-
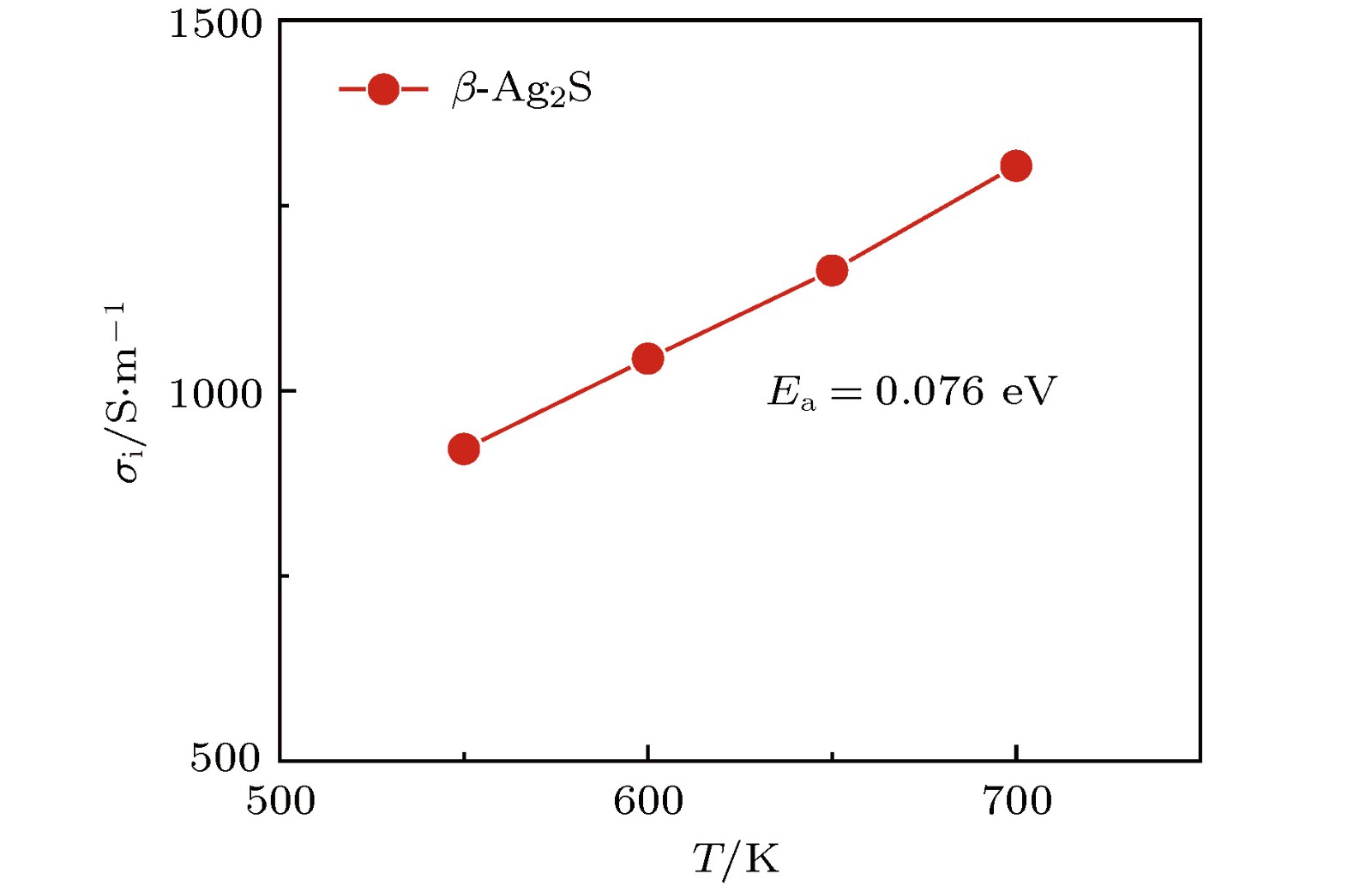
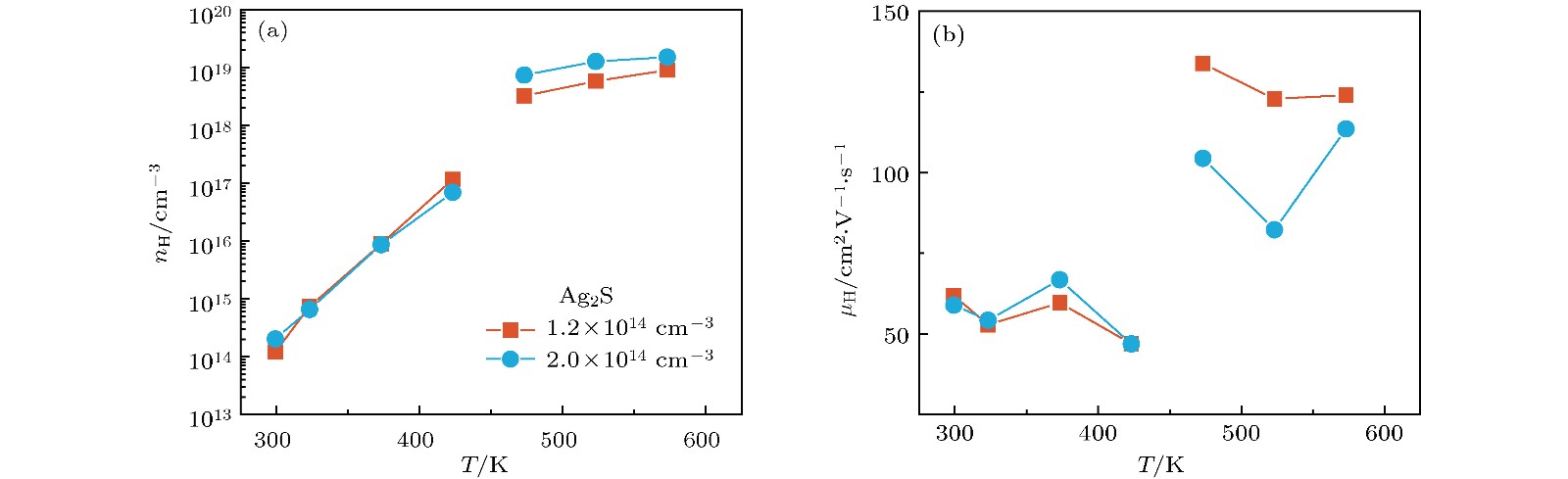
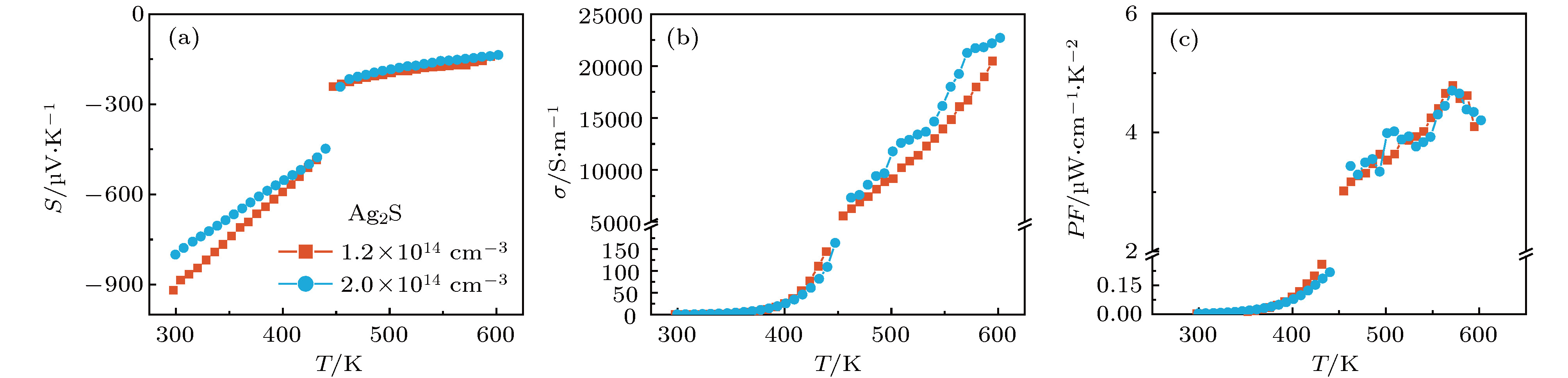
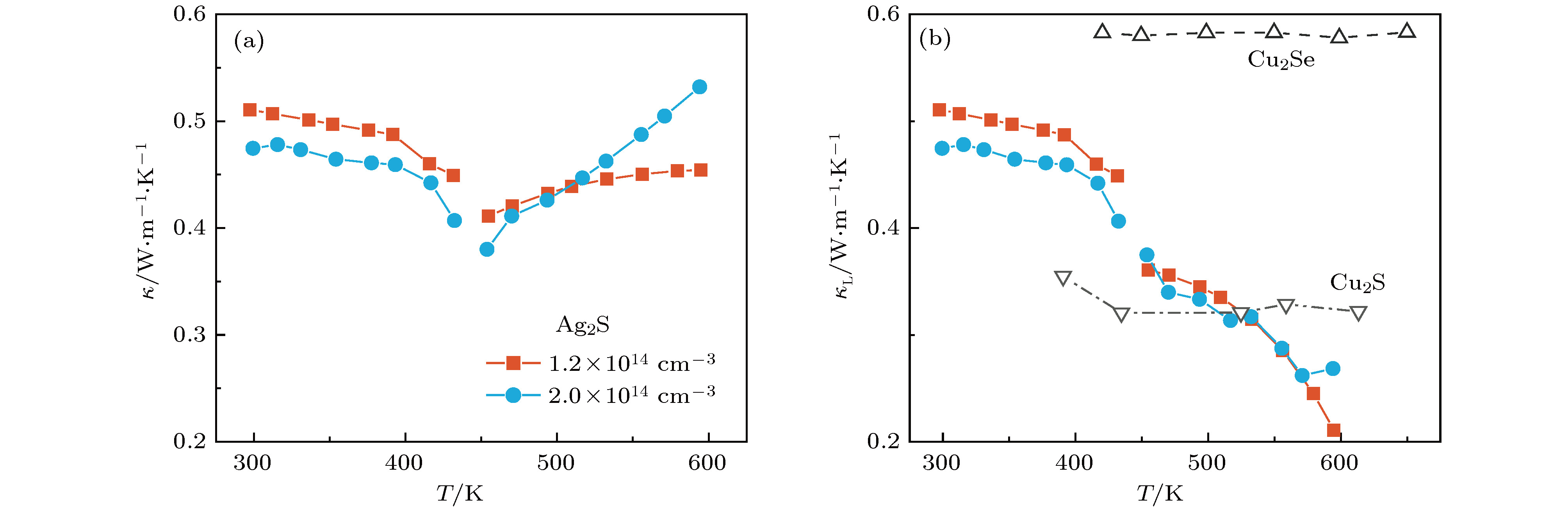
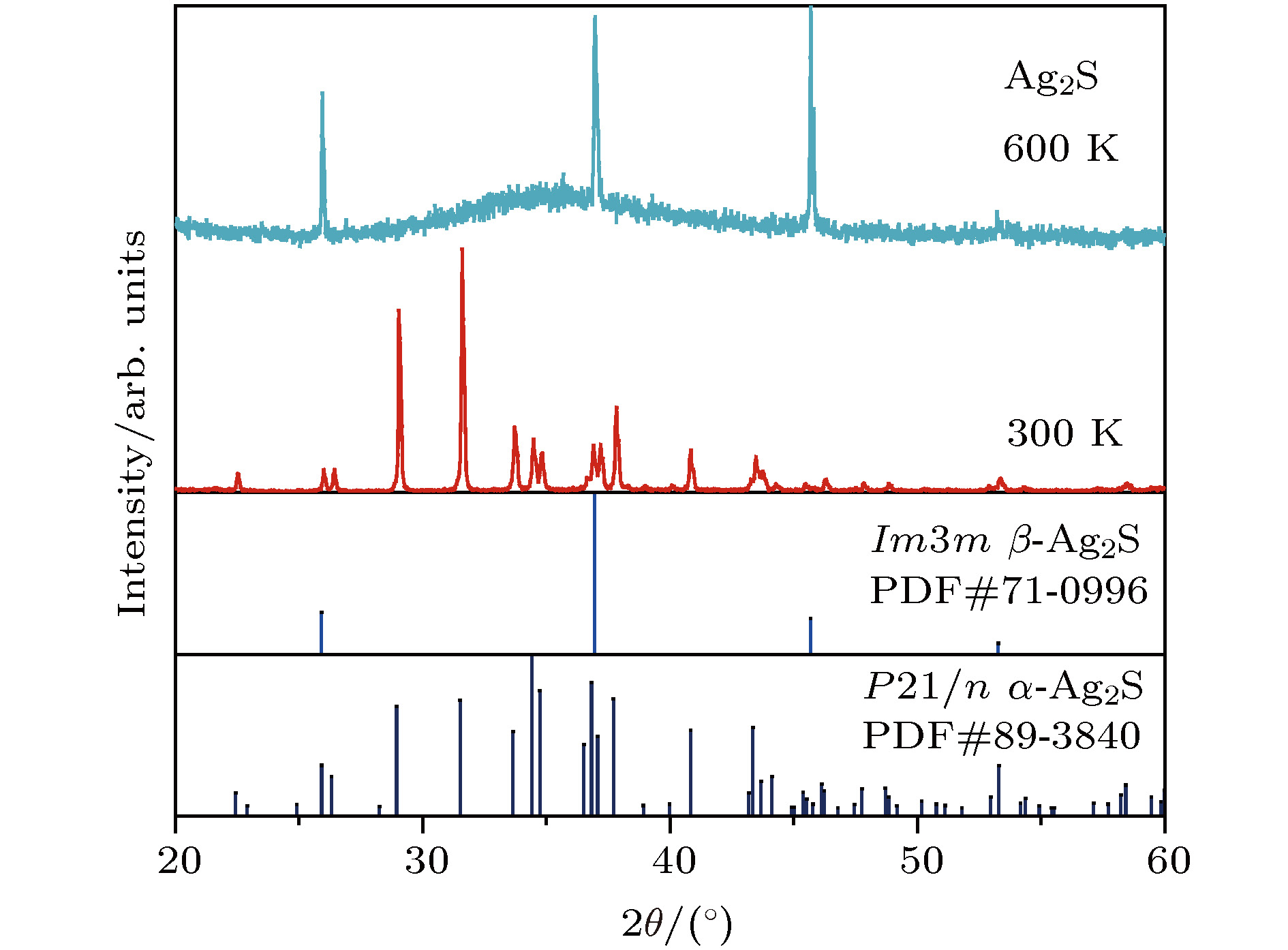
硫化银(Ag2S)是一种典型的快离子导体材料, 前期关于Ag2S的研究主要集中在光电和生物等领域. 最近的研究表明, α-Ag2S具有和金属一样的良好延展性和变形能力. 但是, Ag2S的热电性能尚无公开报道. 本工作合成了单相Ag2S化合物, 系统研究了其在300—600 K范围的物相变化、离子迁移特性和电热输运性质. 研究发现, Ag2S在300—600 K温度区间表现出半导体的电输运性质. 由于单斜-体心立方相晶体结构转变, Ag2S的离子电导率、载流子浓度、迁移率、电导率、泽贝克系数等性质在455 K前后出现急剧变化. 在550 K, Ag2S的功率因子最高可达5 μW·cm–1·K–2. Ag2S在300—600 K温度区间均表现出本征的低晶格热导率(低于0.6 W·m–1·K–1). S亚晶格中随机分布的类液态Ag离子是导致β-Ag2S体心立方相具有低晶格热导率的主要原因. 在573 K, Ag2S的热电优值可达0.55, 与Ag2Se, Ag2Te, CuAgSe等已报道的Ag基快离子导体热电材料的性能相当.Recently, Ag2S superionic conductor has attracted great attention due to its metal-like ductility and deformability. In this work, the single phase Ag2S compound is fabricated by the melting-annealing method. The crystal structure, ionic conduction, and electrical and thermal transports in a temperature range of 300-600 K are systematically investigated. The monoclinic-cubic crystal structure transition occurs around 455 K for Ag2S. Significant reduction in the specific heat at constant volume below the Dulong-Petit limit is observed for Ag2S after the monoclinic-cubic phase transition, which is attributed to the liquid-like Ag ions existing inside the sulfur framework. Ag2S shows typical semiconducting-like electrical transport behavior in the whole measured temperature range. Around 455 K, the ionic conductivity, carrier concentration, carrier mobility, electrical conductivity, and Seebeck coefficient each show an abrupt change. The calculated ionic activation based on the ionic conductivity is 0.076 eV for the body centered cubic Ag2S. The calculated band gap based on the electrical conductivity decreases from 1.1 eV for the monoclinic Ag2S to 0.42 eV for the body centered cubic Ag2S. The abrupt increase of electrical conductivity after the monoclinic-cubic phase transition leads to a maximum power factor around 5 μW·cm–1·K–2 at 550 K. In the whole measured temperature range, Ag2S demonstrates an intrinsically low lattice thermal conductivity (below 0.6 W·m–1·K–1). The calculated phonon dispersion indicates that the weak chemical bonding between Ag and S is responsible for the low lattice thermal conductivity observed in the monoclinic Ag2S. Likewise, the presence of liquid-like Ag ions with low ionic activation energy is responsible for the low lattice thermal conductivity for the cubic Ag2S. Finally, the Ag2S shows the maximum thermoelectric figure of merit of 0.55 at 580 K, which is comparable to the thermoelectric figure of merit reported before in most of Ag-based thermoelectric superionic conductors.
[1] Tan G, Zhao L, Kanatzidis M G 2016 Chem. Rev. 116 12123
 Google Scholar
Google Scholar
[2] Zeier W G, Zevalkink A, Gibbs Z M, Hautier G, Kanatzidis M G, Snyder G J 2016 Angew. Chem: Int. Ed. 55 6826
 Google Scholar
Google Scholar
[3] Shi X, Chen L, Uher C 2016 Int. Mater. Rev. 61 379
 Google Scholar
Google Scholar
[4] Zhu T, Liu Y, Fu C, Heremans J P, Snyder J G, Zhao X 2017 Adv. Mater. 29 1605884
 Google Scholar
Google Scholar
[5] Liu H, Shi X, Xu F, Zhang L, Zhang W, Chen L, Li Q, Uher C, Day T, Snyder J G 2012 Nat. Mater. 11 422
 Google Scholar
Google Scholar
[6] Zhao K, Qiu P, Song Q, Blichfeld A B, Eikeland E, Ren D, Ge B, Iversen B B, Shi X, Chen L 2017 Mater. Today Phys. 1 14
 Google Scholar
Google Scholar
[7] Zhu C, He Y, Lu P, Fu Z, Xu F, Yao H, Zhang L, Shi X, Chen L 2017 Ceram. Int. 43 7866
 Google Scholar
Google Scholar
[8] Zhao K, Guan M, Qiu P, Blichfeld A B, Eikeland E, Zhu C, Ren D, Xu F, Iversen B B, Shi X, Chen L 2018 J. Mater. Chem. A 6 6977
 Google Scholar
Google Scholar
[9] Lü Y, Chen J, Max D, Li Y, Shi X, Chen L 2015 J. Inorg. Mater. 30 1115
 Google Scholar
Google Scholar
[10] Wang X, Qiu P, Zhang T, Ren D, Wu L, Shi X, Yang J, Chen L 2015 J. Mater. Chem. A 3 13662
 Google Scholar
Google Scholar
[11] Bhattacharya S, Basu R, Bhatt R, Pitale S, Singh A, Aswal D K, Gupta S K, Navaneethan M, Hayakawa Y 2013 J. Mater. Chem. A 1 11289
 Google Scholar
Google Scholar
[12] Jiang B, Qiu P, EikelandE, Chen H, Song Q, Ren D, Zhang T, Yang J, Iversen B B, Shi X, Chen L 2017 J. Mater. Chem. C 5 943
 Google Scholar
Google Scholar
[13] Jiang B, Qiu P, Chen H, Zhang Q, Zhao K, Ren D, Shi X, Chen L 2017 Chem. Commun. 53 11658
 Google Scholar
Google Scholar
[14] Shi X, Chen H, Hao F, Liu R, Wang T, Qiu P, Burkhardt U, Grin Y, Chen L 2018 Nat. Mater. 17 421
 Google Scholar
Google Scholar
[15] Rahlfs P 1936 Zeitschrift für Phys. Chem. 31B 157
[16] Skinner B J 1966 Econ. Geol. 61 1
 Google Scholar
Google Scholar
[17] 董占民, 孙红三, 许佳, 李一, 孙家林 2011 60 077304
 Google Scholar
Google Scholar
Dong Z M, Sun H S, Xu J, Li Y, Sun J L 2011 Acta Phys. Sin. 60 077304
 Google Scholar
Google Scholar
[18] Yang J, Ying J Y 2011 Angew. Chem.: Int. Ed. 50 4637
 Google Scholar
Google Scholar
[19] Khanchandani S, Srivastava P K, Kumar S, Ghosh S, Ganguli A K 2014 Inorg. Chem. 53 8902
 Google Scholar
Google Scholar
[20] ZhangY, Hong G, ZhangY, Chen G, Li F, Dai H, Wang Q 2012 ACS Nano 6 3695
 Google Scholar
Google Scholar
[21] Du Y, Xu B, Fu T, Cai M, Li F, Zhang Y, Wang Q 2010 J. Am. Chem. Soc. 132 1470
 Google Scholar
Google Scholar
[22] 邓立儿, 李妍, 巩蕾, 王佳 2018 无机材料学报 33 825
Deng L, Li Y, Gong L, Wang J 2018 J. Inorg. Mater. 33 825
[23] Hong G, Robinson J T, Zhang Y, Diao S, Antaris A L, Wang Q, Dai H 2012 Angew. Chem.: Int. Ed. 51 9818
 Google Scholar
Google Scholar
[24] Pei Y, Shi X, Lalonde A, Wang H, Chen L, Snyder G J 2011 Nature 473 66
 Google Scholar
Google Scholar
[25] 张玉, 吴立华, 曾李骄开, 刘叶烽, 张继业, 王佳, 邢娟娟, 骆军 2016 65 107201
 Google Scholar
Google Scholar
Zhang Y, Wu L H, Zeng L J K, Liu Y F, Zhang J Y, Wang J, Xing J J, Luo J 2016 Acta Phys. Sin. 65 107201
 Google Scholar
Google Scholar
[26] 杨小燕, 吴洁华, 任都迪, 张天松, 陈立东 2016 无机材料学报 31 997
Yang X Y, Wu J H, Ren D D, Zhang T S, Chen L D 2016 J. Inorg. Mater. 31 997
[27] Biswas K, He J, Blum I D, Wu C I, Hogan T P, Seidman D N, Dravid V P, Kanatzidis M G 2012 Nature 489 414
 Google Scholar
Google Scholar
[28] Shi X, Zhang W, Chen L, Yang J 2005 Phys. Rev. Lett. 95 185503
 Google Scholar
Google Scholar
[29] 姚铮, 仇鹏飞, 李小亚, 陈立东 2016 无机材料学报 31 1375
Yao Z, Qiu P F, Li X Y, Chen L D 2016 J. Inorg. Mater. 31 1375
[30] Day T, Drymiotis F, Zhang T, Rhodes D, Shi X, Chen L, Snyder G J 2013 J. Mater. Chem. C 1 7568
 Google Scholar
Google Scholar
[31] Pei Y, Heinz N A, Snyder G J 2011 J. Mater. Chem. 21 18256
 Google Scholar
Google Scholar
[32] Liu Y, Qiu P, Chen H, Chen R, Shi X, Chen L 2017 J. Inorg. Mater. 32 1337
 Google Scholar
Google Scholar
[33] Tsuchiya Y, Tamaki S, Waseda Y, Toguri J M 1978 J. Phys. C: Solid State Phys. 11 651
 Google Scholar
Google Scholar
[34] Blanton T, Misture S, Dontula N, Zdzieszynski S 2011 Powder Diffr. 26 114
 Google Scholar
Google Scholar
[35] Honma K, Iida K 1987 J. Phys. Soc. Japan 56 1828
 Google Scholar
Google Scholar
[36] Ishiwata S, Shiomi Y, Lee J S, Bahramy M S, Suzuki T, Uchida M, Arita R, Taguchi Y, Tokura Y 2013 Nat. Mater. 12 512
 Google Scholar
Google Scholar
[37] He Y, Da yT, Zhang T, Liu H, Shi X, Chen L, Snyder G J 2014 Adv. Mater. 26 3974
 Google Scholar
Google Scholar
[38] Liu H, YuanX, Lu P, Shi X, Xu F, He Y, Tang Y, Bai S, Zhang W, Chen L, Lin Y, Shi L, Lin H, Gao X, Zhang X, Chi H, Uher C 2013 Adv. Mater. 25 6607
 Google Scholar
Google Scholar
[39] Aliev F F, Jafarov M B, Tairov B A, Pashaev G P, Saddinova A A, Kuliev A A 2008 Semiconductors 42 1146
 Google Scholar
Google Scholar
[40] Balapanov M K, Gafurov I G, Mukhamed'yanov U K, Yakshibaev R A, Ishembetov R K 2004 Phys. Status Solidi B 241 114
 Google Scholar
Google Scholar
[41] Mi W, Qiu P, Zhang T, Lü Y, Shi X, Chen L 2014 Appl. Phys. Lett. 104 133903
 Google Scholar
Google Scholar
[42] Xiao C, Xu J, Li K, Feng J, Yang J, Xie Y 2012 J. Am. Chem. Soc. 134 4287
 Google Scholar
Google Scholar
[43] He Y, Lu P, Shi X, Xu F, Zhang T, Snyder G J, Uher C, Chen L 2015 Adv. Mater. 27 3639
 Google Scholar
Google Scholar
[44] Qiu P, Qin Y, Zhang Q, Li R, Yang J, Song Q, Tang Y, Bai S, Shi X, Chen L 2018 Adv. Sci. 5 1700727
 Google Scholar
Google Scholar
-
图 3 Ag2S化合物的(a)定压热容Cp及(b)相同温度下的Cp和定容热容CV计算值的比较, 其中点划线分别为固体的CV理论值3NkB和液体的CV理论值2NkB
Fig. 3. (a) Specific heat at constant pressure Cp of Ag2S compound; (b) comparison of Cp and the calculated specific heat at constant volume CV. The dash-dot lines are the theoretical CV of solid and liquid, respectively.
-
[1] Tan G, Zhao L, Kanatzidis M G 2016 Chem. Rev. 116 12123
 Google Scholar
Google Scholar
[2] Zeier W G, Zevalkink A, Gibbs Z M, Hautier G, Kanatzidis M G, Snyder G J 2016 Angew. Chem: Int. Ed. 55 6826
 Google Scholar
Google Scholar
[3] Shi X, Chen L, Uher C 2016 Int. Mater. Rev. 61 379
 Google Scholar
Google Scholar
[4] Zhu T, Liu Y, Fu C, Heremans J P, Snyder J G, Zhao X 2017 Adv. Mater. 29 1605884
 Google Scholar
Google Scholar
[5] Liu H, Shi X, Xu F, Zhang L, Zhang W, Chen L, Li Q, Uher C, Day T, Snyder J G 2012 Nat. Mater. 11 422
 Google Scholar
Google Scholar
[6] Zhao K, Qiu P, Song Q, Blichfeld A B, Eikeland E, Ren D, Ge B, Iversen B B, Shi X, Chen L 2017 Mater. Today Phys. 1 14
 Google Scholar
Google Scholar
[7] Zhu C, He Y, Lu P, Fu Z, Xu F, Yao H, Zhang L, Shi X, Chen L 2017 Ceram. Int. 43 7866
 Google Scholar
Google Scholar
[8] Zhao K, Guan M, Qiu P, Blichfeld A B, Eikeland E, Zhu C, Ren D, Xu F, Iversen B B, Shi X, Chen L 2018 J. Mater. Chem. A 6 6977
 Google Scholar
Google Scholar
[9] Lü Y, Chen J, Max D, Li Y, Shi X, Chen L 2015 J. Inorg. Mater. 30 1115
 Google Scholar
Google Scholar
[10] Wang X, Qiu P, Zhang T, Ren D, Wu L, Shi X, Yang J, Chen L 2015 J. Mater. Chem. A 3 13662
 Google Scholar
Google Scholar
[11] Bhattacharya S, Basu R, Bhatt R, Pitale S, Singh A, Aswal D K, Gupta S K, Navaneethan M, Hayakawa Y 2013 J. Mater. Chem. A 1 11289
 Google Scholar
Google Scholar
[12] Jiang B, Qiu P, EikelandE, Chen H, Song Q, Ren D, Zhang T, Yang J, Iversen B B, Shi X, Chen L 2017 J. Mater. Chem. C 5 943
 Google Scholar
Google Scholar
[13] Jiang B, Qiu P, Chen H, Zhang Q, Zhao K, Ren D, Shi X, Chen L 2017 Chem. Commun. 53 11658
 Google Scholar
Google Scholar
[14] Shi X, Chen H, Hao F, Liu R, Wang T, Qiu P, Burkhardt U, Grin Y, Chen L 2018 Nat. Mater. 17 421
 Google Scholar
Google Scholar
[15] Rahlfs P 1936 Zeitschrift für Phys. Chem. 31B 157
[16] Skinner B J 1966 Econ. Geol. 61 1
 Google Scholar
Google Scholar
[17] 董占民, 孙红三, 许佳, 李一, 孙家林 2011 60 077304
 Google Scholar
Google Scholar
Dong Z M, Sun H S, Xu J, Li Y, Sun J L 2011 Acta Phys. Sin. 60 077304
 Google Scholar
Google Scholar
[18] Yang J, Ying J Y 2011 Angew. Chem.: Int. Ed. 50 4637
 Google Scholar
Google Scholar
[19] Khanchandani S, Srivastava P K, Kumar S, Ghosh S, Ganguli A K 2014 Inorg. Chem. 53 8902
 Google Scholar
Google Scholar
[20] ZhangY, Hong G, ZhangY, Chen G, Li F, Dai H, Wang Q 2012 ACS Nano 6 3695
 Google Scholar
Google Scholar
[21] Du Y, Xu B, Fu T, Cai M, Li F, Zhang Y, Wang Q 2010 J. Am. Chem. Soc. 132 1470
 Google Scholar
Google Scholar
[22] 邓立儿, 李妍, 巩蕾, 王佳 2018 无机材料学报 33 825
Deng L, Li Y, Gong L, Wang J 2018 J. Inorg. Mater. 33 825
[23] Hong G, Robinson J T, Zhang Y, Diao S, Antaris A L, Wang Q, Dai H 2012 Angew. Chem.: Int. Ed. 51 9818
 Google Scholar
Google Scholar
[24] Pei Y, Shi X, Lalonde A, Wang H, Chen L, Snyder G J 2011 Nature 473 66
 Google Scholar
Google Scholar
[25] 张玉, 吴立华, 曾李骄开, 刘叶烽, 张继业, 王佳, 邢娟娟, 骆军 2016 65 107201
 Google Scholar
Google Scholar
Zhang Y, Wu L H, Zeng L J K, Liu Y F, Zhang J Y, Wang J, Xing J J, Luo J 2016 Acta Phys. Sin. 65 107201
 Google Scholar
Google Scholar
[26] 杨小燕, 吴洁华, 任都迪, 张天松, 陈立东 2016 无机材料学报 31 997
Yang X Y, Wu J H, Ren D D, Zhang T S, Chen L D 2016 J. Inorg. Mater. 31 997
[27] Biswas K, He J, Blum I D, Wu C I, Hogan T P, Seidman D N, Dravid V P, Kanatzidis M G 2012 Nature 489 414
 Google Scholar
Google Scholar
[28] Shi X, Zhang W, Chen L, Yang J 2005 Phys. Rev. Lett. 95 185503
 Google Scholar
Google Scholar
[29] 姚铮, 仇鹏飞, 李小亚, 陈立东 2016 无机材料学报 31 1375
Yao Z, Qiu P F, Li X Y, Chen L D 2016 J. Inorg. Mater. 31 1375
[30] Day T, Drymiotis F, Zhang T, Rhodes D, Shi X, Chen L, Snyder G J 2013 J. Mater. Chem. C 1 7568
 Google Scholar
Google Scholar
[31] Pei Y, Heinz N A, Snyder G J 2011 J. Mater. Chem. 21 18256
 Google Scholar
Google Scholar
[32] Liu Y, Qiu P, Chen H, Chen R, Shi X, Chen L 2017 J. Inorg. Mater. 32 1337
 Google Scholar
Google Scholar
[33] Tsuchiya Y, Tamaki S, Waseda Y, Toguri J M 1978 J. Phys. C: Solid State Phys. 11 651
 Google Scholar
Google Scholar
[34] Blanton T, Misture S, Dontula N, Zdzieszynski S 2011 Powder Diffr. 26 114
 Google Scholar
Google Scholar
[35] Honma K, Iida K 1987 J. Phys. Soc. Japan 56 1828
 Google Scholar
Google Scholar
[36] Ishiwata S, Shiomi Y, Lee J S, Bahramy M S, Suzuki T, Uchida M, Arita R, Taguchi Y, Tokura Y 2013 Nat. Mater. 12 512
 Google Scholar
Google Scholar
[37] He Y, Da yT, Zhang T, Liu H, Shi X, Chen L, Snyder G J 2014 Adv. Mater. 26 3974
 Google Scholar
Google Scholar
[38] Liu H, YuanX, Lu P, Shi X, Xu F, He Y, Tang Y, Bai S, Zhang W, Chen L, Lin Y, Shi L, Lin H, Gao X, Zhang X, Chi H, Uher C 2013 Adv. Mater. 25 6607
 Google Scholar
Google Scholar
[39] Aliev F F, Jafarov M B, Tairov B A, Pashaev G P, Saddinova A A, Kuliev A A 2008 Semiconductors 42 1146
 Google Scholar
Google Scholar
[40] Balapanov M K, Gafurov I G, Mukhamed'yanov U K, Yakshibaev R A, Ishembetov R K 2004 Phys. Status Solidi B 241 114
 Google Scholar
Google Scholar
[41] Mi W, Qiu P, Zhang T, Lü Y, Shi X, Chen L 2014 Appl. Phys. Lett. 104 133903
 Google Scholar
Google Scholar
[42] Xiao C, Xu J, Li K, Feng J, Yang J, Xie Y 2012 J. Am. Chem. Soc. 134 4287
 Google Scholar
Google Scholar
[43] He Y, Lu P, Shi X, Xu F, Zhang T, Snyder G J, Uher C, Chen L 2015 Adv. Mater. 27 3639
 Google Scholar
Google Scholar
[44] Qiu P, Qin Y, Zhang Q, Li R, Yang J, Song Q, Tang Y, Bai S, Shi X, Chen L 2018 Adv. Sci. 5 1700727
 Google Scholar
Google Scholar
计量
- 文章访问数: 19827
- PDF下载量: 589
- 被引次数: 0













 下载:
下载: