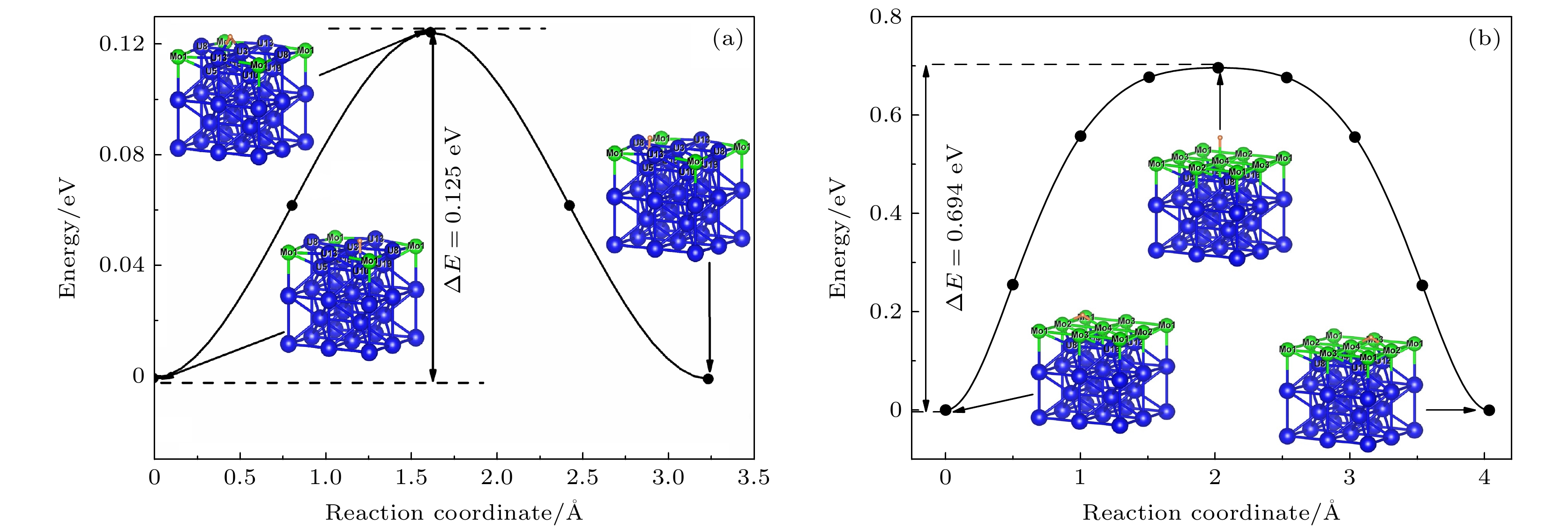
-
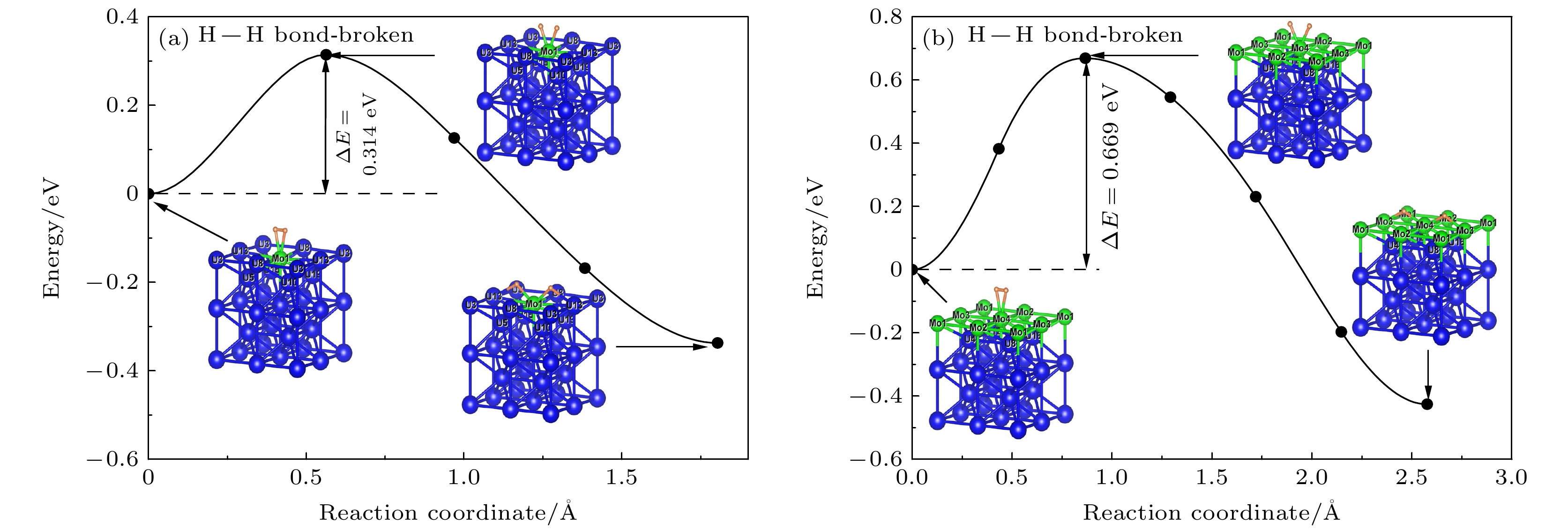
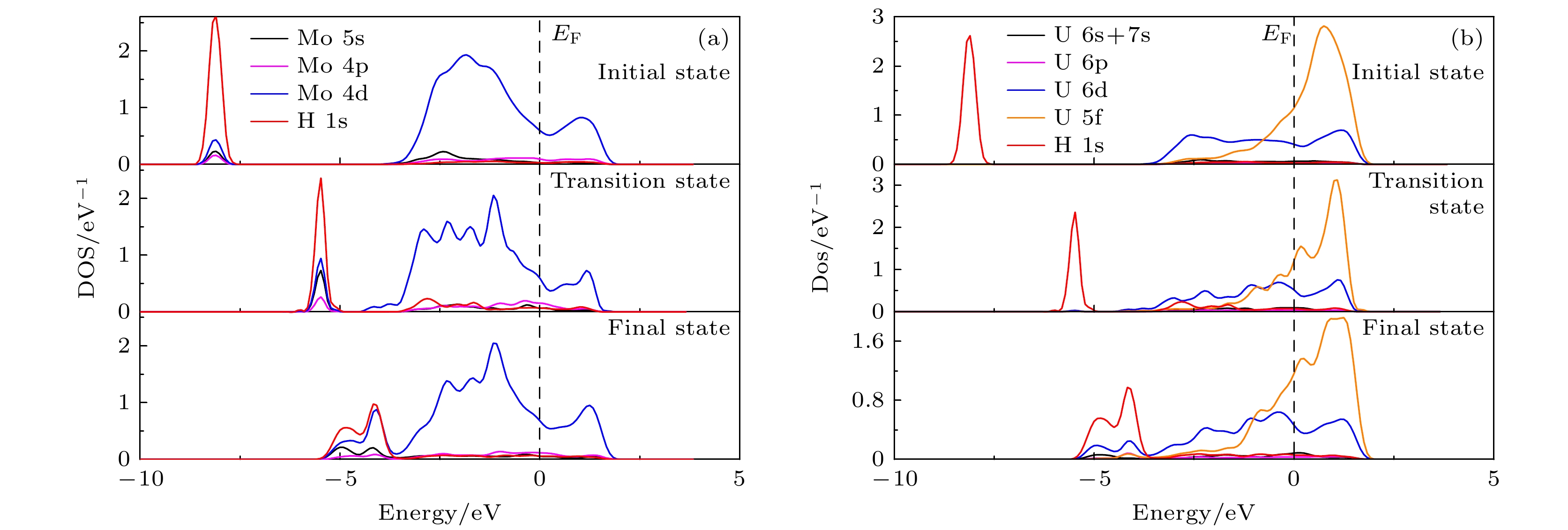
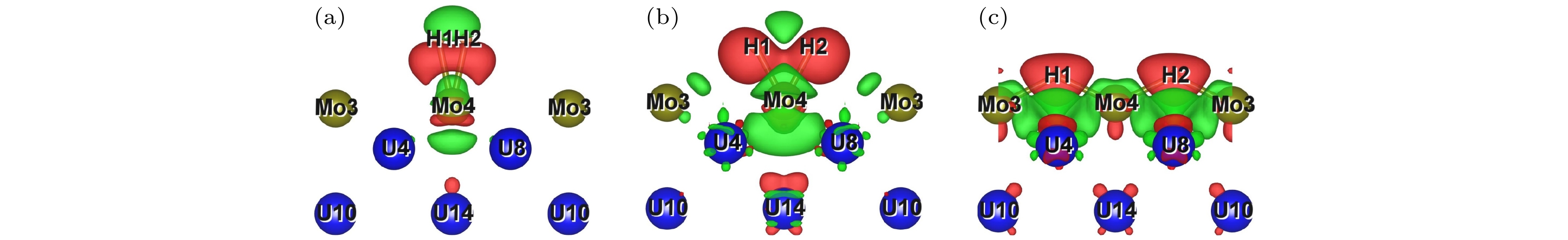
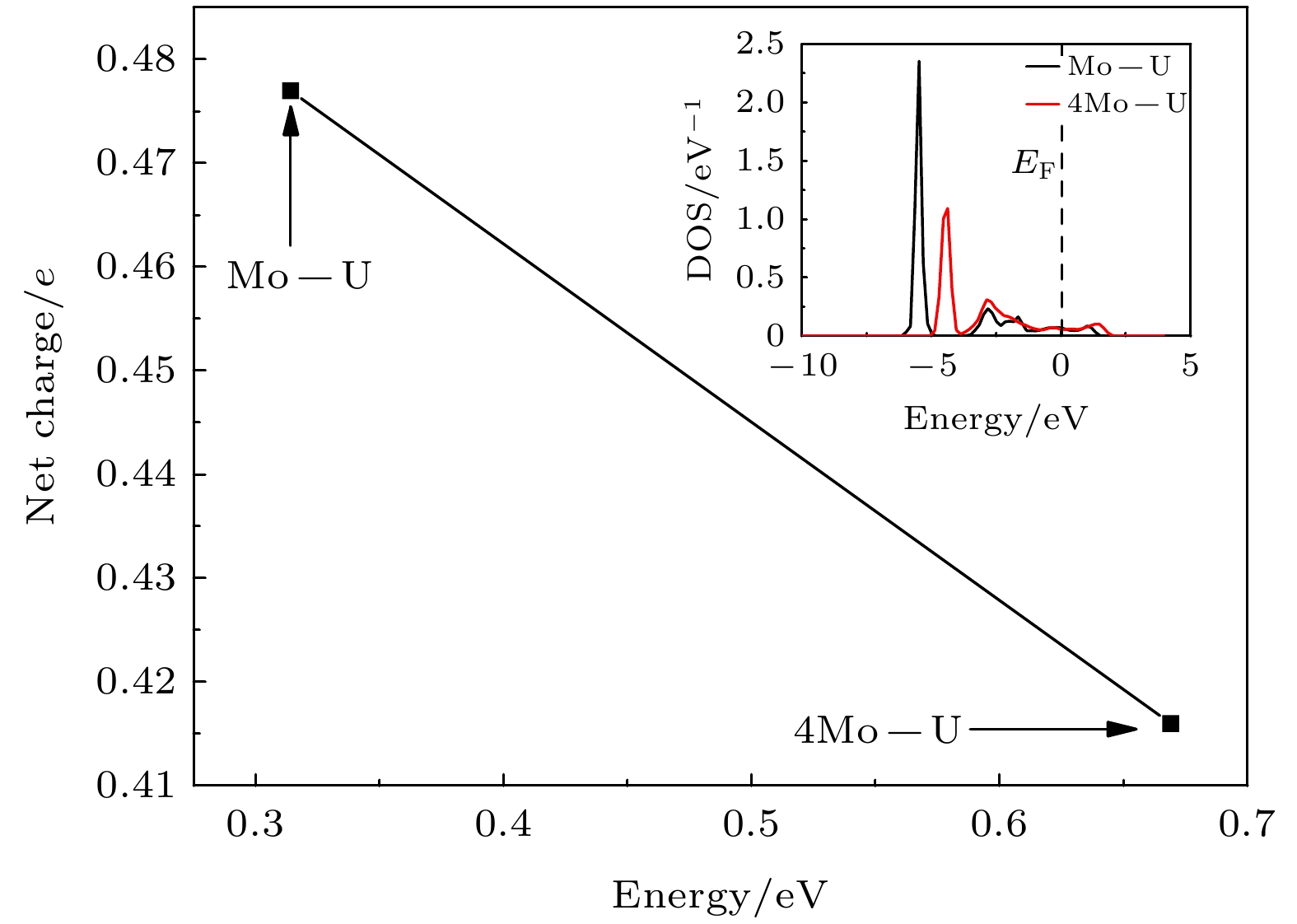
As an important uranium alloy, U-Mo alloy has excellent mechanical properties, structural stability and thermal conductivity, which is an important nuclear reactor fuel and tank armor. However, there exists a serious of fundamental problems of U-Mo alloy which need solving for practical applications. U-Mo alloy is easily subjected to surface corrosion of small molecules including the H2, O2, H2O, and CO2. The hydrogen corrosion and oxidation will have significant influence on it. In order to further investigate the reaction mechanism, based on the density functional theory and the transition state algorithm, the first principles calculation of γ-U (100) with Mo atom doping and Mo coating is carried out. Firstly, the minimum energy path of H2 molecule is calculated for the dissociation adsorption on Mo-U and 4Mo-U surface. Secondly, the transition states of H and O atoms are studied during surface diffusing between adjacent most stable adsorption sites. Thirdly, the bulk phase diffusion of H and O atoms are investigated and the relationship is analyzed between adsorption energy and adsorption height in the bulk phase diffusion. The results show that when H2 molecule is adsorbed at the configuration of top horizontal position, the H atom needs to overcome a barrier to triggering off the H—H bond-broken and then is adsorbed on surface bridge site by the neighboring atoms. The energy barrier for H2 dissociation on 4Mo-U is higher than that of Mo-U. Meanwhile, the lower energy barrier is required for O atom to diffuse in Mo-U, so that it can be adsorbed, dissociated and diffused quickly, and then forming an oxidation film on the surface. Furthermore, both H and O atoms need to cross the energy barrier to diffuse into the body phase, forming chemical bonds with the atoms and staying in the body phase stably finally. In this paper, we comprehensively analyze the dissociation and diffusion of the initial stage for hydrogen corrosion and oxidation on uranium-molybdenum alloy by theoretical studies. The results lay a foundation for theoretically exploring the surface corrosion mechanism of U-Mo alloy. Meanwhile, They provide theoretical support for investigating burn-in and corrosion of uranium-molybdenum alloy, predicting material properties under extreme and special environment, and providing a reference for further research on corrosion resistance of uranium-molybdenum alloy. -
Keywords:
- uranium-molybdenum alloy /
- surface corrosion /
- transient state /
- dissociation and adsorption
[1] 伯格J. J. 著 (石琪 译) 1983 铀合金物理冶金 (北京: 原子能出版社) 第76—79页
Burke J J (translated by Shi Q)1983 Physical Metallurgy of Uranium Alloys (Beijing: Atomic Energy Press) pp76–79 (in Chinese)
[2] Yang X Y, Yang Y, Liu Y, Wang Z W, Wärnå J, Xu Z T, Zhang P 2020 Prog. Nucl. Energy 122 103268
 Google Scholar
Google Scholar
[3] Koelling D D, Freeman A J 1973 Phys. Rev. B 7 4454
 Google Scholar
Google Scholar
[4] Shen Z Y, Kong Y, Du Y, Zhang S Y 2021 Calphad 72 102241
 Google Scholar
Google Scholar
[5] SU Q L, DENG H Q, AO B Y, Xiao S F, Chen P H, Hu W Y 2014 Rsc Advances 4 57308
 Google Scholar
Google Scholar
[6] Bajaj S, Landa A, Söderlind P, Turchi P E A, Arróyave R 2011 J. Nucl. Mater. 419 177
 Google Scholar
Google Scholar
[7] Castellano A, Bottin F, Dorado B, Bouchet J 2020 Phys. Rev. B 101 184111
 Google Scholar
Google Scholar
[8] Losada E L, Garces J E 2019 J. Nucl. Mater. 518 380
 Google Scholar
Google Scholar
[9] Alonso P R, Rubiolo G H 2007 Modell. Simul. Mater. Sci. Eng. 15 263
 Google Scholar
Google Scholar
[10] Powell G L, Kirkpatrick J R 1997 J. Alloys Compd. 253 167
[11] Kautz E J, Lambeets S V, Royer J, Perea D E, Harilal S S 2022 Scr. Mater. 212 114528
 Google Scholar
Google Scholar
[12] Ilton E S, Bagus P S 2011 Surf. Interface Anal. 43 1549
 Google Scholar
Google Scholar
[13] Mclean W, Colmenares C A, Smith R L, Somorjai G A 1982 Phys. Rev. B 25 8
 Google Scholar
Google Scholar
[14] Tian X F, Wang Yu, Li L S, Wu M D, Yu Y 2020 Comput. Mater. Sci. 179 109633
 Google Scholar
Google Scholar
[15] Liu G D, Liu Z X, Ao B Y, Hu W Y, Deng H Q 2018 Comput. Mater. Sci. 144 85
 Google Scholar
Google Scholar
[16] 李俊炜, 贾维敏, 吕沙沙, 魏雅璇, 李正操, 王金涛 2022 71 226601
 Google Scholar
Google Scholar
Li J W, Jia W M, Lü S S, Wei Y X, Li Z C, Wang J T 2022 Acta Phys. Sin. 71 226601
 Google Scholar
Google Scholar
[17] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[18] Kresse G, Hafner J 1993 Phys. Rev. B: Condens. Matter. 48 13115
 Google Scholar
Google Scholar
[19] Perdew J P, Chevary J A, Vosko S H, Jackson K A, Pederson M R, Singh D J, Fiolhais C 1993 Phys. Rev. B: Condens. Matter. 46 6671
[20] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[21] Payne M C, Teter M P, Allan D C, Arias T A, Joannopoulos J D 1992 Rev. Mod. Phys. 64 1045
 Google Scholar
Google Scholar
[22] Henkelman G, Uberuaga B P, Jónsson H 2000 J. Chem. Phys. 113 9901
 Google Scholar
Google Scholar
[23] Pack James D, Monkhorst H J 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[24] Chiotti P, Klepfer H H, White R W 1959 Trans. Am. Soc. Metals 51 772
[25] Henkelman G, Arnaldsson A, Jónsson H 2006 Comput. Mater. Sci. 36 354
 Google Scholar
Google Scholar
[26] Electronegativity of Chemical Elements, Material-properties https://material-properties.org/electronegativity-of-chemical-elements/ [2023-1-1]
[27] Deringer V L, Tchougréeff A L, Dronskowski R 2011 J. Phys. Chem. A 115 5461
 Google Scholar
Google Scholar
[28] Nelson R, ErturalC, George J, Deringer V, Dronskowski R 2020 J. Comput. Chem. 41 1931
 Google Scholar
Google Scholar
-
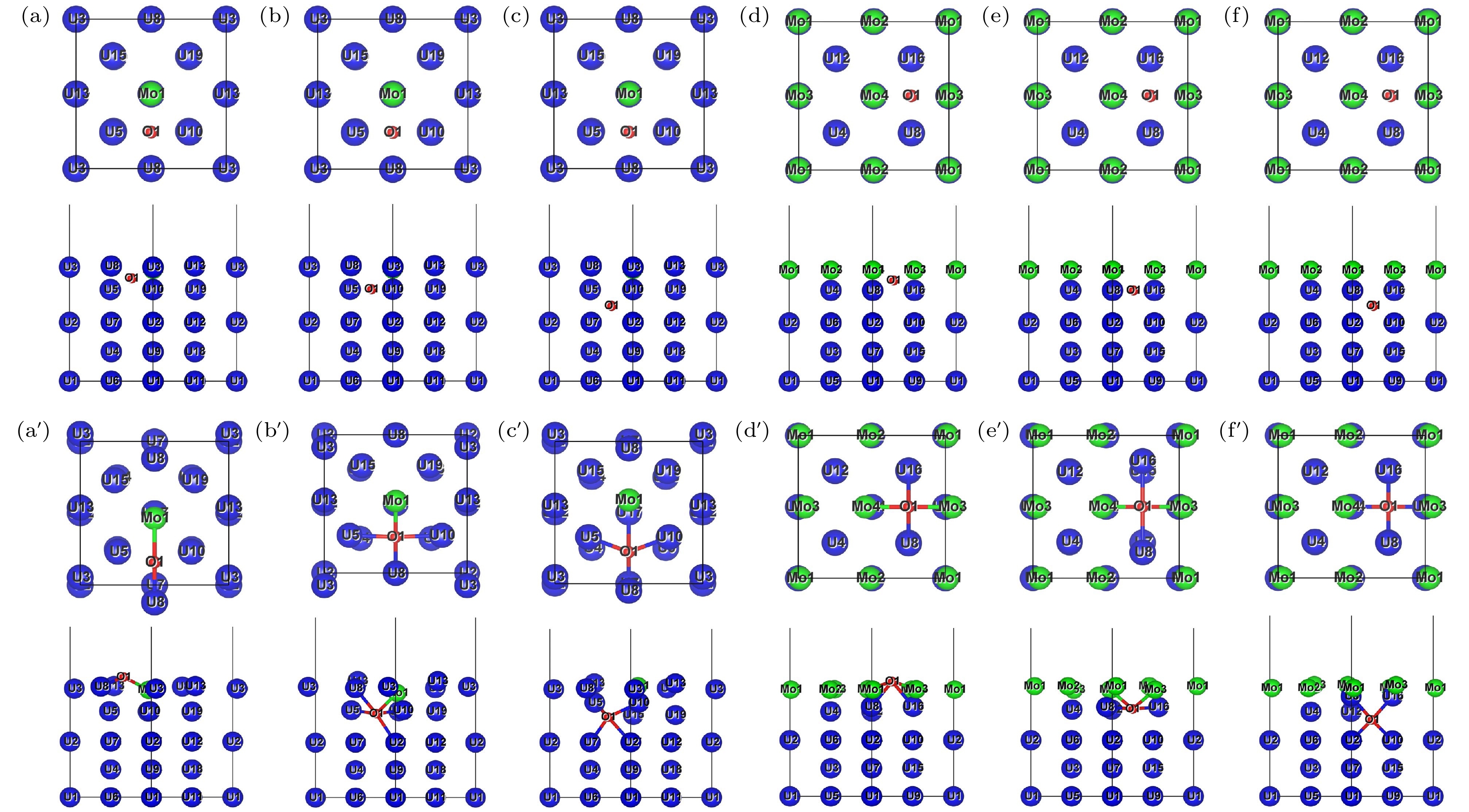
图 10 H原子在Mo-U和4Mo-U体内扩散优化前后的俯视图和侧视图 (a) 优化前H-Mo-U_First; (b)优化前H-Mo-U_Second; (c)优化前H-Mo-U_Third; (d)优化前H-4Mo-U_First; (e) 优化前H-4Mo-U_Second; (f) 优化前H-4Mo-U_Third. 下方对应图像为其优化后结果, 如(a')优化后H-Mo-U_First
Figure 10. Top and side views of H atom bulk diffusion on Mo-U surface before and after structure optimization: (a) H-Mo-U_First before optimization; (b) H-Mo-U_Second before optimization; (c) H-Mo-U_Third before optimization; (d) H-4Mo-U_First before optimization; (e) H-4Mo-U_Second before optimization; (f) H-4Mo-U_Third before optimization. The corresponding image below is optimization result, such as (a') H-Mo-U_First after optimization.
图 11 O原子在Mo-U和4Mo-U体内扩散优化前后的俯视图和侧视图 (a) 优化前O-Mo-U_First; (b)优化前O-Mo-U_Second; (c)优化前O-Mo-U_Third; (d)优化前O-4Mo-U_First; (e) 优化前O-4Mo-U_Second; (f) 优化前O-4Mo-U_Third. 下方对应图像为其优化后结果, 如(a')优化后O-Mo-U_First
Figure 11. Top and side views of O atom bulk diffusion on Mo-U surface before and after structure optimization: (a) O-Mo-U_First before optimization; (b) O-Mo-U_Second before optimization; (c) O-Mo-U_Third before optimization; (d) O-4Mo-U_First before optimization; (e) O-4Mo-U_Second before optimization; (f) O-4Mo-U_Third before optimization. The corresponding image below is optimization result, such as (a') O-Mo-U_First after optimization.
图 12 不同初始吸附构型下, 原子体相扩散稳定吸附高度及吸附能量变化 (a) H原子在Mo-U体相扩散; (b) H原子在4Mo-U体相扩散; (c) O原子在Mo-U体相扩散; (d) O原子在4Mo-U体相扩散
Figure 12. The adsorption height and energy for atom diffusion in bulk phase under different initial adsorption configurations: (a) H atom diffusion in Mo-U bulk phase; (b) H atom diffusion in 4Mo-U bulk phase; (c) O atom diffusion in Mo-U bulk phase; (d) O atom diffusion in 4Mo-U bulk phase.
表 1 H2分子在Mo-U表面不同解离阶段下的净电荷数
Table 1. The net charge number of H2 molecules at different dissociation stages on Mo-U surface.
Configuration H1/e H2/e Mo1/e U3/e U8/e U13/e Initial 0.1277 0.1032 0.2662 –0.1315 –0.3385 –0.2727 Transition 0.2218 0.2552 0.1256 –0.0307 –0.2919 –0.3678 02 0.3442 0.3442 0.0495 –0.0407 –0.3044 –0.4325 03 0.4179 0.4177 0.0034 –0.0411 –0.2918 –0.4775 Final 0.4482 0.4482 0.0130 –0.0436 –0.2637 –0.5015 表 2 H2分子在4Mo-U表面不同解离阶段下的净电荷数
Table 2. The net charge number of H2 molecules at different dissociation stages on 4Mo-U surface.
Configuration H1/e H2/e Mo1/e Mo2/e Mo3/e Mo4/e Initial 0.0267 0.0319 0.2742 0.2484 0.2456 0.1868 01 0.1000 0.1000 0.2685 0.2337 0.2087 0.1183 Transition 0.2080 0.2080 0.2528 0.2073 0.1327 0.0377 03 0.2992 0.2992 0.2527 0.2030 0.0856 –0.0345 04 0.3529 0.3529 0.2518 0.2158 0.0399 –0.0536 05 0.3777 0.3777 0.2408 0.2453 0.0095 –0.0225 Final 0.3926 0.3926 0.2480 0.2632 0.0077 0.0043 表 A1 O原子在体相扩散后与Mo-U结构中部分原子的ICOHP
Table A1. ICOHP between O atom and partial atoms in Mo-U after bulk phase diffusion.
Mo-U_ Second ICOHP/eV Mo-U_ Third ICOHP/eV Mo1-O1 1.6431 U5-O1 4.8108 U5-O1 4.4165 U7-O1 5.0568 U8-O1 2.6392 U10-O1 4.8167 U10-O1 4.4180 U17-O1 2.2676 U17-O1 2.5125 表 A2 O原子在体相扩散后与4Mo-U结构中部分原子的ICOHP
Table A2. ICOHP between O atom and partial atoms in 4Mo-U after bulk phase diffusion.
4Mo-U_Second ICOHP/eV 4Mo-U_Third ICOHP/eV Mo3-O1 2.4263 U8-O1 3.9520 Mo4-O1 1.5266 U10-O1 6.0116 U8-O1 5.0490 U14-O1 5.9456 U16-O1 5.0493 U16-O1 3.9521 -
[1] 伯格J. J. 著 (石琪 译) 1983 铀合金物理冶金 (北京: 原子能出版社) 第76—79页
Burke J J (translated by Shi Q)1983 Physical Metallurgy of Uranium Alloys (Beijing: Atomic Energy Press) pp76–79 (in Chinese)
[2] Yang X Y, Yang Y, Liu Y, Wang Z W, Wärnå J, Xu Z T, Zhang P 2020 Prog. Nucl. Energy 122 103268
 Google Scholar
Google Scholar
[3] Koelling D D, Freeman A J 1973 Phys. Rev. B 7 4454
 Google Scholar
Google Scholar
[4] Shen Z Y, Kong Y, Du Y, Zhang S Y 2021 Calphad 72 102241
 Google Scholar
Google Scholar
[5] SU Q L, DENG H Q, AO B Y, Xiao S F, Chen P H, Hu W Y 2014 Rsc Advances 4 57308
 Google Scholar
Google Scholar
[6] Bajaj S, Landa A, Söderlind P, Turchi P E A, Arróyave R 2011 J. Nucl. Mater. 419 177
 Google Scholar
Google Scholar
[7] Castellano A, Bottin F, Dorado B, Bouchet J 2020 Phys. Rev. B 101 184111
 Google Scholar
Google Scholar
[8] Losada E L, Garces J E 2019 J. Nucl. Mater. 518 380
 Google Scholar
Google Scholar
[9] Alonso P R, Rubiolo G H 2007 Modell. Simul. Mater. Sci. Eng. 15 263
 Google Scholar
Google Scholar
[10] Powell G L, Kirkpatrick J R 1997 J. Alloys Compd. 253 167
[11] Kautz E J, Lambeets S V, Royer J, Perea D E, Harilal S S 2022 Scr. Mater. 212 114528
 Google Scholar
Google Scholar
[12] Ilton E S, Bagus P S 2011 Surf. Interface Anal. 43 1549
 Google Scholar
Google Scholar
[13] Mclean W, Colmenares C A, Smith R L, Somorjai G A 1982 Phys. Rev. B 25 8
 Google Scholar
Google Scholar
[14] Tian X F, Wang Yu, Li L S, Wu M D, Yu Y 2020 Comput. Mater. Sci. 179 109633
 Google Scholar
Google Scholar
[15] Liu G D, Liu Z X, Ao B Y, Hu W Y, Deng H Q 2018 Comput. Mater. Sci. 144 85
 Google Scholar
Google Scholar
[16] 李俊炜, 贾维敏, 吕沙沙, 魏雅璇, 李正操, 王金涛 2022 71 226601
 Google Scholar
Google Scholar
Li J W, Jia W M, Lü S S, Wei Y X, Li Z C, Wang J T 2022 Acta Phys. Sin. 71 226601
 Google Scholar
Google Scholar
[17] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[18] Kresse G, Hafner J 1993 Phys. Rev. B: Condens. Matter. 48 13115
 Google Scholar
Google Scholar
[19] Perdew J P, Chevary J A, Vosko S H, Jackson K A, Pederson M R, Singh D J, Fiolhais C 1993 Phys. Rev. B: Condens. Matter. 46 6671
[20] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[21] Payne M C, Teter M P, Allan D C, Arias T A, Joannopoulos J D 1992 Rev. Mod. Phys. 64 1045
 Google Scholar
Google Scholar
[22] Henkelman G, Uberuaga B P, Jónsson H 2000 J. Chem. Phys. 113 9901
 Google Scholar
Google Scholar
[23] Pack James D, Monkhorst H J 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[24] Chiotti P, Klepfer H H, White R W 1959 Trans. Am. Soc. Metals 51 772
[25] Henkelman G, Arnaldsson A, Jónsson H 2006 Comput. Mater. Sci. 36 354
 Google Scholar
Google Scholar
[26] Electronegativity of Chemical Elements, Material-properties https://material-properties.org/electronegativity-of-chemical-elements/ [2023-1-1]
[27] Deringer V L, Tchougréeff A L, Dronskowski R 2011 J. Phys. Chem. A 115 5461
 Google Scholar
Google Scholar
[28] Nelson R, ErturalC, George J, Deringer V, Dronskowski R 2020 J. Comput. Chem. 41 1931
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 6964
- PDF Downloads: 167
- Cited By: 0















 DownLoad:
DownLoad: