-
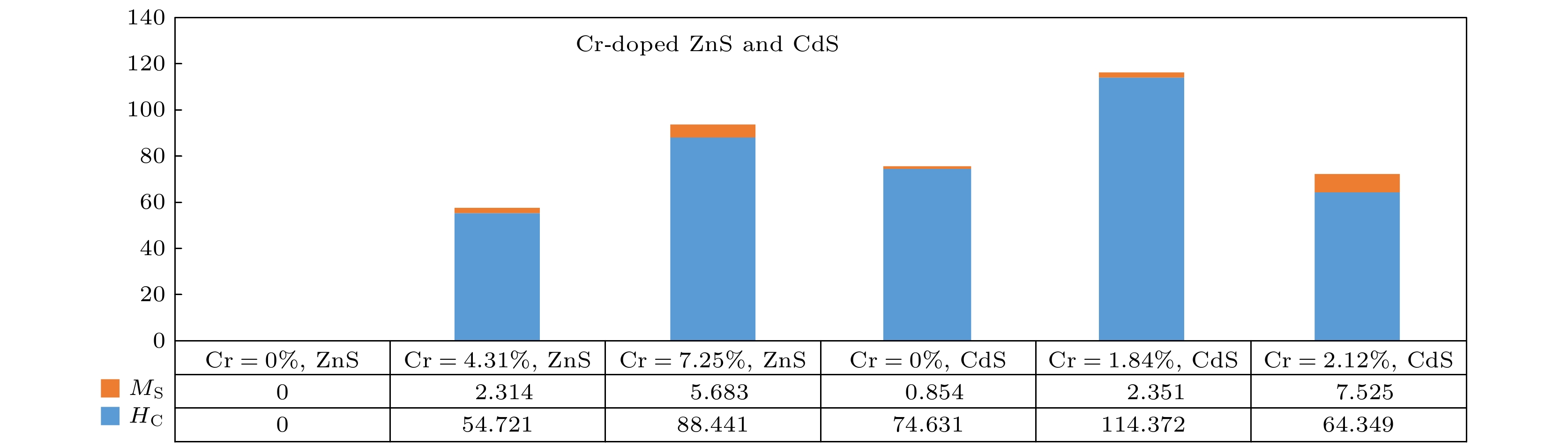
以乙醇胺和乙二胺为混合溶剂, 通过简单溶剂热法, 用S, ZnO和CdO为源, 成功制备了不同量Cr掺杂ZnS和CdS半导体纳米结构. X-射线衍射测试表明, 纳米结构ZnS和CdS具有纤锌矿结构. 扫描电子显微镜给出了不同铬含量的ZnS和CdS的形貌. 用电子能量散射谱观察到产物的成分为Cr, Zn, Cd和S. 振动样品磁强计测量表明, Cr掺杂的ZnS在室温下表现出铁磁性, 而未掺杂的ZnS在室温下表现出抗磁性. 掺杂的ZnS纳米片(Cr原子百分比为4.31%和7.25%)饱和磁化Ms分别为2.314 × 10–3和5.683 × 10–3 emu/g (1 emu/g = 10–3A·m2/g), 矫顽力Hc为54.721, 和88.441 Oe (
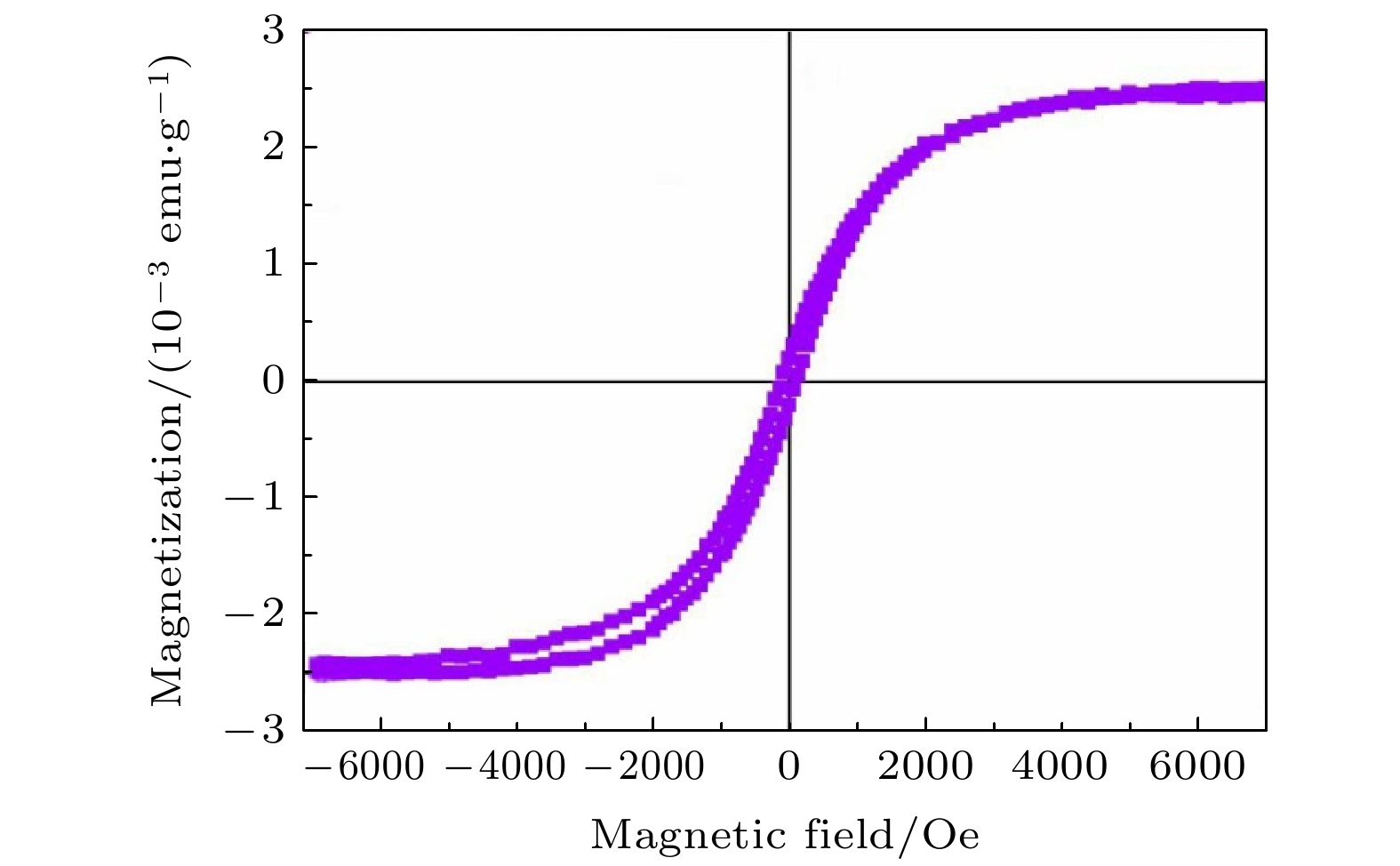
$1\;{\rm{Oe}} = \dfrac{{{{10}^3}}}{{4{\rm{\pi }}}}{\rm{A}}/{\rm{m}}$ ). 未掺杂CdS的铁磁性很弱, 而Cr掺杂CdS的铁磁性较强. CdS纳米片(Cr原子百分比分别为0, 1.84%和2.12%)的饱和磁化Ms分别为0.854 × 10–3, 2.351 × 10–3和7.525 × 10–3 emu/g, 矫顽力Hc 为74.631, 114.372和64.349 Oe. 实验结果表明, 在室温下, Cr掺杂ZnS具有铁磁性, 这与第一性原理计算的Cr掺杂ZnS的铁磁性结果一致. Cr掺杂CdS的铁磁性起源与CdS晶格中Cr的掺杂有关.With the continuous development of nanotechnology, people have higher and higher requirements for the performances of nanomaterials. In the past few decades, researchers have used various methods to prepare nanomaterials with different dopants, and studied their optical and electrical properties. Nanomaterials with ferromagnetic properties have a wide range of applications, and there have been many reports about the ferromagnetic properties of doped magnetic elements. However, there have been few reports about Cr-doped ZnS and CdS. In order to obtain Cr-doped ZnS and CdS nanosheets with room temperature ferromagnetism, in this paper, using ethanolamine (EA) and ethylenediamine (EN) as mixed solvents, ZnS and CdS semiconductor nanostructures doped with different amounts of chromium are successfully prepared in S, ZnO and CdO sources by simple solvent thermal method. The X-ray diffraction (XRD) measurements show that the ZnS and CdS nanostructure have a wurtzite structure. Scanning electron microscopy (SEM) images show the morphologies of ZnS and CdS with different chromium content. When the content of Cr is 4.31 at% or 7.25 at%, the thickness of Cr-doped ZnS nanosheets is about 210–290 nm, and the morphology of undoped ZnS is composed of sub-morphologies of relatively thick nanosheets. The morphologies of CdS doped with different amounts of Cr are composed of sub-morphologies of snowflake like nanosheets with thickness of about 120–190 nm. Energy dispersive spectrometer (EDS) is used to observe the product composed of Cr, Zn, Cd, and S. The EDS measurement and calculation of the Cr content in Cr-doped ZnS nanosheets are 4.31 at% and 7.25 at% respectively, and those of the Cr content in Cr-doped CdS nanosheets are 1.84 at% and 2.12 at%. The vibration sample magnetometer(VSM) measurements show that ZnS doped with chromium exhibits ferromagnetism at room temperature, while the undoped ZnS exhibits diamagnetism at room temperature. The values of saturation magnetization Msof Cr-doped ZnS nanosheets with Cr = 4.31 at% and 7.25 at% are 2.314 and 5.683 (10–3 emu/g), and the coercivity values of Hc are 54.721 and 88.441 Oe, respectively. The ferromagnetism of pure CdS is weak, while that of Cr-doped CdS is enhanced at room temperature. The saturation magnetization Ms values of Cr-doped CdS nanosheets with Cr = 0, 1.84 at% and 2.12 at% are 0.854, 2.351 and 7.525(10–3 emu/g), respectively, and the coercivity values of Hc are 74.631, 114.372 and 64.349 Oe, respectively. The values of saturation magnetization of ZnS and CdS increases with the Cr doping increasing. The ferromagnetism of Cr-doped ZnS at room temperature is confirmed by the experimental result, which is consistent with the ferromagnetism of Cr-doped ZnS calculated by the first principle. The origin of ferromagnetism of Cr-doped CdS is related to the doping of Cr in CdS lattice. -
Keywords:
- ZnS /
- CdS /
- Cr /
- ferromagnetism
[1] Xiang Q J, Cheng B, Yu J G 2015 Angew. Chem. Int. Ed. 54 11350
 Google Scholar
Google Scholar
[2] Jin J, Yu J G, Guo D P, Cui C, Ho W K 2015 Small 11 5262
 Google Scholar
Google Scholar
[3] Zhang J, Xu Q L, Qiao S Z, Yu J G 2013 Chem. Sum. Chem. Full. Papers 6 2009
[4] Diroll B T, Koschitzky A, Murray C B 2014 J. Phys. Chem. Lett. 5 85
 Google Scholar
Google Scholar
[5] Diroll B T, Murray C B 2014 American Chem. Soc. Nano Lett. 8 6466
[6] Zhong W W, Shen S J, He M, Wang D, Wang Z P, Lin Z P, Tu W G, Yu J G 2019 Appl. Catal., B 258 117967
 Google Scholar
Google Scholar
[7] Hu Y, Hao X Q, Cui Z W, Zhou J, Chu S Q, Wang Y, Zou Z G 2020 Appl. Catal., B 260 118131
 Google Scholar
Google Scholar
[8] Liu C H, Yu A F, Peng M Z, Song M, Liu W, Zhang Y, Zhai J Y 2016 J. Mater. Chem. C 120 6971
[9] Purusothaman Y, Alluri N R, Chandrasekhar A, Kim S J 2017 J. Mater. Chem. C 5 415
 Google Scholar
Google Scholar
[10] Yang D C, Qiu Y, Wang T Y, Song W B, Wang Z Z, Xu J, Feng Q X, Zong Y, Sun X L 2016 J. Mater. Sci.-Mater. Electron. 27 6708
 Google Scholar
Google Scholar
[11] Junaid M, Imran M, Ikram M, Naz M, Aqeel M, Afzal H, Maeed H, All S 2019 Appl. Nano Sci. 18 0933
[12] Murugesan R, Sivakumar S, Karthik K, Anandan P, Haris M 2019 Appl. Phys. A 125 281
 Google Scholar
Google Scholar
[13] Kumar B, Sinha N, Ray G, Godara S, Gupta M K 2014 Mater. Res. Bull. 59 267
 Google Scholar
Google Scholar
[14] ChengY J, Brahma S, Liu C P, Huang J L 2017 J. Alloys Compd. 728 1248
 Google Scholar
Google Scholar
[15] Sinha N, Goel S, Joseph A J, Yadav H, Batra K, Gupta M K, Kumar B 2018 Ceram. Int. 44 8582
 Google Scholar
Google Scholar
[16] Shim Y, Mills M E, Borovikov V, Amar J G 2009 Phys. Rev. E 5 1604
[17] Elango M, Gopalakrishnan K, Vairam S, Thamilselvan M 2012 J. Alloys Compd. 538 48
 Google Scholar
Google Scholar
[18] Niwayama Y, Kura H, Sato T, Takahashi M, Ogawa T 2008 Phys. Rev. Lett. 92 202502
[19] Haazen P P, Laloe J B, Nummy T J, Swagten J M, Herrero P J, Heiman D, Moodera J S 2012 Appl. Phys. Lett. 100 082404
 Google Scholar
Google Scholar
[20] Park S O, Lee H J, Cho Y C, Jeong S Y 2002 Appl. Phys. Lett. 80 22
 Google Scholar
Google Scholar
[21] Li Y F, Zhou Z, Jin P, Chen Y S, Zhang S B, Chen Z F 2010 J. Phys. Chem. C 114 12099
[22] Madhu C, Sundaresan A, Rao C N R 2008 Phys. Rev. B 77 201306
 Google Scholar
Google Scholar
[23] Zhang Z F, Li J, Jian J K, Wu R, Sun Y F, Wang S F, Ren Y S, Li J J 2013 J. Cryst. Growth 372 39
 Google Scholar
Google Scholar
[24] Zhang Z F, Han L, Xie G Y, Liao Q L, Zhong B, Yu Y 2016 J. Mater. Sci.- Mater. Electron. 27 12490
 Google Scholar
Google Scholar
[25] Elavarthi P, Kumar A A, Murali G, Reddy D A, Gunasekhar K R 2016 J. Alloys Compd. 656 510
 Google Scholar
Google Scholar
[26] Zhang S X, Ogale S B, Kundaliya D C, Fu L F, Browning N D, Dhar S, Ramadan W, Higgins J S, Greene R L, Venkatesan T 2006 Appl. Phys. Lett. 89 012501
 Google Scholar
Google Scholar
[27] Ozaki N, Nishizawa N, Marcet S, Kuroda S, Eryu O, Takita K 2006 Phys. Rev. Lett. 97 037201
 Google Scholar
Google Scholar
-
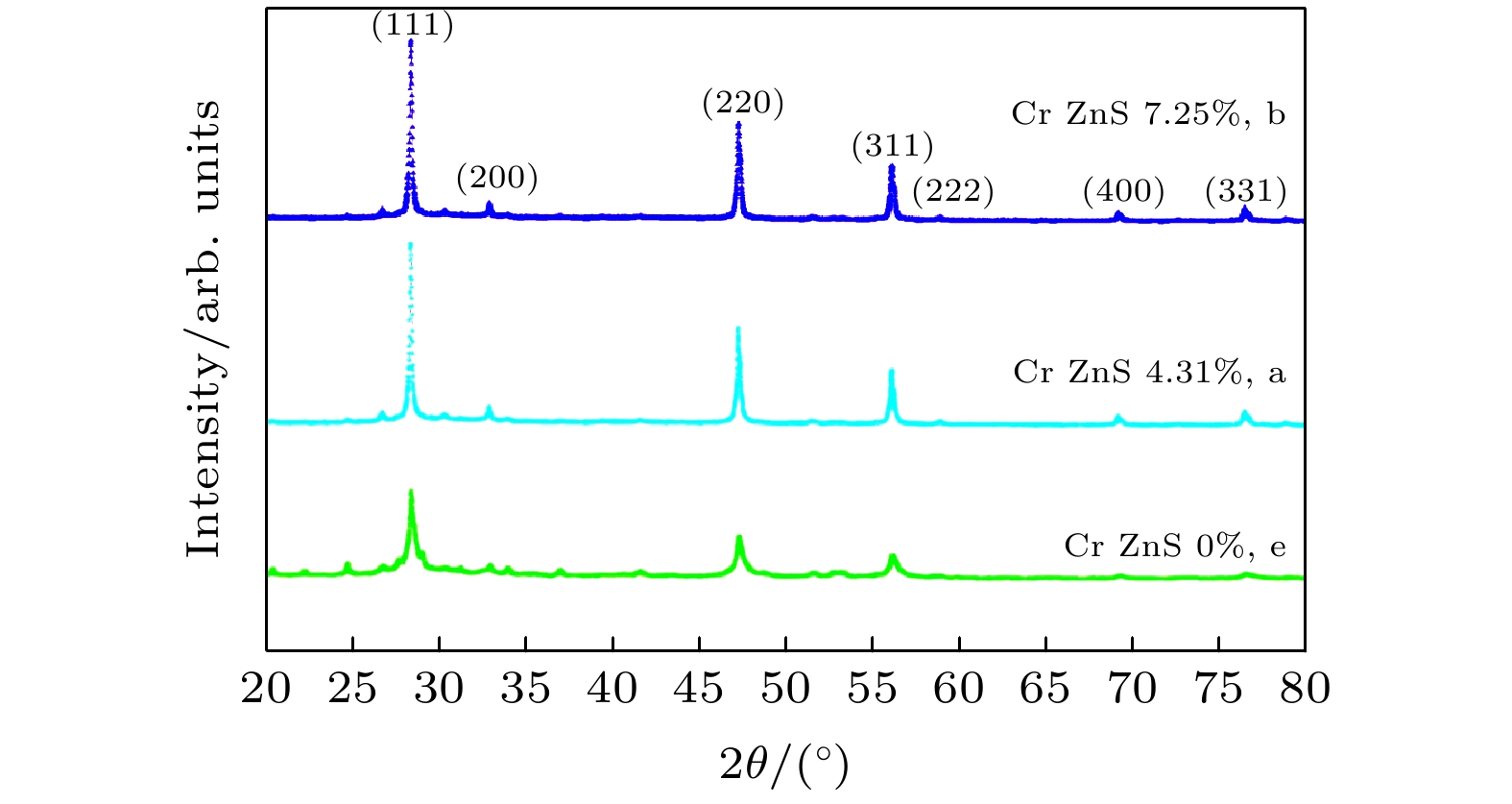
图 1 Cr掺杂ZnS纳米片的XRD图谱, 三条曲线分别对应样本A (Cr原子百分比为4.31%, a), 样本B (Cr原子百分比为7.25%, b)和样本E (Cr原子百分比为0.00%, e)
Fig. 1. Some of the powder XRD patterns of Cr-doped ZnS nanosheets. Three curves correspond to sample A (atomic percentages of Cr is4.31%, a), sample B (atomic percentages of Cr is 7.25%, b), sample E (atomic percentages of Cr is 0%, e), respectively.
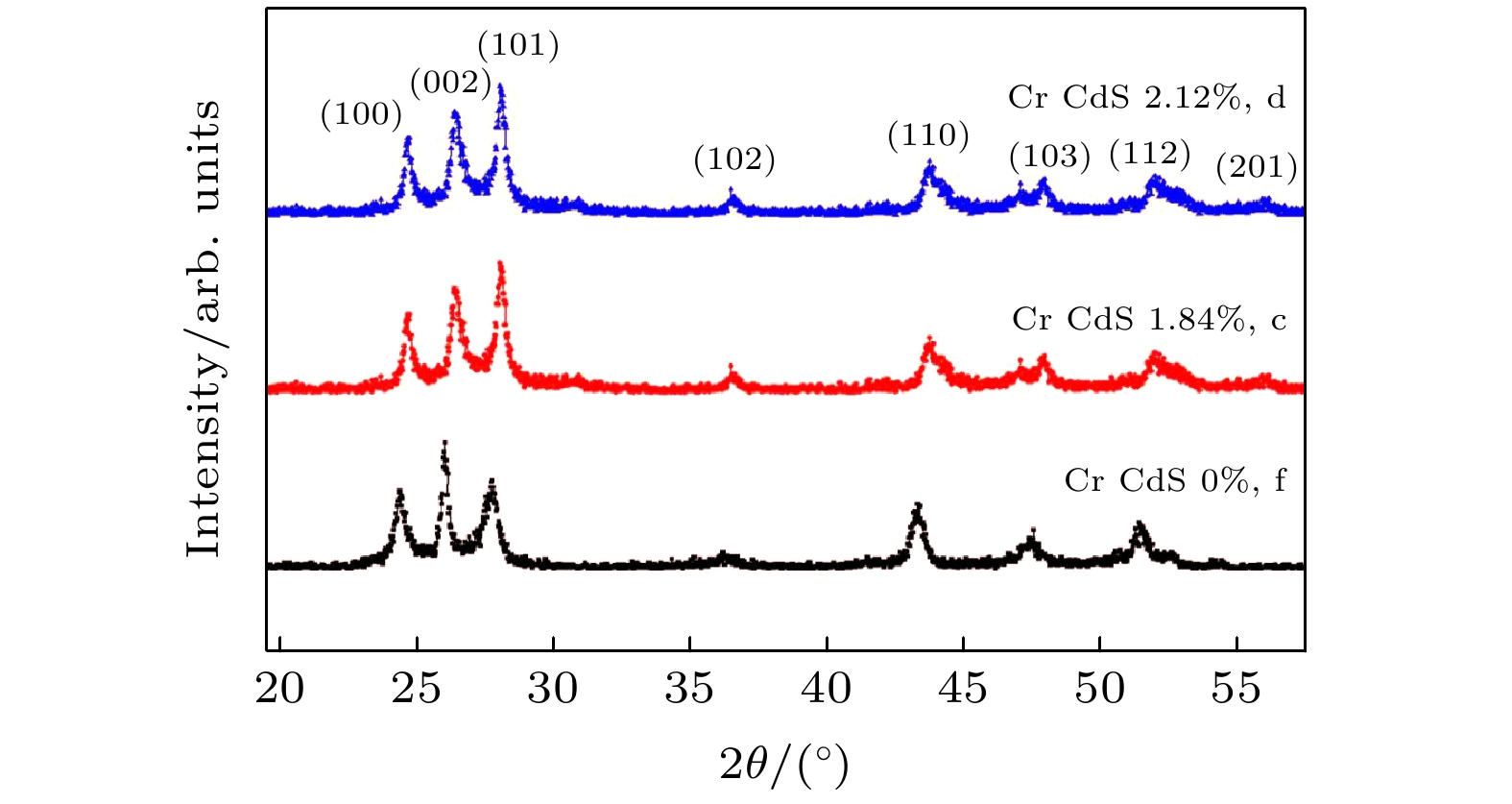
图 2 Cr掺杂CdS纳米片的XRD图谱, 三条曲线分别对应样本C (Cr原子百分比为1.84%, c), 样本D (Cr原子百分比为2.12%, d)和样本F (Cr原子百分比为0.00%, f)
Fig. 2. Some of the powder XRD patterns of the Cr-doped CdS nanosheets. Three curves correspond to sample C (atomic percentages of Cr is 1.84%, c), sample D (atomic percentages of Cr is 2.12%, d) and sample F (atomic percentages of Cr is 0.00%, f), respectively.
图 3 (a), (b), (e) Cr掺杂ZnS纳米片的SEM图案; (c), (d), (f) Cr掺杂CdS纳米片的SEM图案, 掺杂Cr的原子百分比分别为(a) 4.31%, (b) 7.25%, (e) 0, (c) 1.84%, (d) 2.12%, (f) = 0
Fig. 3. (a), (b), (e) The SEM patterns of the Cr-doped ZnS nanosheets; (c), (d), (f) the SEM patterns of the Cr-doped CdS nanosheets. The atomic percentages of Cr doping are (a) 4.31%, (b) 7.25%, (e) 0, (c) 1.84%, (d) 2.12%, (f) 0, respectively.
图 4 (a), (b) Cr掺杂ZnS纳米片的EDS图; (c), (d) Cr掺杂CdS纳米片的EDS图. 掺杂Cr的原子百分比分别为(a) 4.31%, (b) 7.25%, (c) 1.84%, (d) 2.12%
Fig. 4. (a), (b) The EDS patterns of the Cr-doped ZnS nanosheets; (c), (d) the EDS patterns of the Cr-doped CdS nanosheets. The atomic percentages of Cr doping are (a) 4.31%, (b) 7.25%, (c) 1.84%, (d) 2.12%, respectively.
表 1 制备样品(A—F)的反应条件(所有实验在200 ℃下反应24 h)
Table 1. Reaction conditions of the prepared products (All experments are carried out 200 ℃ for 24 h).
Sample Compsition of solvent/mL Sulfur, zinc and cadium
source/mmolChromic chloride hexahydrate/mmol Concentration of oxalic
acid/mmolA 10 EN + 20 EA 1 S + 1 ZnO 0.250 0.025 B 20 EN + 10 EA 1 S + 1 ZnO 0.500 0.025 E 15 EN + 15 EA 1 S + 1 ZnO 0 0.025 C 10 EN + 20 EA 1 S + 1 CdO 0.250 0.025 D 20 EN + 10 EA 1 S + 1 CdO 0.500 0.025 F 15 EN + 15 EA 1 S + 1 CdO 0 0.025 表 2 SEM, EDS和VSM计算Cr掺杂的ZnS和CdS纳米片的Cr含量、形貌、尺寸、磁性、矫顽力和饱和磁化强度图
Table 2. Measured chromium content, morphology, size, magnetic properties, coercivity and saturation magnetization of Cr doped ZnS and CdS nanosheets using SEM, EDS and VSM.
Cr content/% Morphologies Size/nm Magnetic properties Coercivity/Oe Saturation magnetization/
(10–3 emu·g–1)ZnS 0 Hexagonal flake 310—390 Diamagnetism — — 4.31 flower-like sheet 210—290 Ferromagnetism 54.721 2.314 7.25 Flower-like sheet 200—250 Ferromagnetism 88.441 5.683 CdS 0 Snowflake 110—160 Weak ferromagnetism 74.631 0.854 1.84 Snowflake 100—170 strong ferromagnetism 114.372 2.351 2.12 Snowflake 100—200 Strong ferromagnetism 64.349 7.525 -
[1] Xiang Q J, Cheng B, Yu J G 2015 Angew. Chem. Int. Ed. 54 11350
 Google Scholar
Google Scholar
[2] Jin J, Yu J G, Guo D P, Cui C, Ho W K 2015 Small 11 5262
 Google Scholar
Google Scholar
[3] Zhang J, Xu Q L, Qiao S Z, Yu J G 2013 Chem. Sum. Chem. Full. Papers 6 2009
[4] Diroll B T, Koschitzky A, Murray C B 2014 J. Phys. Chem. Lett. 5 85
 Google Scholar
Google Scholar
[5] Diroll B T, Murray C B 2014 American Chem. Soc. Nano Lett. 8 6466
[6] Zhong W W, Shen S J, He M, Wang D, Wang Z P, Lin Z P, Tu W G, Yu J G 2019 Appl. Catal., B 258 117967
 Google Scholar
Google Scholar
[7] Hu Y, Hao X Q, Cui Z W, Zhou J, Chu S Q, Wang Y, Zou Z G 2020 Appl. Catal., B 260 118131
 Google Scholar
Google Scholar
[8] Liu C H, Yu A F, Peng M Z, Song M, Liu W, Zhang Y, Zhai J Y 2016 J. Mater. Chem. C 120 6971
[9] Purusothaman Y, Alluri N R, Chandrasekhar A, Kim S J 2017 J. Mater. Chem. C 5 415
 Google Scholar
Google Scholar
[10] Yang D C, Qiu Y, Wang T Y, Song W B, Wang Z Z, Xu J, Feng Q X, Zong Y, Sun X L 2016 J. Mater. Sci.-Mater. Electron. 27 6708
 Google Scholar
Google Scholar
[11] Junaid M, Imran M, Ikram M, Naz M, Aqeel M, Afzal H, Maeed H, All S 2019 Appl. Nano Sci. 18 0933
[12] Murugesan R, Sivakumar S, Karthik K, Anandan P, Haris M 2019 Appl. Phys. A 125 281
 Google Scholar
Google Scholar
[13] Kumar B, Sinha N, Ray G, Godara S, Gupta M K 2014 Mater. Res. Bull. 59 267
 Google Scholar
Google Scholar
[14] ChengY J, Brahma S, Liu C P, Huang J L 2017 J. Alloys Compd. 728 1248
 Google Scholar
Google Scholar
[15] Sinha N, Goel S, Joseph A J, Yadav H, Batra K, Gupta M K, Kumar B 2018 Ceram. Int. 44 8582
 Google Scholar
Google Scholar
[16] Shim Y, Mills M E, Borovikov V, Amar J G 2009 Phys. Rev. E 5 1604
[17] Elango M, Gopalakrishnan K, Vairam S, Thamilselvan M 2012 J. Alloys Compd. 538 48
 Google Scholar
Google Scholar
[18] Niwayama Y, Kura H, Sato T, Takahashi M, Ogawa T 2008 Phys. Rev. Lett. 92 202502
[19] Haazen P P, Laloe J B, Nummy T J, Swagten J M, Herrero P J, Heiman D, Moodera J S 2012 Appl. Phys. Lett. 100 082404
 Google Scholar
Google Scholar
[20] Park S O, Lee H J, Cho Y C, Jeong S Y 2002 Appl. Phys. Lett. 80 22
 Google Scholar
Google Scholar
[21] Li Y F, Zhou Z, Jin P, Chen Y S, Zhang S B, Chen Z F 2010 J. Phys. Chem. C 114 12099
[22] Madhu C, Sundaresan A, Rao C N R 2008 Phys. Rev. B 77 201306
 Google Scholar
Google Scholar
[23] Zhang Z F, Li J, Jian J K, Wu R, Sun Y F, Wang S F, Ren Y S, Li J J 2013 J. Cryst. Growth 372 39
 Google Scholar
Google Scholar
[24] Zhang Z F, Han L, Xie G Y, Liao Q L, Zhong B, Yu Y 2016 J. Mater. Sci.- Mater. Electron. 27 12490
 Google Scholar
Google Scholar
[25] Elavarthi P, Kumar A A, Murali G, Reddy D A, Gunasekhar K R 2016 J. Alloys Compd. 656 510
 Google Scholar
Google Scholar
[26] Zhang S X, Ogale S B, Kundaliya D C, Fu L F, Browning N D, Dhar S, Ramadan W, Higgins J S, Greene R L, Venkatesan T 2006 Appl. Phys. Lett. 89 012501
 Google Scholar
Google Scholar
[27] Ozaki N, Nishizawa N, Marcet S, Kuroda S, Eryu O, Takita K 2006 Phys. Rev. Lett. 97 037201
 Google Scholar
Google Scholar
计量
- 文章访问数: 6800
- PDF下载量: 59
- 被引次数: 0














 下载:
下载: