-
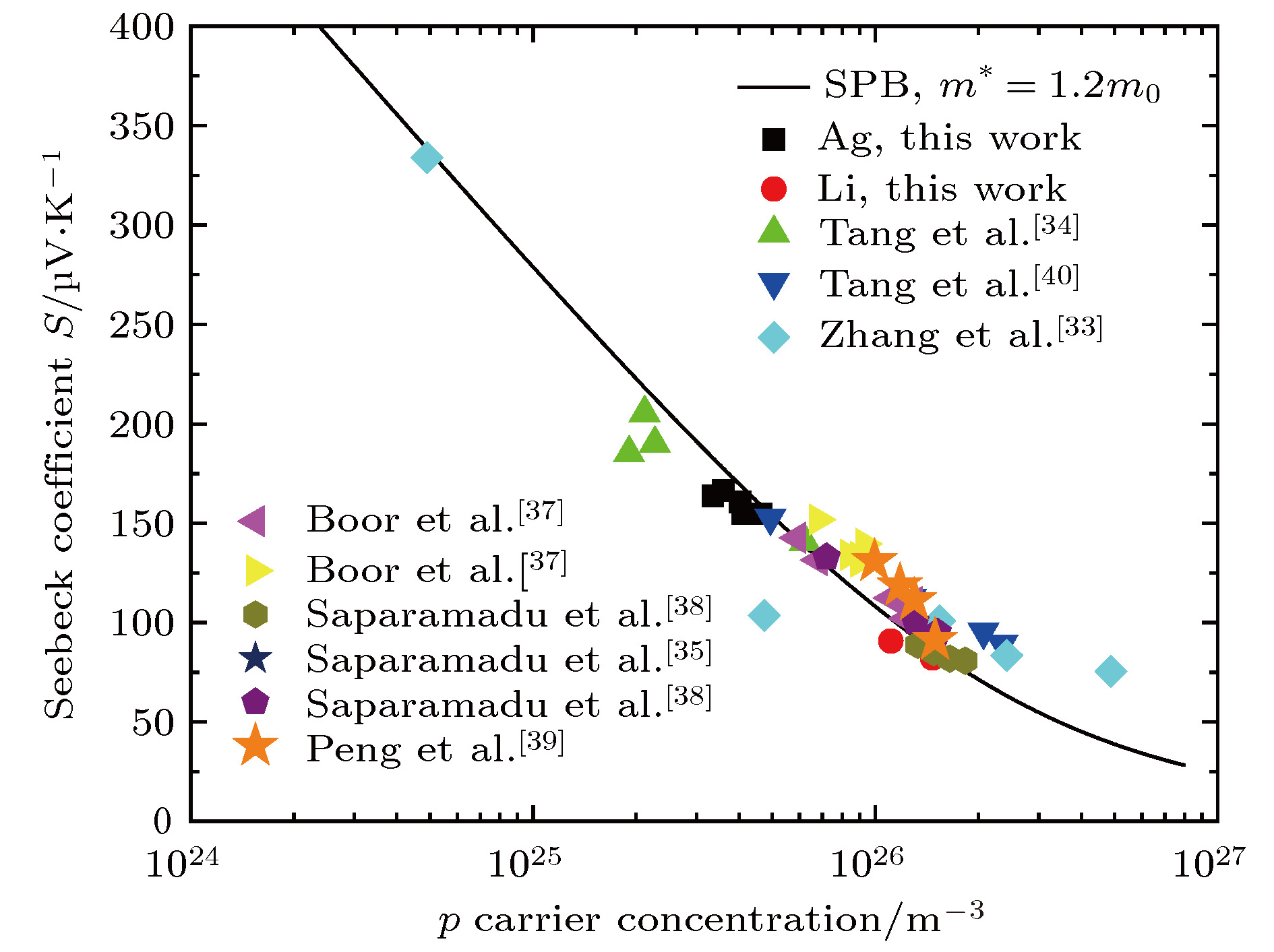
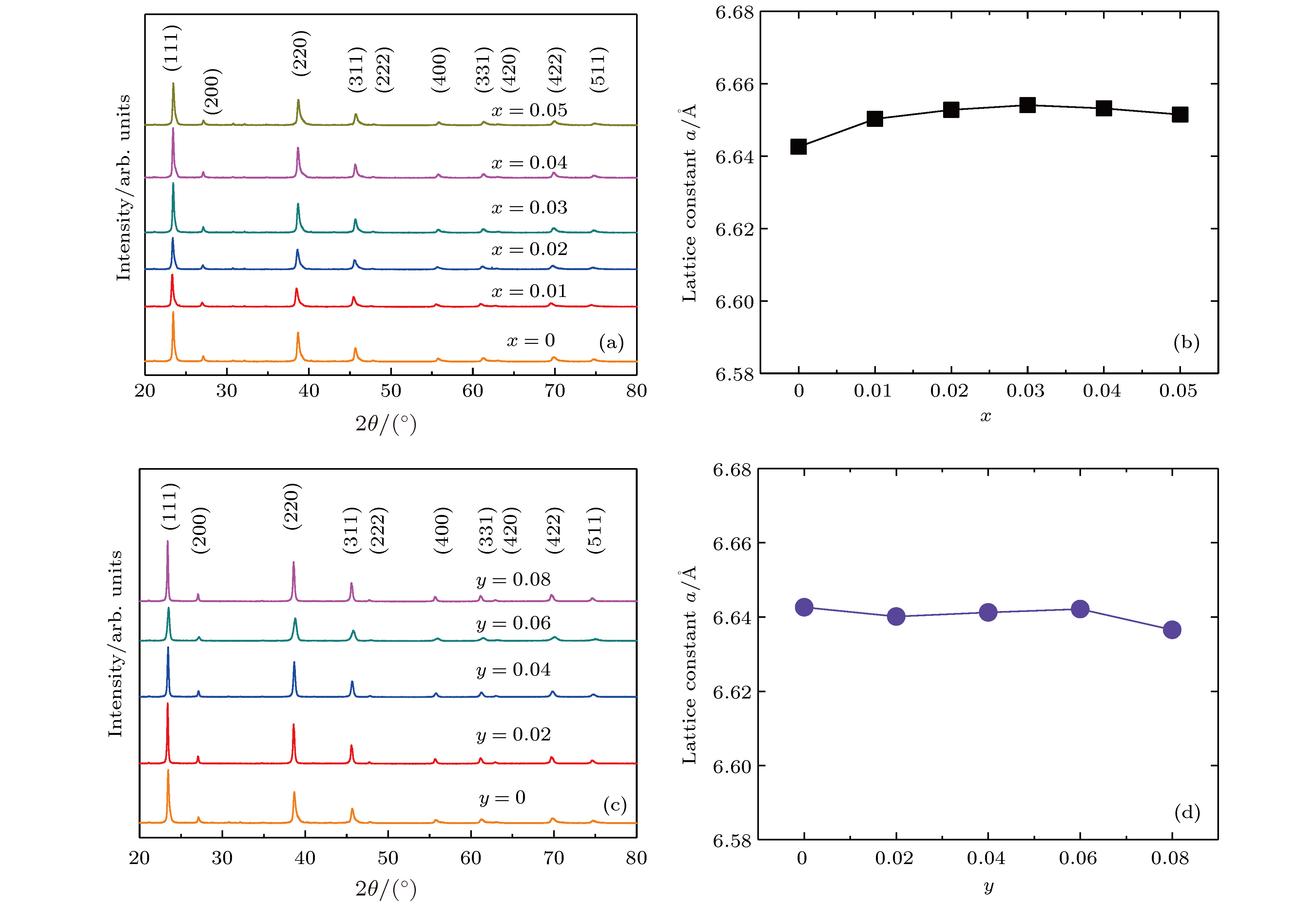
采用两步固相法合成了物相均匀的Mg2(1–x)Ag2xSi0.3Sn0.7 (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05)和Mg2(1–y)Li2ySi0.3Sn0.7 (y = 0, 0.02, 0.04, 0.06, 0.08)热电材料, 测试了室温物理性能和室温至773 K的热电性能, 研究了不同掺杂剂的固溶度、微观结构、载流子浓度、电性能和热输运. X射线衍射图谱和扫描电子显微镜图像显示掺杂Ag和Li的固溶度分别为x = 0.03和y = 0.06. 根据单抛物线模型, p型的Mg2(1–x)Ag2xSi0.3Sn0.7和Mg2(1–y)Li2ySi0.3Sn0.7的有效质量为1.2m0. 对比结果表明: 掺杂Ag或Li的最大载流子浓度分别达到4.64 × 1019 cm–3和15.1 × 1019 cm–3; 掺杂Li元素的样品有较高的固溶度、较高的载流子浓度和较高的功率因子PF约为1.62 × 10–3 W·m–1·K–2; 掺杂Li元素样品中较高的载流子浓度能够有效抑制双极效应, 显著降低双极热导率; Mg1.92Li0.08Si0.3Sn0.7的最大ZT值0.54, 比Mg1.9Ag0.1Si0.3Sn0.7的最大ZT值0.34提高了大约58%. 根据Callaway理论, 由于质量场波动和应变场波动增强声子散射, 掺杂Ag和Li元素样品的晶格热导率比未掺杂样品明显降低.
-
关键词:
- Mg2Si0.3Sn0.7 /
- 热电性能 /
- 掺杂 /
- 晶格热导率
In recent decades, Mg2(Si, Sn) solid solutions have long been considered as one of the most important classes of eco-friendly thermoelectric materials. The thermoelectric performance of Mg2(Si, Sn) solid solutions with outstanding characteristics of low-price, non-toxicity, earth-abundant and low-density has been widely studied. The n-type Mg2(Si, Sn) solid solutions have achieved the dimensionless thermoelectric figure of merit ZT ~1.4 through Bi/Sb doping and convergence of conduction bands. However, the thermoelectric performances for p-type Mg2(Si, Sn) solid solutions are mainly improved by optimizing the carrier concentration. In this work, the thermoelectric properties for p-type Mg2Si0.3Sn0.7 are investigated and compared with those for different p-type dopant Ag or Li. The homogeneous Mg2Si0.3Sn0.7 with Ag or Li doping is synthesized by two-step solid-state reaction method at temperatures of 873 K and 973 K for 24 h, respectively. The transport parameters and the thermoelectric properties are measured at temperatures ranging from room temperature to 773 K for Mg2(1–x)Ag2xSi0.3Sn0.7 (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05) and Mg2(1–y)Li2ySi0.3Sn0.7 (y = 0, 0.02, 0.04, 0.06, 0.08) samples. The influences of different dopants on solid solubility, microstructure, carrier concentration, electrical properties and thermal transport are also investigated. The X-ray diffraction (XRD) patterns and scanning electron microscopy (SEM) images show that the solid solubility for Ag and for Li are x = 0.03 and y = 0.06, respectively. Based on the assumption of single parabolic band model, the value of effective mass ~1.2m0 of p-type Mg2(1–x)Ag2xSi0.3Sn0.7 and Mg2(1–y)Li2ySi0.3Sn0.7 are similar to that reported in the literature. The comparative results demonstrate that the maximum carrier concentration for Ag doping and for Li doping are 4.64×1019 cm–3 for x = 0.01 and 15.1×1019 cm–3 for y = 0.08 at room temperature, respectively; the Li element has higher solid solubility in Mg2(Si, Sn), which leads to higher carrier concentration and power factor PF ~1.62×10–3${\rm W}\cdot{\rm m^{–1}}\cdot{\rm K^{–2}}$ in Li doped samples; the higher carrier concentration of Li doped samples effectively suppresses the bipolar effect; the maximum of ZT ~0.54 for Mg1.92Li0.08Si0.3Sn0.7 is 58% higher than that of Mg1.9Ag0.1Si0.3Sn0.7 samples. The lattice thermal conductivity of Li or Ag doped sample decreases obviously due to the stronger mass and strain field fluctuations in phonon transport.-
Keywords:
- Mg2Si0.3Sn0.7 /
- thermoelectric performance /
- doping /
- lattice thermal conductivity
[1] Dresselhaus M S, Chen G, Tang M Y, Yang R G, Lee H, Wang D Z, Ren Z F, Fleurial J P, Gogna P 2007 Adv. Mater. 19 1043
 Google Scholar
Google Scholar
[2] Snyder G J, Toberer E S 2008 Nat. Mater. 7 105
 Google Scholar
Google Scholar
[3] Bell L E 2008 Science 321 1457
 Google Scholar
Google Scholar
[4] Zhu T, Liu Y, Fu C, Heremans J P, Snyder G J, Zhao X 2017 Adv. Mater. 29 1605884
 Google Scholar
Google Scholar
[5] 朱航天, 任武洋, 张勤勇, 任志锋 2018 西华大学学报(自然科学版) 37 15
 Google Scholar
Google Scholar
Zhu H T, Ren W Y, Zhang Q Y, Ren Z F 2018 J. Xihua Univ. (Natural Science Edition)
37 15  Google Scholar
Google Scholar
[6] Pei Y, Shi X, Lalonde A, Wang H, Chen L, Snyder G J 2011 Nature 473 66
 Google Scholar
Google Scholar
[7] Mao J, Wang Y, Ge B, Jie Q, Liu Z, Saparamadu U, Liu W, Ren Z 2016 Phys. Chem. Chem. Phys. 18 20726
 Google Scholar
Google Scholar
[8] Lu Q, Wu M, Wu D, Chang C, Guo Y P, Zhou C S, Li W, Ma X M, Wang G, Zhao L D, Huang L, Liu C, He J 2017 Phys. Rev. Lett. 119 116401
 Google Scholar
Google Scholar
[9] Pei Y, Lalonde A D, Wang H, Snyder G J 2012 Energy Environ. Sci. 5 7963
 Google Scholar
Google Scholar
[10] 张勤勇, 袁国才, 王俊臣, 毛俊西, 雷晓波 2018 西华大学学报(自然科学版) 37 1
 Google Scholar
Google Scholar
Zhang Q Y, Yuan G C, Wang J C, Mao J X, Lei X B 2018 J. Xihua Univ. (Natural Science Edition)
37 1  Google Scholar
Google Scholar
[11] Paul B, Ajay Kumar V, Banerji P 2010 J. Appl. Phys. 108 064322
 Google Scholar
Google Scholar
[12] Xie W J, Yan Y G, Zhu S, Zhou M, Populoh S, Gałązka K, Poon S J, Weidenkaff A, He J, Tang X F, Tritt T M 2013 Acta Mater. 61 2087
 Google Scholar
Google Scholar
[13] Heremans J P, Wiendlocha B, Chamoire A M 2012 Energy Environ. Sci. 5 5510
 Google Scholar
Google Scholar
[14] Zhang Q, Wang H, Liu W, Wang H, Yu B, Zhang Q, Tian Z, Ni G, Lee S, Esfarjani K, Chen G, Ren Z 2012 Energy Environ. Sci. 5 5246
 Google Scholar
Google Scholar
[15] Zhou B Q, Li S, Li W, Li J, Zhang X Y, Lin S Q, Chen Z W, Pei Y Z 2017 ACS Appl. Mater. Interfaces 9 34033
 Google Scholar
Google Scholar
[16] Xiao Y, Wu H, Li W, Yin M, Pei Y, Zhang Y, Fu L, Chen Y, Pennycook S J, Huang L, He J, Zhao L D 2017 J. Am. Chem. Soc. 139 18732
 Google Scholar
Google Scholar
[17] 王浚臣, 袁国才, 禹劲秋, 莫小波, 金应荣, 黄丽宏 2018 西华大学学报(自然科学版) 37 68
 Google Scholar
Google Scholar
Wang J C, Yuan G C, Yu J Q, Mo X B, Jin Y R, Huang L H 2018 Journal of Xihua University (Natural Science Edition)
37 68  Google Scholar
Google Scholar
[18] de Boor J, Dasgupta T, Saparamadu U, Müller E, Ren Z F 2017 Mater. Today Energy 4 105
 Google Scholar
Google Scholar
[19] Bashir M B A, Mohd Said S, Sabri M F M, Shnawah D A, Elsheikh M H 2014 Renewable and Sustainable Energy Reviews 37 569
 Google Scholar
Google Scholar
[20] Santos R, Aminorroaya Yamini S, Dou S X 2018 J. Mater. Chem. A 6 3328
 Google Scholar
Google Scholar
[21] Liu W, Yin K, Zhang Q, Uher C, Tang X 2017 Nat. Sci. Rev. 4 611
 Google Scholar
Google Scholar
[22] Pulikkotil J J, Singh D J, Auluck S, Saravanan M, Misra D K, Dhar A, Budhani R C 2012 Phys. Rev. B 86 155204
 Google Scholar
Google Scholar
[23] Sun J, Singh D J 2016 Phys. Rev. Appl. 5 024006
 Google Scholar
Google Scholar
[24] Tani J I, Kido H 2008 Intermetallics 16 418
 Google Scholar
Google Scholar
[25] Tani J I, Kido H 2012 Physica B 407 3493
 Google Scholar
Google Scholar
[26] Imai Y, Mori Y, Nakamura S, Takarabe K I 2013 J. Alloys Compd. 549 175
 Google Scholar
Google Scholar
[27] Tani J I, Kido H 2008 J. Alloys Compd. 466 335
 Google Scholar
Google Scholar
[28] Zhang Q, He J, Zhao X B, Zhang S N, Zhu T J, Yin H, Tritt T M 2008 J. Phys. D: Appl. Phys. 41 185103
 Google Scholar
Google Scholar
[29] Luo W J, Yang M J, Fei C, Shen Q, Jiang H G, Zhang L M 2010 Mater. Trans. 51 288
 Google Scholar
Google Scholar
[30] Liu W, Tang X, Li H, Yin K, Sharp J, Zhou X, Uher C 2012 J. Mater. Chem. 22 13653
 Google Scholar
Google Scholar
[31] Ihou-Mouko H, Mercier C, Tobola J, Pont G, Scherrer H 2011 J. Alloys Compd. 509 6503
 Google Scholar
Google Scholar
[32] Tada S, Isoda Y, Udono H, Fujiu H, Kumagai S, Shinohara Y 2014 J. Electron. Mater. 43 1580
[33] Zhang Q, Cheng L, Liu W, Zheng Y, Su X, Chi H, Liu H, Yan Y, Tang X, Uher C 2014 Phys. Chem. Chem. Phys. 16 23576
 Google Scholar
Google Scholar
[34] Tang X, Zhang Y, Zheng Y, Peng K, Huang T, Lu X, Wang G, Wang S, Zhou X 2017 Appl. Therm. Eng. 111 1396
 Google Scholar
Google Scholar
[35] Yin K, Zhang Q, Zheng Y, Su X, Tang X, Uher C 2015 J. Mater. Chem. C 3 10381
 Google Scholar
Google Scholar
[36] Liu W, Chi H, Sun H, Zhang Q, Yin K, Tang X, Zhang Q, Uher C 2014 Phys. Chem. Chem. Phys. 16 6893
 Google Scholar
Google Scholar
[37] de Boor J, Saparamadu U, Mao J, Dahal K, Müller E, Ren Z 2016 Acta Mater. 120 273
 Google Scholar
Google Scholar
[38] Saparamadu U, de Boor J, Mao J, Song S, Tian F, Liu W, Zhang Q, Ren Z 2017 Acta Mater. 141 154
 Google Scholar
Google Scholar
[39] Gao P, Davis J D, Poltavets V V, Hogan T P 2016 J. Mater. Chem. C 4 929
 Google Scholar
Google Scholar
[40] Tang X, Wang G, Zheng Y, Zhang Y, Peng K, Guo L, Wang S, Zeng M, Dai J, Wang G, Zhou X 2016 Scripta Mater. 115 52
 Google Scholar
Google Scholar
[41] Kim H S, Gibbs Z M, Tang Y, Wang H, Snyder G J 2015 APL Mater. 3 041506
 Google Scholar
Google Scholar
[42] Yang J, Meisner G P, Chen L 2004 Appl. Phys. Lett. 85 1140
 Google Scholar
Google Scholar
[43] 覃玉婷, 仇鹏飞, 史迅, 陈立东 2017 无机材料学报 32 1171
Qin Y T, Qiu P F, Shi X, Chen L D 2017 J. Inorg. Mater. 32 1171
[44] Slack G A 1957 Phys. Rev. 105 832
 Google Scholar
Google Scholar
[45] Abeles B 1963 Phys. Rev. 131 1906
 Google Scholar
Google Scholar
-
图 4 (a)—(f) Mg2(1-x)Ag2xSi0.3Sn0.7 (0 ≤ x ≤ 0.05)和Mg2(1-y)Li2ySi0.3Sn0.7 (0 ≤ y ≤ 0.08)的电导率、Seebeck系数和功率因子与温度的关系
Fig. 4. The temperature dependence of (a), (d) electrical conductivity, (b), (e) Seebeck coefficient and (c), (f) power factor for Mg2(1-x)Ag2xSi0.3Sn0.7 (0 ≤ x ≤ 0.05) and Mg2(1-y)Li2ySi0.3Sn0.7 (0 ≤ y ≤ 0.08)
图 5 (a)—(f) Mg2(1-x)Ag2xSi0.3Sn0.7 (0 ≤ x ≤ 0.05)和Mg2(1-y)Li2ySi0.3Sn0.7 (0 ≤ y ≤ 0.08)的热导率, 晶格热导率和ZT值与温度的关系图
Fig. 5. The temperature dependence of (a), (d) thermal conductivity, (b), (e) lattice thermal conductivity and (c), (f) ZT for Mg2(1-x)Ag2xSi0.3Sn0.7 (0 ≤ x ≤ 0.05) and Mg2(1-y)Li2ySi0.3Sn0.7 (0 ≤ y ≤ 0.08)
表 1 Mg2(1-x)Ag2xSi0.3Sn0.7 (0 ≤ x ≤ 0.05)和Mg2(1-y)Li2ySi0.3Sn0.7 (0 ≤ y ≤ 0.08)在300 K的物理参数
Table 1. Physical parameters of Mg2(1-x)Ag2xSi0.3Sn0.7 (0 ≤ x ≤ 0.05) and Mg2(1-y)Li2ySi0.3Sn0.7 (0 ≤ y ≤ 0.08) at 300 K
Composition σ/104 S·m–1 RH/cm3·C–1 p/1019 cm–3 μ/cm2·V–1·s–1 S/μV·K–1 m*(m0) x = 0 0.15 –3.22 –0.19 48.3 –458.0 1.6 x = 0.01 3.48 0.135 4.64 46.8 154.7 1.2 x = 0.02 3.33 0.173 3.60 57.7 166.4 1.1 x = 0.03 3.73 0.186 3.35 69.5 163.8 1.0 x = 0.04 3.11 0.155 4.03 48.2 160.6 1.1 x = 0.05 3.62 0.153 4.09 53.7 154.7 1.1 y = 0.02 11.20 0.056 11.10 63.1 90.7 1.0 y = 0.04 9.65 0.044 14.00 42.8 91.3 1.2 y = 0.06 13.14 0.042 14.70 55.8 82.2 1.1 y = 0.08 9.74 0.041 15.10 40.2 83.9 1.2 -
[1] Dresselhaus M S, Chen G, Tang M Y, Yang R G, Lee H, Wang D Z, Ren Z F, Fleurial J P, Gogna P 2007 Adv. Mater. 19 1043
 Google Scholar
Google Scholar
[2] Snyder G J, Toberer E S 2008 Nat. Mater. 7 105
 Google Scholar
Google Scholar
[3] Bell L E 2008 Science 321 1457
 Google Scholar
Google Scholar
[4] Zhu T, Liu Y, Fu C, Heremans J P, Snyder G J, Zhao X 2017 Adv. Mater. 29 1605884
 Google Scholar
Google Scholar
[5] 朱航天, 任武洋, 张勤勇, 任志锋 2018 西华大学学报(自然科学版) 37 15
 Google Scholar
Google Scholar
Zhu H T, Ren W Y, Zhang Q Y, Ren Z F 2018 J. Xihua Univ. (Natural Science Edition)
37 15  Google Scholar
Google Scholar
[6] Pei Y, Shi X, Lalonde A, Wang H, Chen L, Snyder G J 2011 Nature 473 66
 Google Scholar
Google Scholar
[7] Mao J, Wang Y, Ge B, Jie Q, Liu Z, Saparamadu U, Liu W, Ren Z 2016 Phys. Chem. Chem. Phys. 18 20726
 Google Scholar
Google Scholar
[8] Lu Q, Wu M, Wu D, Chang C, Guo Y P, Zhou C S, Li W, Ma X M, Wang G, Zhao L D, Huang L, Liu C, He J 2017 Phys. Rev. Lett. 119 116401
 Google Scholar
Google Scholar
[9] Pei Y, Lalonde A D, Wang H, Snyder G J 2012 Energy Environ. Sci. 5 7963
 Google Scholar
Google Scholar
[10] 张勤勇, 袁国才, 王俊臣, 毛俊西, 雷晓波 2018 西华大学学报(自然科学版) 37 1
 Google Scholar
Google Scholar
Zhang Q Y, Yuan G C, Wang J C, Mao J X, Lei X B 2018 J. Xihua Univ. (Natural Science Edition)
37 1  Google Scholar
Google Scholar
[11] Paul B, Ajay Kumar V, Banerji P 2010 J. Appl. Phys. 108 064322
 Google Scholar
Google Scholar
[12] Xie W J, Yan Y G, Zhu S, Zhou M, Populoh S, Gałązka K, Poon S J, Weidenkaff A, He J, Tang X F, Tritt T M 2013 Acta Mater. 61 2087
 Google Scholar
Google Scholar
[13] Heremans J P, Wiendlocha B, Chamoire A M 2012 Energy Environ. Sci. 5 5510
 Google Scholar
Google Scholar
[14] Zhang Q, Wang H, Liu W, Wang H, Yu B, Zhang Q, Tian Z, Ni G, Lee S, Esfarjani K, Chen G, Ren Z 2012 Energy Environ. Sci. 5 5246
 Google Scholar
Google Scholar
[15] Zhou B Q, Li S, Li W, Li J, Zhang X Y, Lin S Q, Chen Z W, Pei Y Z 2017 ACS Appl. Mater. Interfaces 9 34033
 Google Scholar
Google Scholar
[16] Xiao Y, Wu H, Li W, Yin M, Pei Y, Zhang Y, Fu L, Chen Y, Pennycook S J, Huang L, He J, Zhao L D 2017 J. Am. Chem. Soc. 139 18732
 Google Scholar
Google Scholar
[17] 王浚臣, 袁国才, 禹劲秋, 莫小波, 金应荣, 黄丽宏 2018 西华大学学报(自然科学版) 37 68
 Google Scholar
Google Scholar
Wang J C, Yuan G C, Yu J Q, Mo X B, Jin Y R, Huang L H 2018 Journal of Xihua University (Natural Science Edition)
37 68  Google Scholar
Google Scholar
[18] de Boor J, Dasgupta T, Saparamadu U, Müller E, Ren Z F 2017 Mater. Today Energy 4 105
 Google Scholar
Google Scholar
[19] Bashir M B A, Mohd Said S, Sabri M F M, Shnawah D A, Elsheikh M H 2014 Renewable and Sustainable Energy Reviews 37 569
 Google Scholar
Google Scholar
[20] Santos R, Aminorroaya Yamini S, Dou S X 2018 J. Mater. Chem. A 6 3328
 Google Scholar
Google Scholar
[21] Liu W, Yin K, Zhang Q, Uher C, Tang X 2017 Nat. Sci. Rev. 4 611
 Google Scholar
Google Scholar
[22] Pulikkotil J J, Singh D J, Auluck S, Saravanan M, Misra D K, Dhar A, Budhani R C 2012 Phys. Rev. B 86 155204
 Google Scholar
Google Scholar
[23] Sun J, Singh D J 2016 Phys. Rev. Appl. 5 024006
 Google Scholar
Google Scholar
[24] Tani J I, Kido H 2008 Intermetallics 16 418
 Google Scholar
Google Scholar
[25] Tani J I, Kido H 2012 Physica B 407 3493
 Google Scholar
Google Scholar
[26] Imai Y, Mori Y, Nakamura S, Takarabe K I 2013 J. Alloys Compd. 549 175
 Google Scholar
Google Scholar
[27] Tani J I, Kido H 2008 J. Alloys Compd. 466 335
 Google Scholar
Google Scholar
[28] Zhang Q, He J, Zhao X B, Zhang S N, Zhu T J, Yin H, Tritt T M 2008 J. Phys. D: Appl. Phys. 41 185103
 Google Scholar
Google Scholar
[29] Luo W J, Yang M J, Fei C, Shen Q, Jiang H G, Zhang L M 2010 Mater. Trans. 51 288
 Google Scholar
Google Scholar
[30] Liu W, Tang X, Li H, Yin K, Sharp J, Zhou X, Uher C 2012 J. Mater. Chem. 22 13653
 Google Scholar
Google Scholar
[31] Ihou-Mouko H, Mercier C, Tobola J, Pont G, Scherrer H 2011 J. Alloys Compd. 509 6503
 Google Scholar
Google Scholar
[32] Tada S, Isoda Y, Udono H, Fujiu H, Kumagai S, Shinohara Y 2014 J. Electron. Mater. 43 1580
[33] Zhang Q, Cheng L, Liu W, Zheng Y, Su X, Chi H, Liu H, Yan Y, Tang X, Uher C 2014 Phys. Chem. Chem. Phys. 16 23576
 Google Scholar
Google Scholar
[34] Tang X, Zhang Y, Zheng Y, Peng K, Huang T, Lu X, Wang G, Wang S, Zhou X 2017 Appl. Therm. Eng. 111 1396
 Google Scholar
Google Scholar
[35] Yin K, Zhang Q, Zheng Y, Su X, Tang X, Uher C 2015 J. Mater. Chem. C 3 10381
 Google Scholar
Google Scholar
[36] Liu W, Chi H, Sun H, Zhang Q, Yin K, Tang X, Zhang Q, Uher C 2014 Phys. Chem. Chem. Phys. 16 6893
 Google Scholar
Google Scholar
[37] de Boor J, Saparamadu U, Mao J, Dahal K, Müller E, Ren Z 2016 Acta Mater. 120 273
 Google Scholar
Google Scholar
[38] Saparamadu U, de Boor J, Mao J, Song S, Tian F, Liu W, Zhang Q, Ren Z 2017 Acta Mater. 141 154
 Google Scholar
Google Scholar
[39] Gao P, Davis J D, Poltavets V V, Hogan T P 2016 J. Mater. Chem. C 4 929
 Google Scholar
Google Scholar
[40] Tang X, Wang G, Zheng Y, Zhang Y, Peng K, Guo L, Wang S, Zeng M, Dai J, Wang G, Zhou X 2016 Scripta Mater. 115 52
 Google Scholar
Google Scholar
[41] Kim H S, Gibbs Z M, Tang Y, Wang H, Snyder G J 2015 APL Mater. 3 041506
 Google Scholar
Google Scholar
[42] Yang J, Meisner G P, Chen L 2004 Appl. Phys. Lett. 85 1140
 Google Scholar
Google Scholar
[43] 覃玉婷, 仇鹏飞, 史迅, 陈立东 2017 无机材料学报 32 1171
Qin Y T, Qiu P F, Shi X, Chen L D 2017 J. Inorg. Mater. 32 1171
[44] Slack G A 1957 Phys. Rev. 105 832
 Google Scholar
Google Scholar
[45] Abeles B 1963 Phys. Rev. 131 1906
 Google Scholar
Google Scholar
计量
- 文章访问数: 10298
- PDF下载量: 90
- 被引次数: 0














 下载:
下载: