-
水系碱金属离子电池因具有高安全性、低成本和环境友好等优势而成为前沿研究的热点之一, 在大规模储能领域具有良好的应用前景. 然而, 许多水系碱金属离子电池在低温条件下出现运行故障或展现出极低的放电比容量, 严重限制了其在恶劣的严寒气候条件下的广泛应用. 本综述首先梳理了近年来低温水系碱金属离子电池的研究进展. 随后从电解液、电极和界面三个方面分别探讨了水系碱金属离子电池在低温下运行所面对的挑战和相应的失效机制, 同时系统地介绍了提高电池低温性能的改性策略并加以评述, 以期为水系碱金属离子电池低温性能的进一步提升及其实际应用提供参考并指明方向.Aqueous alkali-metal-ion batteries are a popular frontier research area, expected to apply for large-scale energy storage due to their high safety, low cost, and environmental friendliness. Depending on diversified social development, batteries ought to function in various ambient, including polar regions and high-altitude locales. Delivering excellent electrochemical performance at low temperatures is crucial to develop aqueous alkali-metal-ion batteries. This review summarizes the representative research progress in the field of aqueous low-temperature alkali-metal-ion batteries in recent years, based on the subjects of electrolyte, electrode, and interface. Firstly, we discussed the challenges of aqueous alkali-metal-ion batteries operated at low temperatures and the corresponding failure mechanisms. At subzero temperatures, aqueous alkali-metal-ion batteries couldn't work or exhibit little capacity, arising from the frozen electrolytes, electrode materials with slow kinetics, and huge interface impedances, which seriously limits their wide application in low-temperature conditions. Then, combined with the latest research work, various strategies have been investigated to improve the electrochemical performance of batteries at low temperatures. To date, the strategies for reducing the freezing point of electrolytes have primarily focused on breaking H-bonds between free water molecules by increasing salt concentration, adding organic/inorganic additives, and using hydrogel as electrolytes. In terms of electrodes, the related studies have concentrated on regulating the structure and morphology of electrodes, introducing the dual ion battery mechanism, and using organic materials and Zn electrodes to alleviate the slow ion dynamics of electrodes. In addition, adding appropriate organic solvents that can generate protective layers with low interface impedance on the electrode surface in the electrolyte can also improve the low-temperature performance of aqueous alkali-metal-ion batteries. Finally, we evaluated multi-dimensionally all strategies, expected to provide a comprehensive reference and point out the direction for the further improvement and practical application of the aqueous alkali-metal-ion batteries at low temperatures.
-
Keywords:
- aqueous low-temperature alkali-metal-ion batteries /
- electrolytes /
- electrodes /
- interface
[1] Dunn B, Kamath H, Tarascon J M 2011 Science 334 928
 Google Scholar
Google Scholar
[2] Larcher D, Tarascon J M 2015 Nat. Chem. 7 19
 Google Scholar
Google Scholar
[3] Goodenough J B 2013 Acc. Chem. Res. 46 1053
 Google Scholar
Google Scholar
[4] Abada S, Marlair G, Lecocq A, Petit M, Sauvant-Moynot V, Huet F 2016 J. Power Sources 306 178
 Google Scholar
Google Scholar
[5] Kim H, Hong J, Park K Y, Kim H, Kim S W, Kang K 2014 Chem. Rev. 114 11788
 Google Scholar
Google Scholar
[6] Tang W, Zhu Y, Hou Y, Liu L, Wu Y, Loh K P, Zhang H, Zhu K 2013 Energy Environ. Sci. 6 2093
 Google Scholar
Google Scholar
[7] Suo L, Borodin O, Gao T, Olguin M, Ho J, Fan X, Luo C, Wang C, Xu K 2015 Science 350 938
 Google Scholar
Google Scholar
[8] Yue J, Suo L 2021 Energy Fuels 35 9228
 Google Scholar
Google Scholar
[9] Zhang S, Xu K, Jow T 2003 J. Power Sources 115 137
 Google Scholar
Google Scholar
[10] Gao H N, Zhao Z G, Cai Y D, Zhou J J, Hua W D, Chen L, Wang L, Zhang J Q, Han D, Liu M J, Jiang L 2017 Nat. Commun. 8 15911
 Google Scholar
Google Scholar
[11] Jiang L, Lu Y, Zhao C, Liu L, Zhang J, Zhang Q, Shen X, Zhao J, Yu X, Li H, Huang X, Chen L, Hu Y S 2019 Nat. Energy 4 495
 Google Scholar
Google Scholar
[12] Wang H, Zhang H, Cheng Y, Feng K, Li X, Zhang H 2018 Electrochim. Acta 278 279
 Google Scholar
Google Scholar
[13] Zhang Y, Xu J, Li Z, Wang Y, Wang S, Dong X, Wang Y 2022 Sci. Bull. 67 161
 Google Scholar
Google Scholar
[14] Zhu K J, Sun Z Q, Jin T, Chen X C, Si Y C, Li H X, Jiao L F 2022 Batteries Supercaps 5 202200308
[15] Nian Q, Wang J, Liu S, Sun T, Zheng S, Zhang Y, Tao Z, Chen J 2019 Angew. Chem. Int. Ed. 58 16994
 Google Scholar
Google Scholar
[16] Ma Z, Chen J, Vatamanu J, Borodin O, Bedrov D, Zhou X, Zhang W, Li W, Xu K, Xing L 2022 Energy Storage Materials 45 903
[17] Liu T, Liu K-T, Wang J, Ji X, Lan P, Mu Z, Pan Y, Cheng S, Liu M 2021 Energy Storage Materials 41 133
[18] Hu Y, Shi R W, Ren Y Y, Peng W S, Feng C D, Zhao Y, Zheng S J, Li W Z, Sun Z, Guo J N, Guo S Y, Wang X L, Yan F 2022 Adv. Funct. Mater. 32 2203081
 Google Scholar
Google Scholar
[19] Tron A, Jeong S, Park Y D, Mun J 2019 ACS Sustainable Chem. Eng. 7 14531
 Google Scholar
Google Scholar
[20] Liang G, Gan Z, Wang X, Jin X, Xiong B, Zhang X, Chen S, Wang Y, He H, Zhi C 2021 ACS Nano 15 17717
 Google Scholar
Google Scholar
[21] Zhu K, Sun Z, Li Z, Liu P, Chen X, Jiao L 2022 Energy Storage Mater. 53 523
 Google Scholar
Google Scholar
[22] Sun T, Liu C, Wang J, Nian Q, Feng Y, Zhang Y, Tao Z, Chen J 2020 Nano Res. 13 676
 Google Scholar
Google Scholar
[23] Sun Y, Zhang Y, Xu Z, Gou W, Han X, Liu M, Li CM 2022 ChemSusChem 202201362
[24] Zhu M, Wang X, Tang H, Wang J, Hao Q, Liu L, Li Y, Zhang K, Schmidt O G 2020 Adv. Funct. Mater. 30 1907218
 Google Scholar
Google Scholar
[25] Yan C, Wang Y, Deng X, Xu Y 2022 Nano-Micro Lett. 14 98
 Google Scholar
Google Scholar
[26] Wang M, Li T, Yin Y, Yan J, Zhang H, Li X 2022 Adv. Energy Mater. 12 2200728
 Google Scholar
Google Scholar
[27] Nian Q, Liu S, Liu J, Zhang Q, Shi J, Liu C, Wang R, Tao Z, Chen J 2019 ACS Appl. Energy Mater. 2 4370
 Google Scholar
Google Scholar
[28] Liu S, Lei T, Song Q, Zhu J, Zhu C 2022 ACS Appl. Mater. Interfaces 14 11425
 Google Scholar
Google Scholar
[29] Pipolo S, Salanne M, Ferlat G, Klotz S, Saitta AM, Pietrucci F 2017 Phys. Rev. Lett. 119 245701
 Google Scholar
Google Scholar
[30] Leadbetter A, Ward R, Clark J, Tucker P, Matsuo T, Suga H 1985 J. Chem. Phys. 82 424
 Google Scholar
Google Scholar
[31] Reber D, Kühnel R S, Battaglia C 2019 ACS Mater. Lett. 1 44
 Google Scholar
Google Scholar
[32] Becker M, Kühnel R S, Battaglia C 2019 ChemComm 55 12032
 Google Scholar
Google Scholar
[33] Liu J, Yang C, Chi X, Wen B, Wang W, Liu Y 2022 Adv. Funct. Mater. 32 2106811
 Google Scholar
Google Scholar
[34] Reber D, Borodin O, Becker M, Rentsch D, Thienenkamp J H, Grissa R, Zhao W, Aribia A, Brunklaus G, Battaglia C 2022 Adv. Funct. Mater. 32 2112138
 Google Scholar
Google Scholar
[35] Bi H, Wang X, Liu H, He Y, Wang W, Deng W, Ma X, Wang Y, Rao W, Chai Y, Ma H, Li R, Chen J, Wang Y, Xue M 2020 Adv. Mater. 32 2000074
 Google Scholar
Google Scholar
[36] Zhu K, Li Z, Sun Z, Liu P, Jin T, Chen X, Li H, Lu W, Jiao L 2022 Small 18 2107662
 Google Scholar
Google Scholar
[37] Ao H, Zhao Y, Zhou J, Cai W, Zhang X, Zhu Y, Qian Y 2019 J. Mater. Chem. A 7 18708
 Google Scholar
Google Scholar
[38] Ramanujapuram A, Yushin G 2018 Adv. Energy Mater. 8 1802624
 Google Scholar
Google Scholar
[39] Chen J, Vatamanu J, Xing L, Borodin O, Chen H, Guan X, Liu X, Xu K, Li W 2020 Adv. Energy Mater. 10 1902654
 Google Scholar
Google Scholar
[40] Liu T, Zhang M, Wang Y L, Wang Q Y, Lü C, Liu K X, Suresh S, Yin Y H, Hu Y Y, Li Y S, Liu X B, Zhong X W, Xia B Y, Wu Z P 2018 Adv. Energy Mater. 8 1802349
 Google Scholar
Google Scholar
[41] Lee J, Lee C L, Park K, Kim I D 2014 J. Power Sources 248 1211
 Google Scholar
Google Scholar
[42] Madzvamuse A, Hamenu L, Mohammed L, Ko JM 2018 ChemistrySelect 3 10805
 Google Scholar
Google Scholar
-
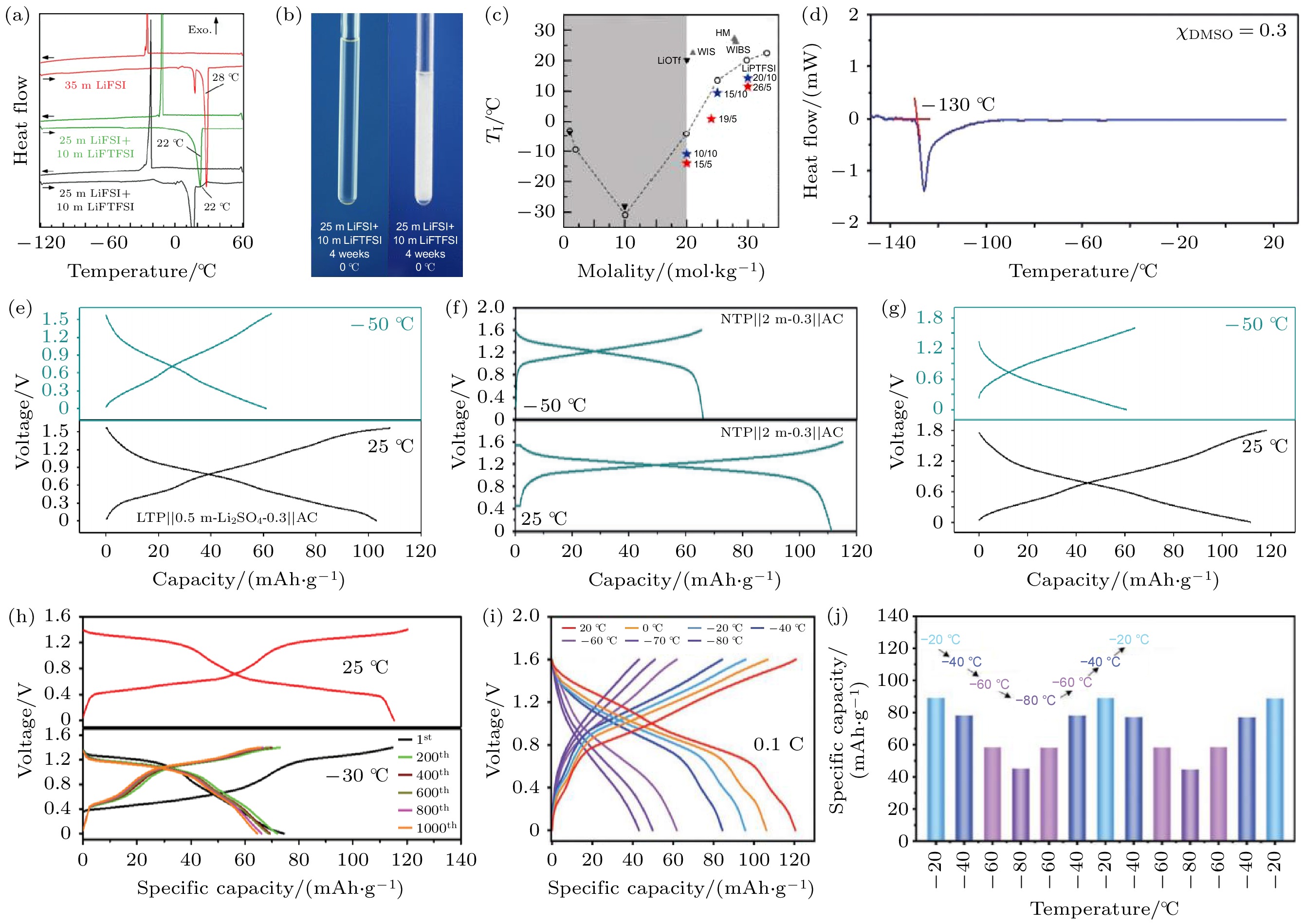
图 2 (a) 35 m LiFSI, 25 m LiFSI+10 m LiFTFSI, 25 m LiFSI+10 m LiTFSI的DSC曲线[31]; (b) 25 m LiFSI+10 m LiFTFSI电解液和25 m LiFSI+10 m LiTFSI电解液在0 °C下保存4周后的照片[31]; (c) LiPTFSI, LiOTf及其二元混合物的水溶液的相图[32]; (d) 水/DMSO (χDMSO = 0.30)混合物的DSC曲线(在水溶液中加入摩尔分数为0.3的DMSO)[15]; (e)—(g) 三种全电池在25和–50 ℃的充放电曲线[15]; (h) Na2CoFe(CN)6//AC 全电池在25和–30 ℃下的充放电曲线[36]; (i) LTP@C //PIL-OH 水凝胶//AC 全电池在不同温度下的充放电曲线[18]; (j) 全电池连续在不同温度下循环的容量变化[18]
Fig. 2. (a) DSC curves of different electrolytes (35 m LiFSI, 25 m LiFSI+10 m LiFTFSI, 25 m LiFSI+10 m LiTFSI) between 60 and –120 ℃[31]; (b) the optical photographs of 25 m LiFSI+10 m LiFTFSI and 25 m LiFSI+10 m LiTFSI after storage at 0 ℃ for 4 weeks[31]; (c) the phase diagrams of aqueous solutions of LiPTFSI, LiOTf, and their binary mixtures[32]; (d) the DSC result of χDMSO = 0.3 electrolyte solvent[15]; (e)–(g) GCD curves of different batteries at 25 and –50 ℃[15]; (h) the GCD curves of the Na2CoFe(CN)6//AC full cells at 25 and –30 ℃ for different cycles[36]; (i) the GCD curves of LTP@C //PIL-OH hydrogel//AC full cells at different temperatures[18]; (j) the capacity change for the full cells cycled at different temperatures continuously[18]
图 3 (a) α-V2O5和δ-K0.5V2O5 (KVO) 两种电极的CV曲线[20]; (b)—(d) δ-K0.5V2O5 (KVO) 电极的三种可能的离子扩散路径示意图[20]; (e)—(g) 三种路径对应的K+传输势垒[20]; (h) α-V2O5和δ0-V2O5 (KVO) 两种电极的电子结构[20]; (i) 不同温度下的δ-K0.5V2O5 (KVO)//PTCDI 软包全电池充放电曲线[20]
Fig. 3. (a) The CV curves of α-V2O5 and reconstructed δ-K0.5V2O5 (KVO) electrodes[20]; (b)–(d) the schematics of three possible pathways for K-ion diffusion[20]; (e)–(g) the transport energy batteries of three kinds of K+ pathways[20]; (h) the electronic structure results of α-V2O5 and δ0-V2O5 (KVO) electrodes from DOS calculating[20]; (i) the GCD curves of δ-K0.5V2O5 (KVO)//PTCDI full batteries at different temperatures[20].
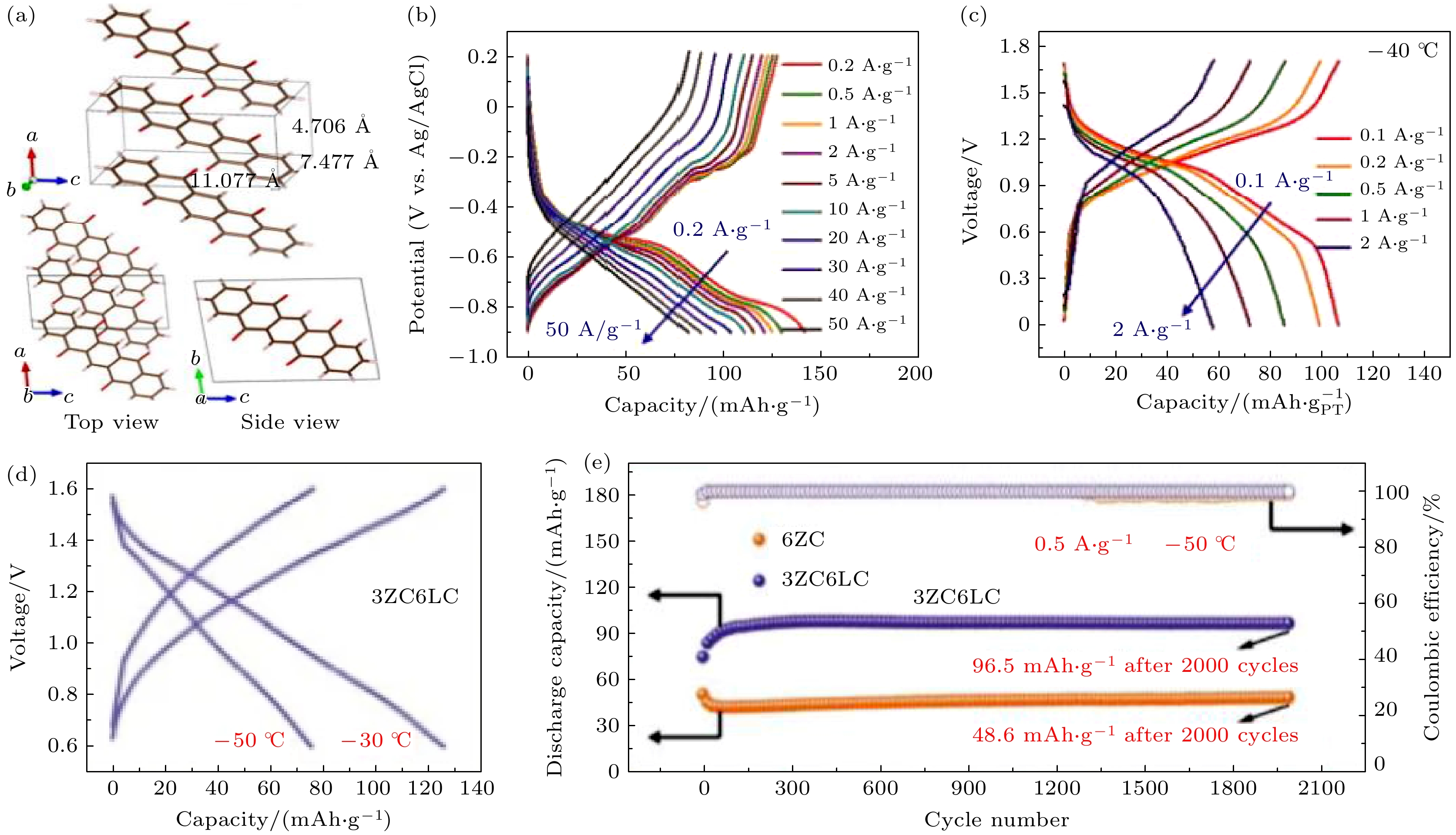
图 4 (a) PT电极的晶体结构[13]; (b) PT电极在不同电流密度下的充放电曲线[13]; (c) NiHCF//17 m NaClO4//PT全电池在不同电流密度下的充放电曲线[13]; (d) PANI//3 ZC6 LC//Zn全电池在–30和–50 ℃下的充放电曲线[25]; (e) PANI//Zn全电池在–50 ℃下的循环性能[25]
Fig. 4. (a) The crystal structure of the PT electrode[13]; (b) the GCD curves of PT electrode at different current densities[13]; (c) the GCD curves of NiHCF//17 m NaClO4//PT full cells at different current densities[13]; (d) the GCD curves of PANI//3 ZC6 LC//Zn full cells at –30 and –50 ℃[25]; (e) the cycle performance of PANI//Zn full cells at –50 ℃[25].
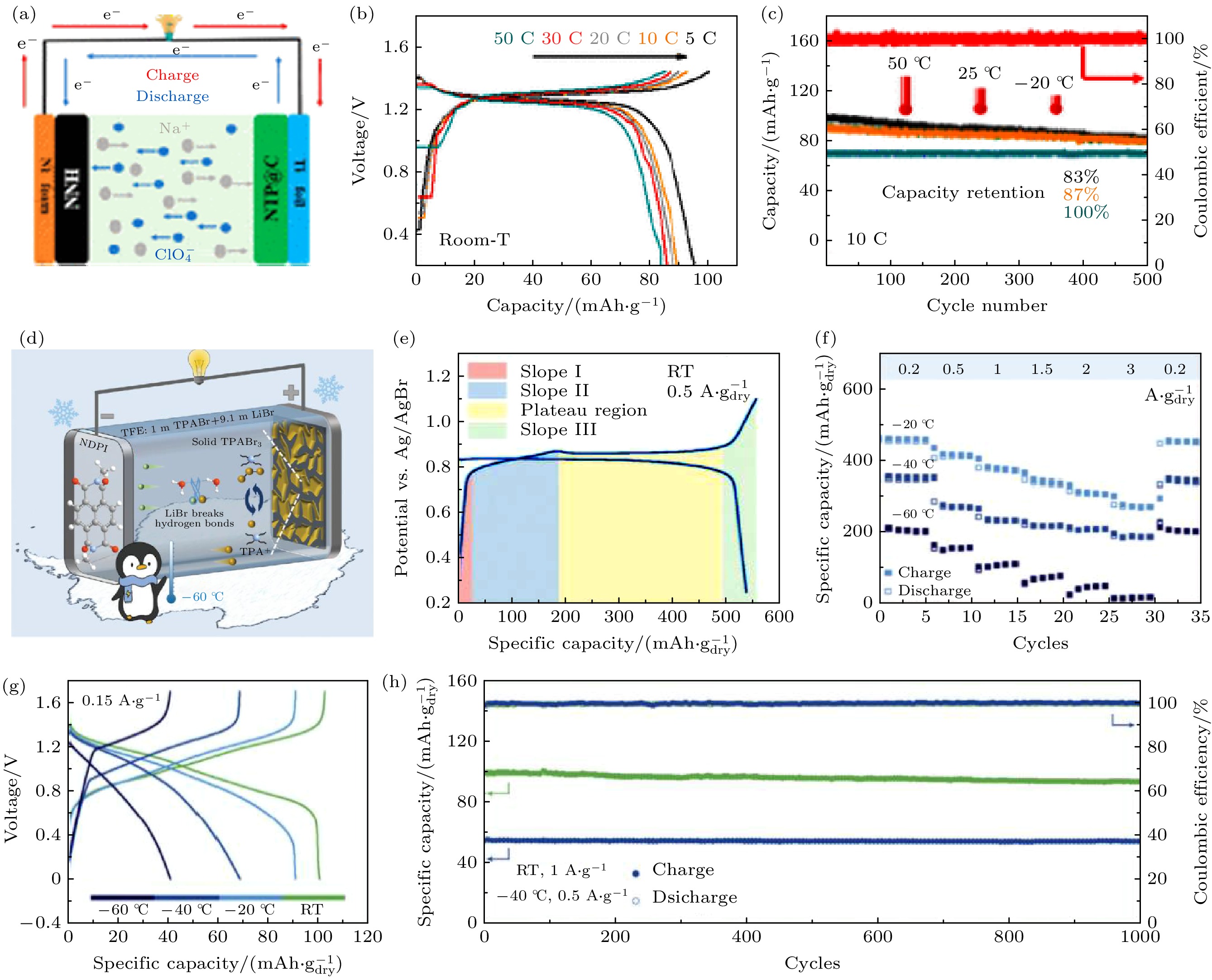
图 5 (a) Ni(OH)2//2 m NaClO4//NaTi2(PO4)3(NTP@C)双离子电池机理示意图[27]; (b) Ni(OH)2//NaTi2(PO4)3(NTP@C)全电池在不同电流密度下的充放电曲线[27]; (c) Ni(OH)2//NaTi2(PO4)3(NTP@C)全电池在不同温度下的循环性能[27]; (d) PC//1 m TPABr+9.1 m LiBr//NDPI全电池机理图[26]; (e) PC电极在室温下的充放电曲线[26]; (f) PC电极在低温下的倍率性能[26]; (g) PC//1 m TPABr+9.1 m LiBr//NDPI全电池在不同温度下的充放电曲线[26]; (h) PC//1 m TPABr+9.1 m LiBr//NDPI全电池的循环性能[26]
Fig. 5. (a) The schematic illustration of Ni(OH)2//2 m NaClO4//NaTi2(PO4)3(NTP@C) full cell[27]; (b) the GCD curves of Ni(OH)2//NaTi2(PO4)3(NTP@C) full cells at different current densities[27]; (c) the cycle performances of Ni(OH)2//NaTi2(PO4)3(NTP@C) full cells at different temperatures[27]; (d) the schematic illustration of PC//1 m TPABr+9.1 m LiBr//NDPI full cell[26]; (e) the GCD curves of PC electrodes at room temperature[26]; (f) the rate performances of PC electrodes from –60 to –20 ℃[26]; (g) the GCD curves of PC//1 m TPABr+9.1 m LiBr//NDPI full cells at different temperatures[26]; (h) the cycle performances of PC//1 m TPABr+9.1 m LiBr//NDPI full cells[26].
图 8 (a) Na3V2(PO4)3 //10 m NaClO4–0.17 m Zn(CH3COO)2–2 wt% VC//Zn全电池的机理示意图[28]; (b) 两种电解液的Na3V2(PO4)3//Zn全电池在–10 ℃下循环前后的阻抗结果[28]; (c) Na3V2(PO4)3 //10 m NaClO4-0.17 m Zn(CH3COO)2–2 wt% VC//Zn全电池低温下的倍率性能[28]; (d) Na3V2(PO4)3 //10 m NaClO4-0.17 m Zn(CH3COO)2-2 wt% VC//Zn全电池在–10 ℃的循环性能[28]
Fig. 8. (a) The schematic illustration of Na3V2(PO4)3 //10 m NaClO4-0.17 m Zn(CH3COO)2-2 wt% VC//Zn full cell[28]; (b) the EIS results of Na3V2(PO4)3//Zn full batteries before and after 30 cycles at –10 ℃ in the two electrolytes[28]; (c) the rate performances of Na3V2(PO4)3 //10 m NaClO4-0.17 m Zn(CH3COO)2-2 wt% VC//Zn full cells at low temperatures[28]; (d) the cycle performances of Na3V2(PO4)3 //10 m NaClO4-0.17 m Zn(CH3COO)2-2 wt% VC//Zn full cells at –10 ℃[28].
-
[1] Dunn B, Kamath H, Tarascon J M 2011 Science 334 928
 Google Scholar
Google Scholar
[2] Larcher D, Tarascon J M 2015 Nat. Chem. 7 19
 Google Scholar
Google Scholar
[3] Goodenough J B 2013 Acc. Chem. Res. 46 1053
 Google Scholar
Google Scholar
[4] Abada S, Marlair G, Lecocq A, Petit M, Sauvant-Moynot V, Huet F 2016 J. Power Sources 306 178
 Google Scholar
Google Scholar
[5] Kim H, Hong J, Park K Y, Kim H, Kim S W, Kang K 2014 Chem. Rev. 114 11788
 Google Scholar
Google Scholar
[6] Tang W, Zhu Y, Hou Y, Liu L, Wu Y, Loh K P, Zhang H, Zhu K 2013 Energy Environ. Sci. 6 2093
 Google Scholar
Google Scholar
[7] Suo L, Borodin O, Gao T, Olguin M, Ho J, Fan X, Luo C, Wang C, Xu K 2015 Science 350 938
 Google Scholar
Google Scholar
[8] Yue J, Suo L 2021 Energy Fuels 35 9228
 Google Scholar
Google Scholar
[9] Zhang S, Xu K, Jow T 2003 J. Power Sources 115 137
 Google Scholar
Google Scholar
[10] Gao H N, Zhao Z G, Cai Y D, Zhou J J, Hua W D, Chen L, Wang L, Zhang J Q, Han D, Liu M J, Jiang L 2017 Nat. Commun. 8 15911
 Google Scholar
Google Scholar
[11] Jiang L, Lu Y, Zhao C, Liu L, Zhang J, Zhang Q, Shen X, Zhao J, Yu X, Li H, Huang X, Chen L, Hu Y S 2019 Nat. Energy 4 495
 Google Scholar
Google Scholar
[12] Wang H, Zhang H, Cheng Y, Feng K, Li X, Zhang H 2018 Electrochim. Acta 278 279
 Google Scholar
Google Scholar
[13] Zhang Y, Xu J, Li Z, Wang Y, Wang S, Dong X, Wang Y 2022 Sci. Bull. 67 161
 Google Scholar
Google Scholar
[14] Zhu K J, Sun Z Q, Jin T, Chen X C, Si Y C, Li H X, Jiao L F 2022 Batteries Supercaps 5 202200308
[15] Nian Q, Wang J, Liu S, Sun T, Zheng S, Zhang Y, Tao Z, Chen J 2019 Angew. Chem. Int. Ed. 58 16994
 Google Scholar
Google Scholar
[16] Ma Z, Chen J, Vatamanu J, Borodin O, Bedrov D, Zhou X, Zhang W, Li W, Xu K, Xing L 2022 Energy Storage Materials 45 903
[17] Liu T, Liu K-T, Wang J, Ji X, Lan P, Mu Z, Pan Y, Cheng S, Liu M 2021 Energy Storage Materials 41 133
[18] Hu Y, Shi R W, Ren Y Y, Peng W S, Feng C D, Zhao Y, Zheng S J, Li W Z, Sun Z, Guo J N, Guo S Y, Wang X L, Yan F 2022 Adv. Funct. Mater. 32 2203081
 Google Scholar
Google Scholar
[19] Tron A, Jeong S, Park Y D, Mun J 2019 ACS Sustainable Chem. Eng. 7 14531
 Google Scholar
Google Scholar
[20] Liang G, Gan Z, Wang X, Jin X, Xiong B, Zhang X, Chen S, Wang Y, He H, Zhi C 2021 ACS Nano 15 17717
 Google Scholar
Google Scholar
[21] Zhu K, Sun Z, Li Z, Liu P, Chen X, Jiao L 2022 Energy Storage Mater. 53 523
 Google Scholar
Google Scholar
[22] Sun T, Liu C, Wang J, Nian Q, Feng Y, Zhang Y, Tao Z, Chen J 2020 Nano Res. 13 676
 Google Scholar
Google Scholar
[23] Sun Y, Zhang Y, Xu Z, Gou W, Han X, Liu M, Li CM 2022 ChemSusChem 202201362
[24] Zhu M, Wang X, Tang H, Wang J, Hao Q, Liu L, Li Y, Zhang K, Schmidt O G 2020 Adv. Funct. Mater. 30 1907218
 Google Scholar
Google Scholar
[25] Yan C, Wang Y, Deng X, Xu Y 2022 Nano-Micro Lett. 14 98
 Google Scholar
Google Scholar
[26] Wang M, Li T, Yin Y, Yan J, Zhang H, Li X 2022 Adv. Energy Mater. 12 2200728
 Google Scholar
Google Scholar
[27] Nian Q, Liu S, Liu J, Zhang Q, Shi J, Liu C, Wang R, Tao Z, Chen J 2019 ACS Appl. Energy Mater. 2 4370
 Google Scholar
Google Scholar
[28] Liu S, Lei T, Song Q, Zhu J, Zhu C 2022 ACS Appl. Mater. Interfaces 14 11425
 Google Scholar
Google Scholar
[29] Pipolo S, Salanne M, Ferlat G, Klotz S, Saitta AM, Pietrucci F 2017 Phys. Rev. Lett. 119 245701
 Google Scholar
Google Scholar
[30] Leadbetter A, Ward R, Clark J, Tucker P, Matsuo T, Suga H 1985 J. Chem. Phys. 82 424
 Google Scholar
Google Scholar
[31] Reber D, Kühnel R S, Battaglia C 2019 ACS Mater. Lett. 1 44
 Google Scholar
Google Scholar
[32] Becker M, Kühnel R S, Battaglia C 2019 ChemComm 55 12032
 Google Scholar
Google Scholar
[33] Liu J, Yang C, Chi X, Wen B, Wang W, Liu Y 2022 Adv. Funct. Mater. 32 2106811
 Google Scholar
Google Scholar
[34] Reber D, Borodin O, Becker M, Rentsch D, Thienenkamp J H, Grissa R, Zhao W, Aribia A, Brunklaus G, Battaglia C 2022 Adv. Funct. Mater. 32 2112138
 Google Scholar
Google Scholar
[35] Bi H, Wang X, Liu H, He Y, Wang W, Deng W, Ma X, Wang Y, Rao W, Chai Y, Ma H, Li R, Chen J, Wang Y, Xue M 2020 Adv. Mater. 32 2000074
 Google Scholar
Google Scholar
[36] Zhu K, Li Z, Sun Z, Liu P, Jin T, Chen X, Li H, Lu W, Jiao L 2022 Small 18 2107662
 Google Scholar
Google Scholar
[37] Ao H, Zhao Y, Zhou J, Cai W, Zhang X, Zhu Y, Qian Y 2019 J. Mater. Chem. A 7 18708
 Google Scholar
Google Scholar
[38] Ramanujapuram A, Yushin G 2018 Adv. Energy Mater. 8 1802624
 Google Scholar
Google Scholar
[39] Chen J, Vatamanu J, Xing L, Borodin O, Chen H, Guan X, Liu X, Xu K, Li W 2020 Adv. Energy Mater. 10 1902654
 Google Scholar
Google Scholar
[40] Liu T, Zhang M, Wang Y L, Wang Q Y, Lü C, Liu K X, Suresh S, Yin Y H, Hu Y Y, Li Y S, Liu X B, Zhong X W, Xia B Y, Wu Z P 2018 Adv. Energy Mater. 8 1802349
 Google Scholar
Google Scholar
[41] Lee J, Lee C L, Park K, Kim I D 2014 J. Power Sources 248 1211
 Google Scholar
Google Scholar
[42] Madzvamuse A, Hamenu L, Mohammed L, Ko JM 2018 ChemistrySelect 3 10805
 Google Scholar
Google Scholar
计量
- 文章访问数: 9042
- PDF下载量: 146
- 被引次数: 0
















 下载:
下载: