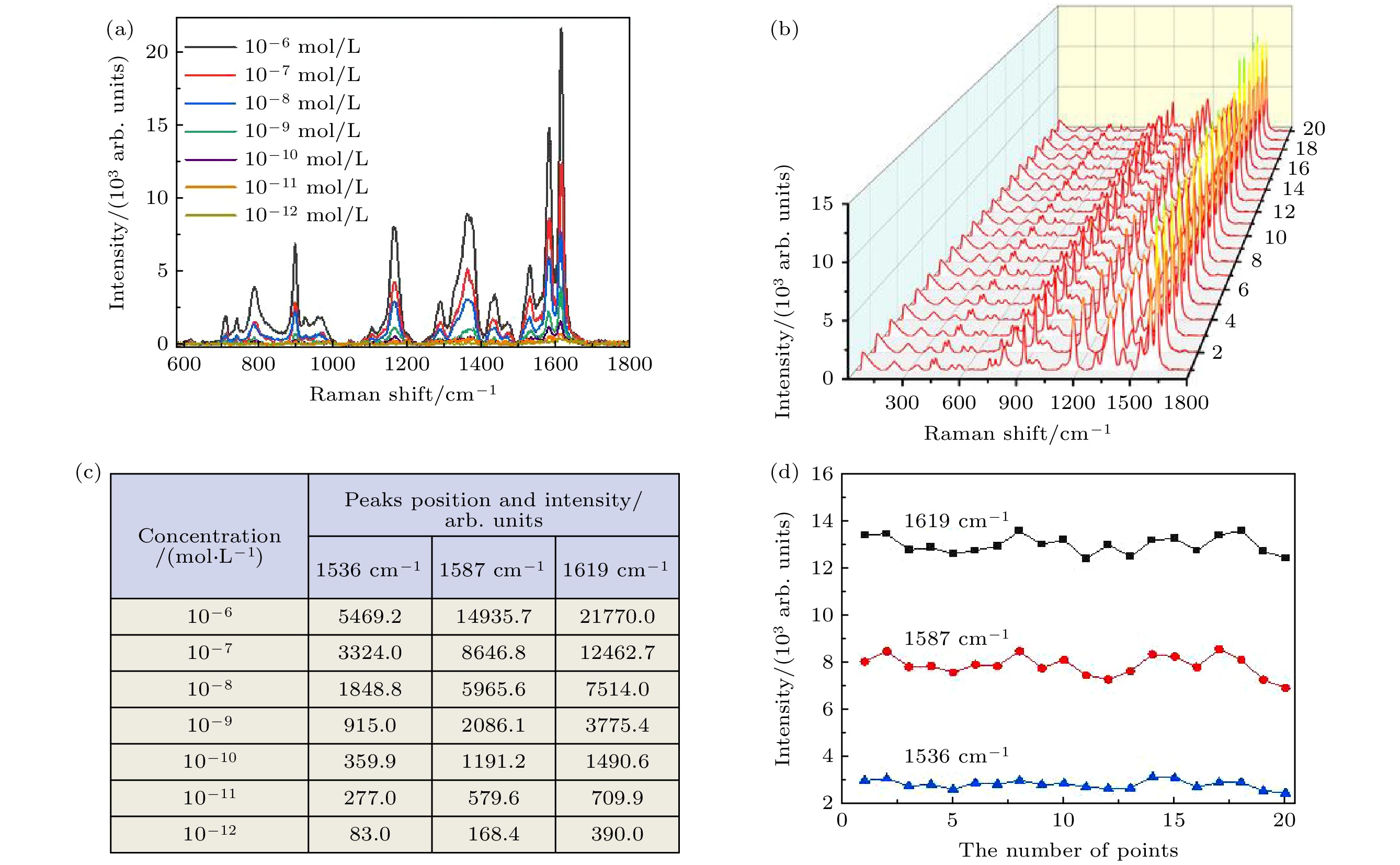
图 5 (a)不同浓度(10–6—10–12 mol/L)的CV分子SERS光谱; (b) 10–7 mol/L的CV分子随机采集20个点的SERS光谱; (c)对应图(a)中3个峰的强度值; (d)对应图(b)中3个最高峰的强度变化曲线
Fig. 5. (a) SERS spectra of CV molecules with different concentrations (10–6–10–12 mol/L); (b) SERS spectra of 20 points of CV molecule (10–7 mol/L) randomly collected; (c) intensity values of the three peaks in panel (a); (d) variation curve of the intensity values of the three peaks in panel (b).
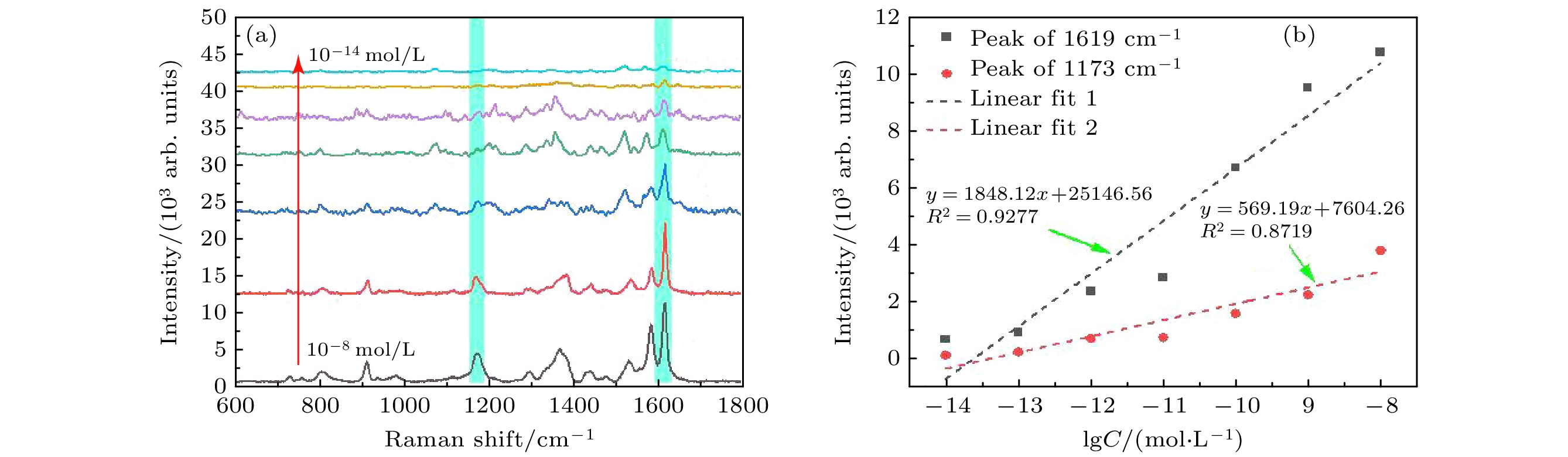
图 8 (a) 低温98 K时不同浓度(10–8—10–14 mol/L)的CV分子的SERS光谱; (b) 1173和1619 cm–1特征峰的SERS强度与CV分子浓度的线性关系
Fig. 8. (a) SERS spectra of CV molecules with different concentrations (10–8–10–14 mol/L) at low temperature 98 K; (b) SERS intensity of 1173 and 1619 cm–1 peaks proportional to the concentration of CV molecule.
图 9 不同浓度(10–3—10–6 mol/L)的肌酐分子的SERS光谱 (a) 296 K; (c) 98 K. 1178和1619 cm–1特征峰强度与肌酐分子浓度的线性关系 (b) 296 K; (d) 98 K
Fig. 9. SERS spectra of creatinine molecules at different concentrations (10–3–10–6 mol/L): (a) 296 K; (c) 98 K. The variation of the intensity of peaks at 1178 and 1619 cm–1 versus different the molecular concentration of creatinine: (b) 296 K; (d) 98 K.
-
[1] Noorul A, Mahmood R T, Asad M J, Zafar M, Mehmood R A 2014 J. Cardiovasc. Dis. 2 2330
[2] Theadom A, Rodrigues M, Roxburgh R, Balalla S, Higgins C, Bhattacharjee R, Jones K, Krishnamurthi R, Feigin V 2014 Neuroepidemiology 43 259
 Google Scholar
Google Scholar
[3] Bikbov B, Purcell C A, Levey A S, Smith M, Abdoli A, Abebe M, Adebayo O M, Afarideh M, Agarwal S K, AgudeloBotero M 2020 Lancet 395 709
 Google Scholar
Google Scholar
[4] Du H, Chen R, Du J, Fan J, Peng X 2016 Ind. Eng. Chem. Res. 55 12334
 Google Scholar
Google Scholar
[5] Erenas M M, Ortiz-Gómez I, De Orbe-Payá I, Hernández Alonso D, Ballester P, Blondeau P, Andrade F J, Salinas-Castillo A, Capitán-Vallvey L F 2019 ACS Sensors 4 421
 Google Scholar
Google Scholar
[6] Sierra A F, Hernández-Alonso D, Romero M A, González Delgado J A, Pischel U, Ballester P 2020 J. Am. Chem. Soc. 142 4276
 Google Scholar
Google Scholar
[7] Titilope J J, Ma J, Thitima R 2021 J. Environ. Chem. Eng. 9 105770
 Google Scholar
Google Scholar
[8] Cialla-May D, Zheng X S, Weber K, Popp J 2017 Chem. Soc. Rev. 46 3945
 Google Scholar
Google Scholar
[9] Pang S, Yang T, He L 2016 Trends Analyt. Chem. 85 73
 Google Scholar
Google Scholar
[10] Zhang R, Xu B B, Liu X Q, Zhang Y L, Xu Y, Chen Q D, Sun H B 2012 Chem. Commun. 48 5913
 Google Scholar
Google Scholar
[11] Jensen L, Aikens C M, Schatz G C 2008 Chem. Soc. Rev. 37 1061
 Google Scholar
Google Scholar
[12] Wu D Y, Li J F, Ren B, Tian Z Q 2008 Chem. Soc. Rev. 37 1025
 Google Scholar
Google Scholar
[13] Morton S M, Jensen L 2009 J. Am. Chem. Soc. 131 4090
 Google Scholar
Google Scholar
[14] Cai Q, Gan W, Falin A, Kenji W, Takashi T, Zhuang J C, Hao W C, Huang S M, Tao T, Cheng Y, Li L H 2020 ACS Appl. Mater. Interfaces 12 21985
 Google Scholar
Google Scholar
[15] Li G H, Gong W B, Qiu T L, Cong S, Zhao Z G, Ma R Z, Yuichi M, Takayoshi S, Geng F X 2020 ACS Appl. Mater. Interfaces 12 23523
 Google Scholar
Google Scholar
[16] Lin J, Yu J, Akakuru O U, Wang X, Yuan B, Chen T, Guo L, Wu A 2020 Chem. Sci. 11 9414
 Google Scholar
Google Scholar
[17] Lohar A A, Shinde A, Gahlaut R, Sagdeo A, Mahamuni S 2018 J. Phys. Chem. C 122 25014
 Google Scholar
Google Scholar
[18] Milot R L, Klug M T, Davies C L, Wang Z, Kraus H, Snaith H J, Johnston M B, Herz L M 2018 Adv. Mater. 30 1804506
 Google Scholar
Google Scholar
[19] Wen P, Yang F, Ge C, Li S B, Xu Y, Chen L 2021 Nanotechnology 32 395502
 Google Scholar
Google Scholar
[20] Zhu W, Wen B Y, Jie L J, Tian X D, Yang Z L, Petar M R, Luo S Y, Tian Z Q, Li J F 2020 Biosens. Bioelectron. 154 112067
 Google Scholar
Google Scholar
[21] Kullavadee K O, Aroonsri N 2021 Appl. Surf. Sci. 546 149092
 Google Scholar
Google Scholar
[22] Nabadweep C, Ankita S, Aneesh M J, Pabitra N 2019 Sens. Actuators, B 285 108
 Google Scholar
Google Scholar
[23] Jiang Y N, Cai Y Z, Hu S, Guo X Y, Ying Y, Wen Y, Wu Y P, Yang H F 2021 J. Innovative Opt. Heal. Sci. 14 2141003
 Google Scholar
Google Scholar
[24] Moskovits M 1985 Rev. Mod. Phys. 57 783
 Google Scholar
Google Scholar
[25] Aravind P, Nitzan A, Metiu H 1981 Surf. Sci. 110 189
 Google Scholar
Google Scholar
[26] 刘小红, 姜珊, 常林, 张炜 2020 69 190701
 Google Scholar
Google Scholar
Liu X H, Jiang S, Chang L, Zhang W 2020 Acta Phys. Sin. 69 190701
 Google Scholar
Google Scholar
[27] Tian Y, Zhang H, Xu L, Chen M, Chen F 2018 Opt. Lett. 43 635
 Google Scholar
Google Scholar
[28] Xu L, Zhang H, Tian Y, Jiao A X, Chen F, Chen M 2019 Talanta 194 680
 Google Scholar
Google Scholar
[29] Tian Y, Li G H, Zhang H, Xu L, Jiao A X, Chen F, Chen M 2018 Opt. Express 26 23347
 Google Scholar
Google Scholar
[30] Zhang H, Xu L, Tian Y, Chen M, Liu X D, Chen F 2017 Opt. Express 25 29389
 Google Scholar
Google Scholar
[31] Liu Z, Yang Z B, Peng B, Cao C, Zhang C, You H J, Xiong Q H, Li Z Y, Fang J X 2014 Adv. Mater. 26 2431
 Google Scholar
Google Scholar
[32] Lin J, Shang Y, Li X, Yu J, Wang X T, Guo L 2017 Adv. Mater. 29 1604797
 Google Scholar
Google Scholar
[33] Ma C, Gao Q, Hong W, Fan J, Fang J X 2017 Adv. Funct. Mater. 27 1603233
 Google Scholar
Google Scholar
[34] Tamarat P, Bodnarchuk M I, rebbia J, Erni R, Kovalenko M V, Even J, Lounis B 2019 Nat. Mater. 18 717
 Google Scholar
Google Scholar
[35] Sultan B J, William J P, Raul Q C, Emiliano C, Carlos S V, Nadia A K, Stefan A M, Ivan P P 2016 Nat. Commun. 7 12189
 Google Scholar
Google Scholar
[36] Nicola´s G T, Emiliano C, Alexander D H N, Pilar C, Gonzalo U, Carlos A B, Marı´a E V, Roberto C S, Alejandro F 2011 ACS Nano 5 5433
 Google Scholar
Google Scholar
[37] Wang X, Huang S C, Hu S, Yan S, Ren B 2020 Nat. Rev. Phys. 2 253
 Google Scholar
Google Scholar
[38] Gao J, Hu Y J, Li S X, Zhang Y J, Chen X 2013 Chem. Phys. 410 81
 Google Scholar
Google Scholar
计量
- 文章访问数: 7416
- PDF下载量: 109
- 被引次数: 0














 下载:
下载: