-
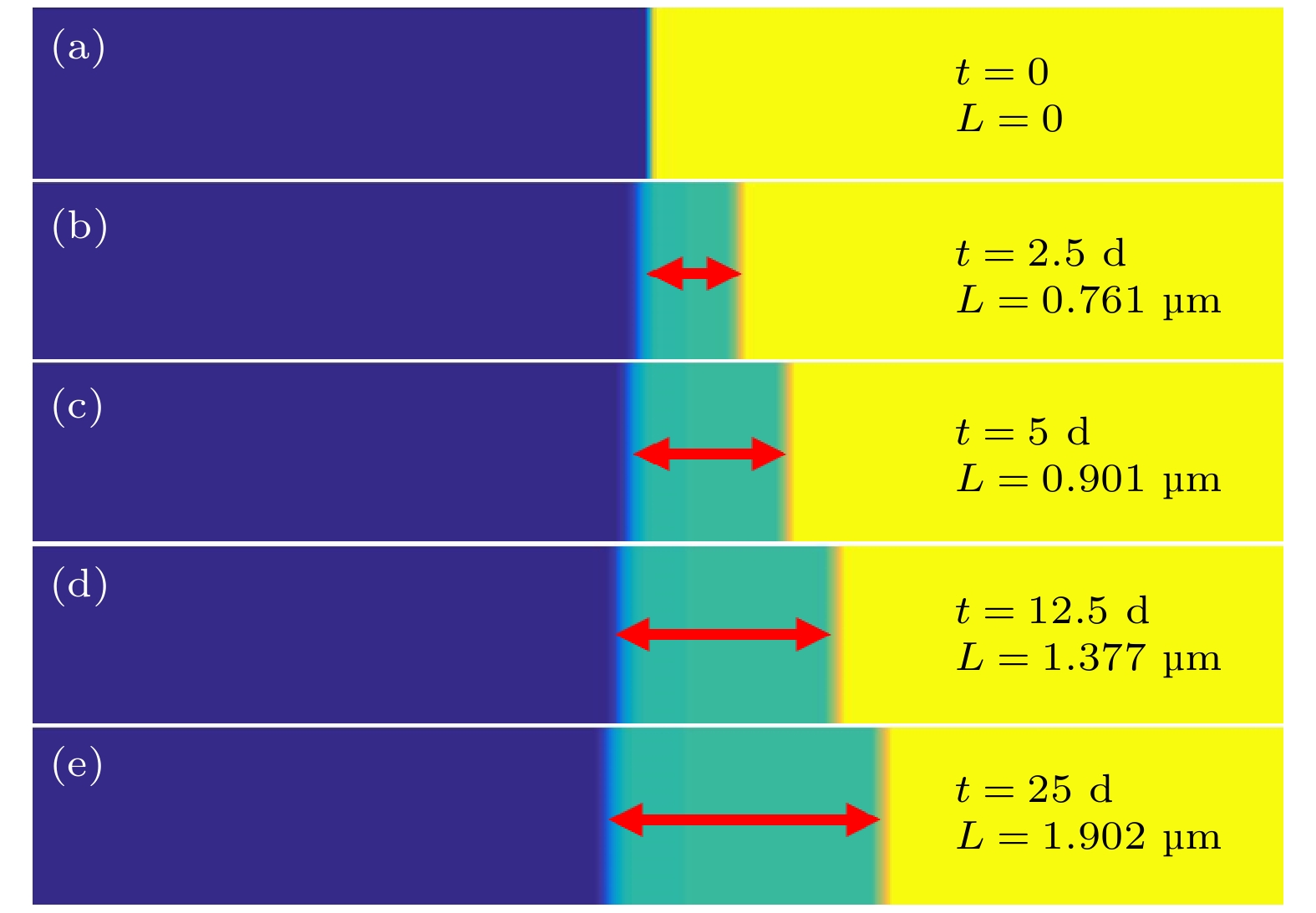
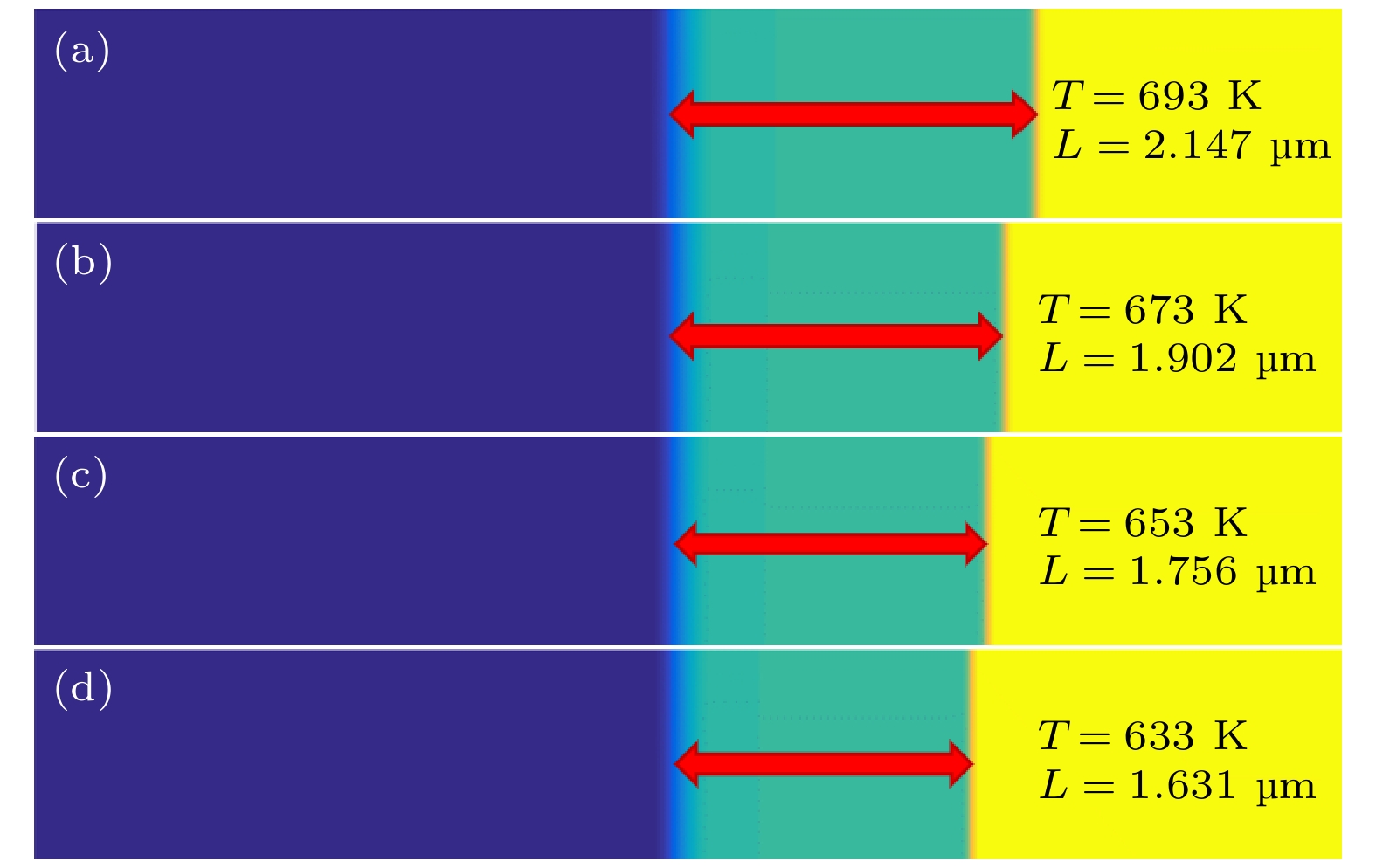
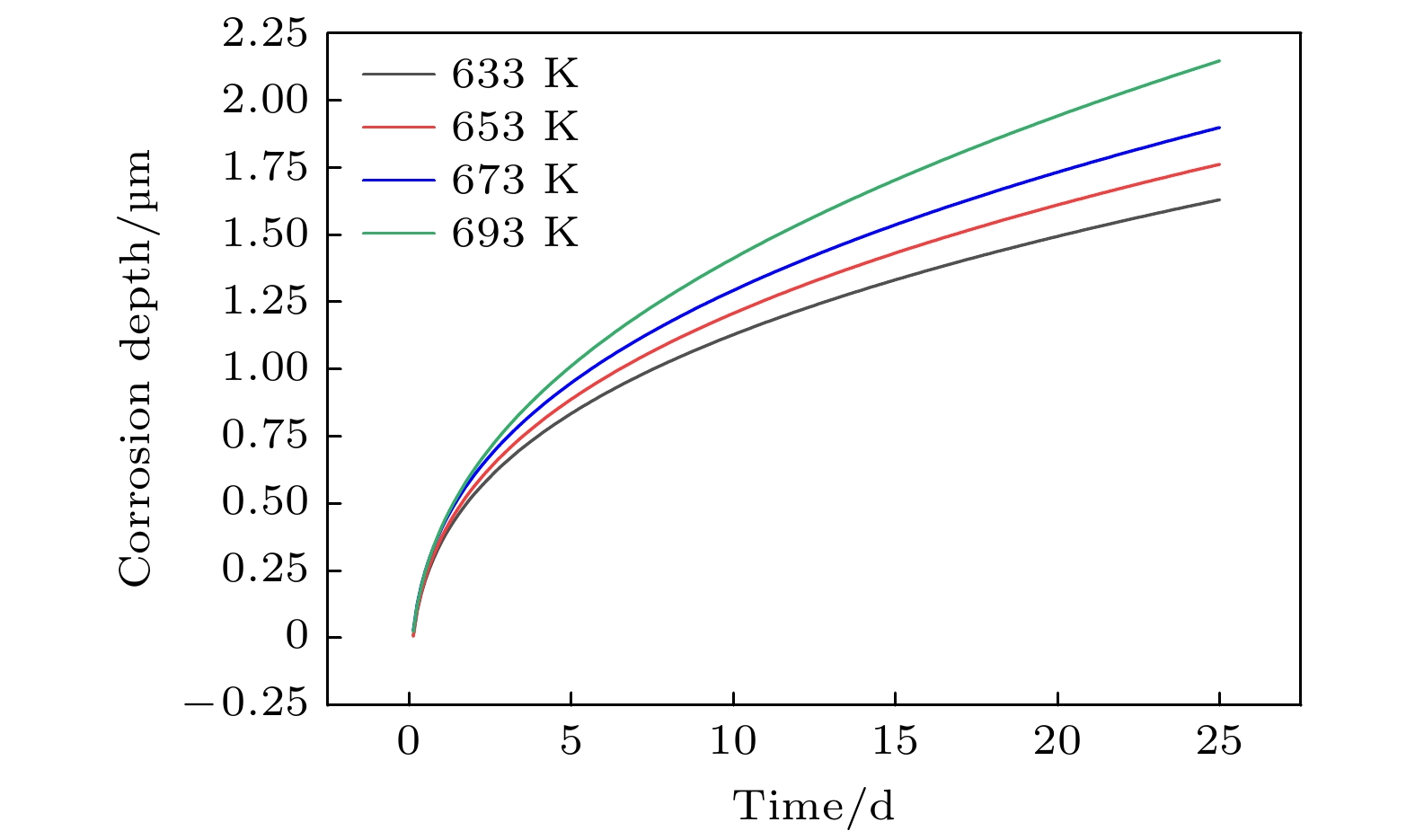
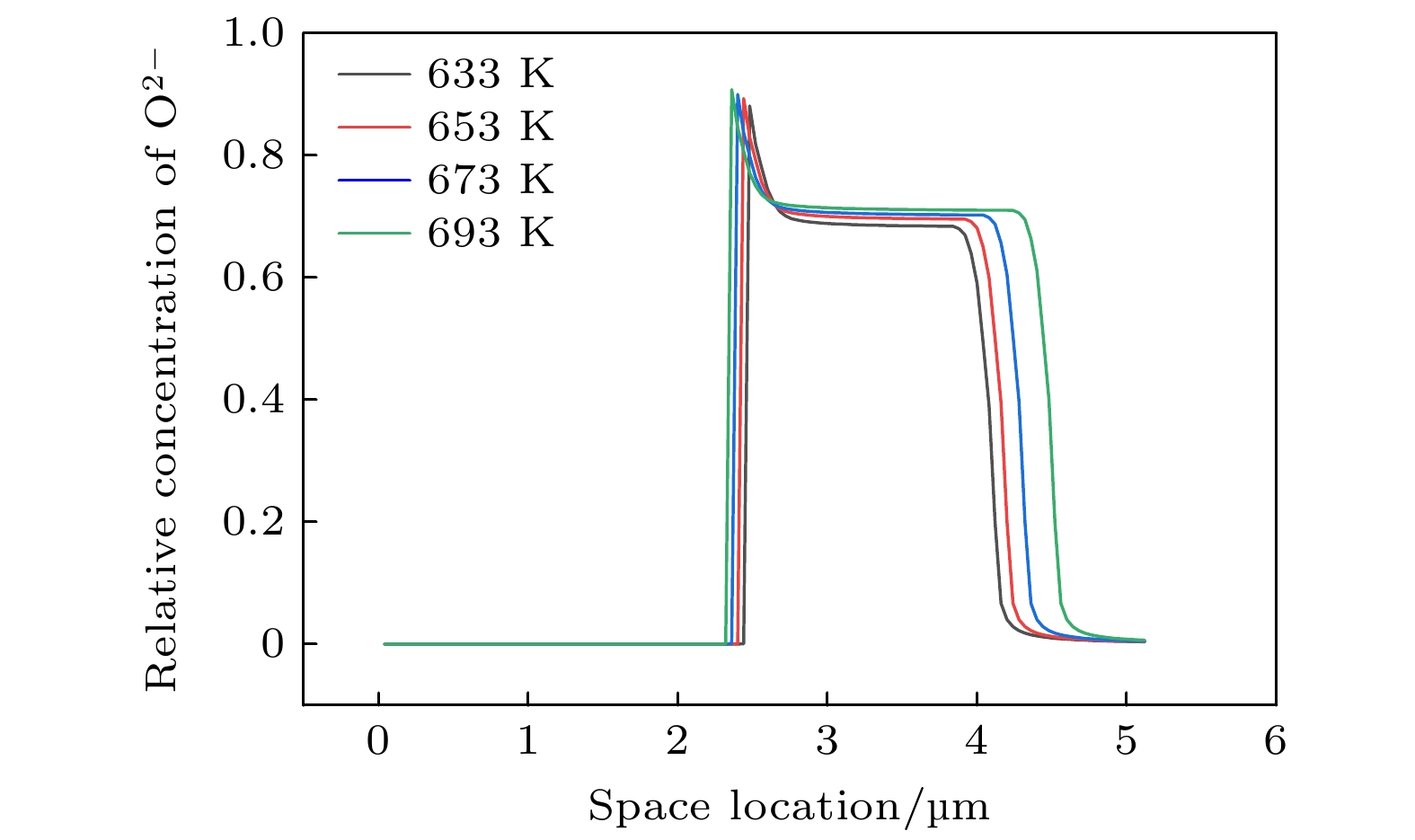
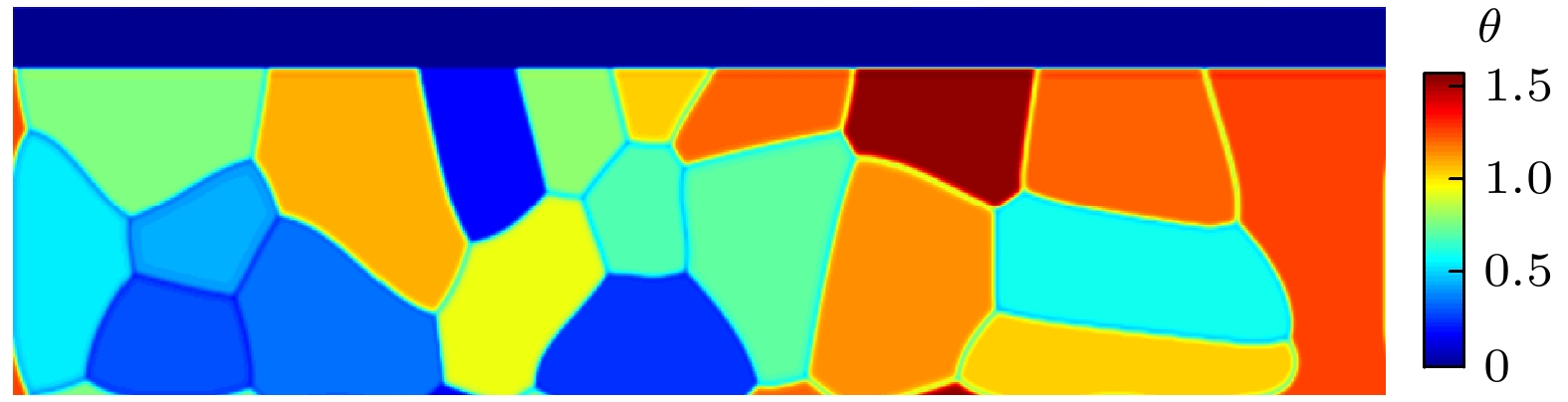
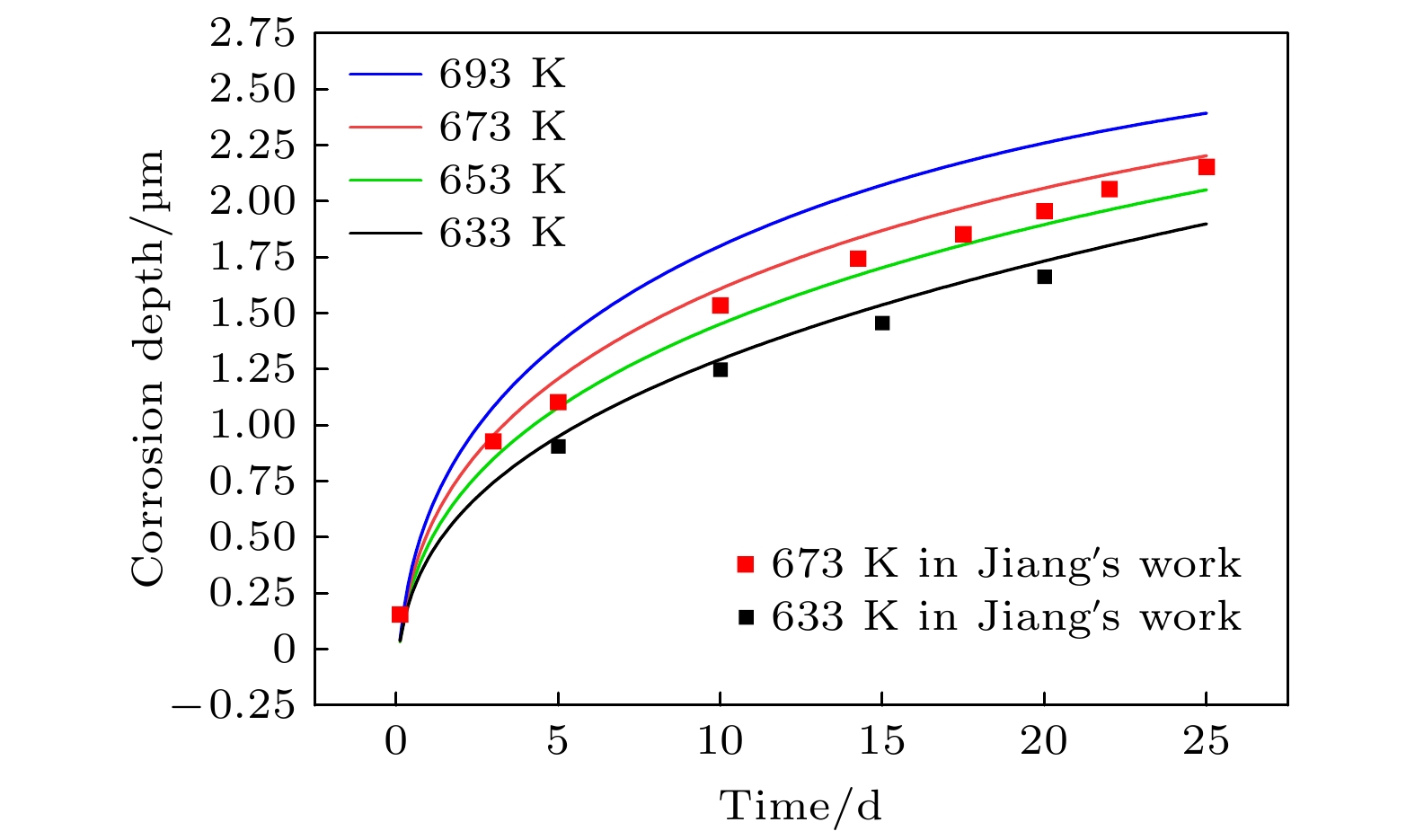
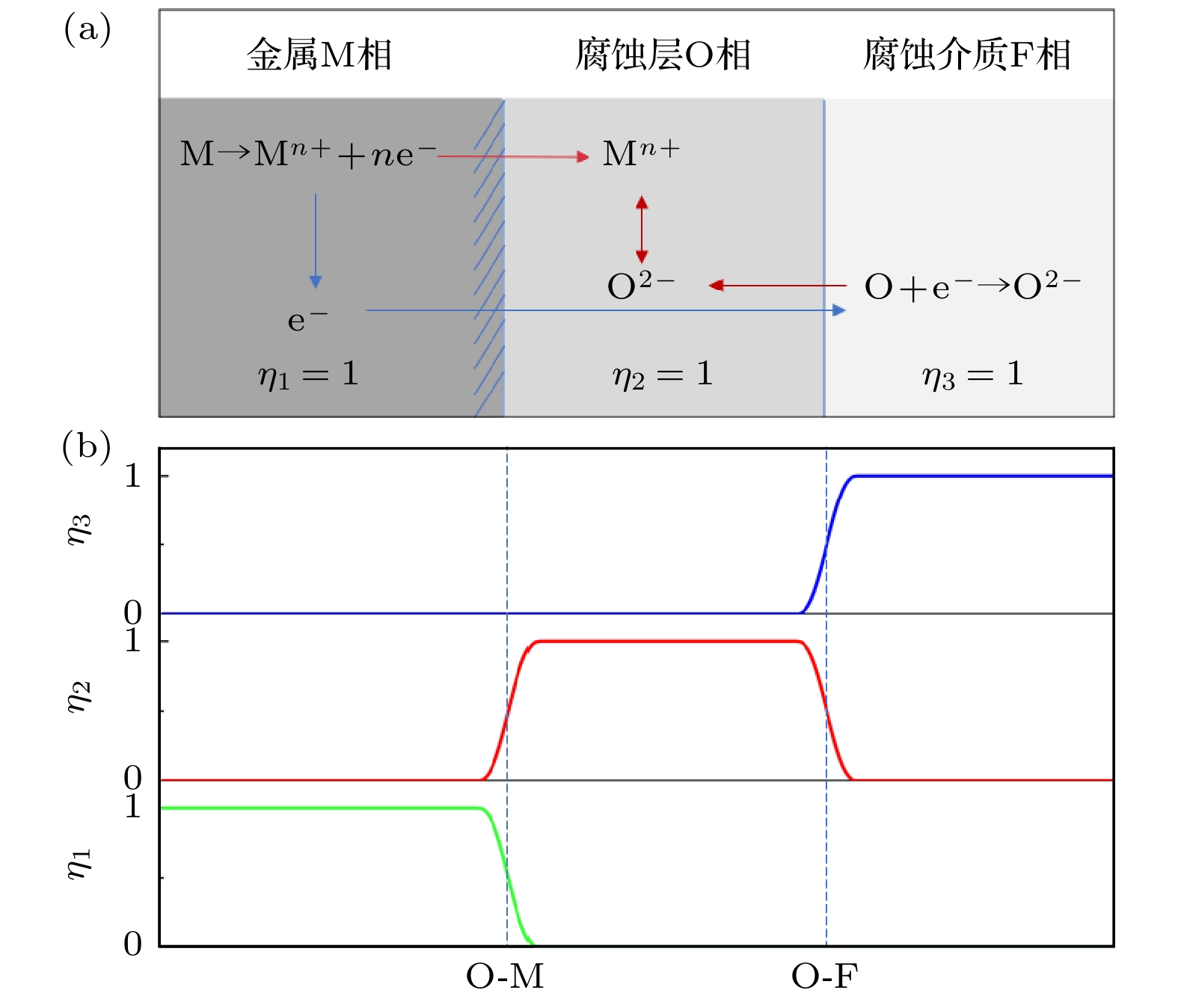
本工作利用腐蚀电化学计算腐蚀界面能, 构建了锆合金腐蚀过程的相场模型. 首先, 利用所建立的模型模拟了Zr-2.5Sn合金均匀腐蚀过程, 模拟结果显示该合金的腐蚀动力学曲线符合立方规律, 与实验结果一致. 分析发现, 在氧化层生成的初期, 氧化层生长速率很高, 但是受温度的影响不明显; 随着氧化层厚度的增长, 温度对氧化层生长曲线的影响变大, 温度越高腐蚀速率越快. 多晶Zr-2.5Sn合金腐蚀行为的模拟结果表明, 在锆合金基体晶界处由于具有更大的氧扩散速率, 氧化速率加快, 并在金属-氧化层界面朝向金属基体一侧形成了沿晶界的具有更高浓度的O2–带, 且对氧化腐蚀速率的影响主要表现在氧化初期, 相场模拟获得的腐蚀动力学曲线与实验结果符合非常好.
-
关键词:
- 相场模拟 /
- Zr-2.5Sn合金 /
- 腐蚀动力学 /
- 晶界腐蚀
Due to the small neutron absorption cross section and excellent thermal creep performance, zirconium alloy is one of the most important cladding materials for fuel rods in commercial fission reactors. However, quantitative analysis of the effects of temperature and grain boundaries on the corrosion microstructure evolution of zirconium alloys is still needed. The establishing of a phase field simulation for the corrosion process of polycrystalline zirconium alloy and the systematical investigating of the thermodynamic influence are both very important. In this study, the phase field model of the corrosion process in zirconium alloys is developed by combining corrosion electrochemistry through calculating the interfacial energy at the metal-oxide and oxide-fluid boundaries. Then the model is used to investigate the uniform corrosion behavior on the surface of Zr-2.5Sn alloy, which demonstrates that the corrosion kinetic curve follows a cubic rule. Subsequently, the influence of temperature on the corrosion thickening curve of zirconium alloy is examined, and good agreement between simulation and experimental results is achieved. It is observed that during early stage of oxide layer formation, there is a high growth rate with minimal temperature dependence; however, as the oxide layer thickness increases, temperature becomes a significant factor affecting its growth rate, with higher temperatures resulting in faster corrosion rates. Furthermore, the effect of polycrystalline zirconium alloy matrices on corrosion rate is investigated, revealing that the grain boundaries accelerate oxide layer thickening due to enhanced oxygen diffusion rates. At metal-oxide interface, O2– bands are formed in areas with higher O2– concentration along these grain boundaries towards the metal matrix, which mainly influences oxidation-corrosion rate during the initial oxidation stage.-
Keywords:
- phase-field simulation /
- Zr-Sn alloy /
- corrosion kinetics /
- grain boundary corrosion
[1] Kai J J, Huang W I, Chou H Y 1990 J. Nucl. Mater. 170 193
 Google Scholar
Google Scholar
[2] Zhao W Z, Quan Q W, Zhang S M, Yang X Y, Wen H Y, Wu Y C, Liu X B, Cao X Z, Wang B Y 2023 Radiat. Phys. Chem. 209 110986
 Google Scholar
Google Scholar
[3] Jones R H, Simonen E P 1994 Mater. Sci. Eng. 176 211
 Google Scholar
Google Scholar
[4] Hu J, Liu J L, Lozano P S, Grovenor C R M, Christensen M, Wolf W, Wimmer E, Mader E V 2019 Acta Mater. 180 105
 Google Scholar
Google Scholar
[5] Cann C D, So C B, Styles R C, Coleman C E 1993 J. Nucl. Mater. 205 267
 Google Scholar
Google Scholar
[6] Wei T G, Dai X, Long C S, Sun C, Long S J, Zheng J Y, Wang P F, Jia Y Z, Zhang J S 2021 Corros. Sci. 192 109808
 Google Scholar
Google Scholar
[7] Tian Z, Peng J C, Lin X D, Hu Y Y, Yao M Y, Xie Y P, Liang X, Zhou B X 2024 Corros. Sci. 202 111937
 Google Scholar
Google Scholar
[8] Ferreirós P A, Polack E C S, Lanzani L A, Alonso P R, Quirós P D, Mieza J I, Rubiolo G H 2021 J. Nucl. Mater. 553 153039
 Google Scholar
Google Scholar
[9] Jiang G Y, Xu D H, Yang W P, Liu L, Zhi Y W, Yang J Q 2022 Prog. Nucl. Energy 154 104490
 Google Scholar
Google Scholar
[10] Yuan R, Xie Y P, Li T, Xu C H, Yao M Y, Xu J X, Guo H B, Zhou B X 2021 Acta Mater. 209 116804
 Google Scholar
Google Scholar
[11] Mcdeavitt S M, Billings G W, Indacochea J E 2002 J. Mater. Sci. 37 3765
 Google Scholar
Google Scholar
[12] Jerlerud P R, Toffolon M C, Joubert J M, Sundman B 2008 CALPHAD 32 593
 Google Scholar
Google Scholar
[13] Asle Zaeem M, El Kadiri H 2014 Comput. Mater. Sci. 89 122
 Google Scholar
Google Scholar
[14] 张更, 王巧, 沙立婷, 李亚捷, 王达, 施思齐 2020 69 226401
 Google Scholar
Google Scholar
Zhang G, Wang Q, Sha L T, Li Y J, Wang D, Shi S Q 2020 Acta Phys. Sin 69 226401
 Google Scholar
Google Scholar
[15] Chen L Q, Zhao Y 2022 Prog. Mater. Sci. 124 100868
 Google Scholar
Google Scholar
[16] Chen L Q 2002 Annu. Rev. Mater. Res. 32 113
 Google Scholar
Google Scholar
[17] 刘续希, 柳文波, 李博岩, 贺新福, 杨朝曦, 恽迪 2022 金属学报 58 943
Liu X X, Liu W B, Li B Y, He X F, Yang Z X, Yun D 2022 Acta Metal. Sin 58 943
[18] Mai W, Soghrati S 2017 Corros. Sci. 125 87
 Google Scholar
Google Scholar
[19] Fang X R, Pan Z C, Ma R J, Chen A R 2023 Comuput. Methods Appl. Mech. Engrg. 414 116196
 Google Scholar
Google Scholar
[20] 冯力, 王智平, 路阳, 朱昌盛 2008 57 1084
 Google Scholar
Google Scholar
Feng L, Wang Z P, Lu Y, Zhu C S 2008 Acta Phys. Sin 57 1084
 Google Scholar
Google Scholar
[21] Yang C, Huang H B, Liu W B, Wang J S, Wang J, Jafri H M, Liu Y, Han G M, Song H F, Chen L Q 2021 Adv. Theor. Simul. 4 2000162
 Google Scholar
Google Scholar
[22] Zhou F Y, Qiu K J, Bian D, Zheng Y F, Lin J P 2014 J. Mater. Sci. Technol. 30 299
 Google Scholar
Google Scholar
[23] Dinsdale A T 1991 CALPHAD 15 317
 Google Scholar
Google Scholar
[24] Aricó S F, Gribaudo L M, 1999 Scripta Mater. 41 159
 Google Scholar
Google Scholar
[25] Wang C, Zinkevich M, Aldinger F 2004 CALPHAD 28 281
 Google Scholar
Google Scholar
[26] Yin T, Lee J, Moosavi K E, Jung I H 2021 Ceram. Int. 47 29267
 Google Scholar
Google Scholar
[27] Isomäki I, Hämäläinen M, Gierlotka W, Onderka B, Fitzner K 2006 J. Alloys Compd. 422 173
 Google Scholar
Google Scholar
[28] Fang X R, Pan Z C, Chen A R, Tian H, Ma R J 2023 Eng. Fract. Mech. 281 109131
 Google Scholar
Google Scholar
[29] Allen S M, Cahn J W 1979 Acta Metall. 27 1085
 Google Scholar
Google Scholar
[30] Larché F C, Cahn J W 1985 Acta Metall. 33 331
 Google Scholar
Google Scholar
[31] Cahn J W 1961 Acta Metall. 9 795
 Google Scholar
Google Scholar
[32] Cox B, Pemsler J P 1968 J. Nucl. Mater. 28 73
 Google Scholar
Google Scholar
[33] Ritchie I G, Atrens A 1977 J. Nucl. Mater. 67 254
 Google Scholar
Google Scholar
[34] Keneshea F J, Douglass D L 1971 Oxid. Met. 3 1
 Google Scholar
Google Scholar
[35] 姜彦博, 柳文波, 孙志鹏, 喇永孝, 恽迪 2022 71 026103
 Google Scholar
Google Scholar
Jiang Y B, Liu W B, Sun Z P, La Y X, Yun D 2022 Acta Phys. Sin 71 026103
 Google Scholar
Google Scholar
[36] Qiu K, Wang R, Peng C, Lu X, Wang N 2015 CALPHAD 48 175
 Google Scholar
Google Scholar
[37] Fisher E, Renken C 1964 Phys. Rev. 135 A482
 Google Scholar
Google Scholar
[38] Fogaing E Y, Lorgouilloux Y, Huger M, Gault C P 2006 J. Mater. Sci. 41 7663
 Google Scholar
Google Scholar
[39] Mirgorodsky A, Smirnov M, Quintard P 1997 Phys. Rev. B 55 19
 Google Scholar
Google Scholar
[40] 刘建章 2007 核结构材料 (北京: 化学工业出版社) 第89页
Liu J Z 2007 Nuclear Structure Material (Beijing: Chemical Industry Press) p89
[41] Fan D, Chen L Q 1997 Acta Mater. 45 611
 Google Scholar
Google Scholar
[42] Bi Y B, Zhang X L, Lu L, Xu Z F, Xie Z G, Chen B B, Liang Z X, Sun Z Q, Luo Z 2023 J. Mater. Res. Technol. 26 5888
 Google Scholar
Google Scholar
[43] Gwinner B, Bataillon C, Chelagemdib R, Gruet N, Lorentz V, Puga B 2023 Electrochim. Acta 470 143334
 Google Scholar
Google Scholar
[44] Zhang M H, Liu S D, Jiang J Y, Wei W C 2023 T Nonferr Metal. Soc. 33 1963
 Google Scholar
Google Scholar
[45] Liu J L, Yu H B, Karamched P, Hu J, He G Z, Goran D, Hughes G M, Wilkinson A J, Sergio L P, Grovenor C R 2019 Acta Mater. 179 328
 Google Scholar
Google Scholar
-
表 1 Zr-Sn合金自由能参数
Table 1. Free energy parameters of Zr-Sn alloys.
Parameter Value Reference EZr/(J·m–3) $-7827.6 + 125.65T - 24.1618T\ln T - 4.38{\rm e}^{-3} T^3 + 34971T^{-1} $ [23] ESn/(J·m–3) $2524.7 + 4.00T - 8.26T \lnT - 16.81{\rm e}^{-3} T^2 + 2.62 {\rm e}^{-6}T^3 - 1.08{\rm e}^6 T^{-1} $ [23] EO/(J·m–3) $-3480.9 - 25.50T - 11.13T\ln T - 5.10 {\rm e}^{-3}T^2 + 0.66 {\rm e}^{-6}T^3 - 38365T^{-1} $ [23] AZrSn $-148022.5+19.41T+(173681.9-22T)(c_{\rm Zr}-c_{\rm Sn}) + 104271.96(c_{\rm Zr}-c_{Sn})^2 $ [24] AZrO $-37876.66+17.2915T - 4471.4(c_{\rm Zr}-c_{\rm O}) $ [25] ASnO 140878 – 23.9326T [26, 35] $g_2^0$ $-1117869+420.3T - 69.6T\ln T - 0.003766T^2 + 702910T^{-1} +4.59 {\rm e}^{-21}T^7 $ [25] $ g_3^0 $ 0 k2/(J·m–3) 2.5 × 105 [13] k3/(J·m–3) 2.5 × 105 [13] Temperature/K D1/(m2·s–1) D2/(m2·s–1) D3/(m2·s–1) DGB/(m2·s–1) 633 3.99×10–17 1.61×10–17 2.98×10–17 1.46×10–16 653 1.26×10–16 5.18×10–17 8.77×10–17 8.11×10–16 673 3.09×10–16 1.11×10–16 2.25×10–16 3.76×10–15 693 1.04×10–15 3.69×10–16 7.64×10–16 2.82×10–14 表 3 锆合金基体及氧化层的弹性模量分量
Table 3. Elastic modulus of Zr alloy and oxide layer.
-
[1] Kai J J, Huang W I, Chou H Y 1990 J. Nucl. Mater. 170 193
 Google Scholar
Google Scholar
[2] Zhao W Z, Quan Q W, Zhang S M, Yang X Y, Wen H Y, Wu Y C, Liu X B, Cao X Z, Wang B Y 2023 Radiat. Phys. Chem. 209 110986
 Google Scholar
Google Scholar
[3] Jones R H, Simonen E P 1994 Mater. Sci. Eng. 176 211
 Google Scholar
Google Scholar
[4] Hu J, Liu J L, Lozano P S, Grovenor C R M, Christensen M, Wolf W, Wimmer E, Mader E V 2019 Acta Mater. 180 105
 Google Scholar
Google Scholar
[5] Cann C D, So C B, Styles R C, Coleman C E 1993 J. Nucl. Mater. 205 267
 Google Scholar
Google Scholar
[6] Wei T G, Dai X, Long C S, Sun C, Long S J, Zheng J Y, Wang P F, Jia Y Z, Zhang J S 2021 Corros. Sci. 192 109808
 Google Scholar
Google Scholar
[7] Tian Z, Peng J C, Lin X D, Hu Y Y, Yao M Y, Xie Y P, Liang X, Zhou B X 2024 Corros. Sci. 202 111937
 Google Scholar
Google Scholar
[8] Ferreirós P A, Polack E C S, Lanzani L A, Alonso P R, Quirós P D, Mieza J I, Rubiolo G H 2021 J. Nucl. Mater. 553 153039
 Google Scholar
Google Scholar
[9] Jiang G Y, Xu D H, Yang W P, Liu L, Zhi Y W, Yang J Q 2022 Prog. Nucl. Energy 154 104490
 Google Scholar
Google Scholar
[10] Yuan R, Xie Y P, Li T, Xu C H, Yao M Y, Xu J X, Guo H B, Zhou B X 2021 Acta Mater. 209 116804
 Google Scholar
Google Scholar
[11] Mcdeavitt S M, Billings G W, Indacochea J E 2002 J. Mater. Sci. 37 3765
 Google Scholar
Google Scholar
[12] Jerlerud P R, Toffolon M C, Joubert J M, Sundman B 2008 CALPHAD 32 593
 Google Scholar
Google Scholar
[13] Asle Zaeem M, El Kadiri H 2014 Comput. Mater. Sci. 89 122
 Google Scholar
Google Scholar
[14] 张更, 王巧, 沙立婷, 李亚捷, 王达, 施思齐 2020 69 226401
 Google Scholar
Google Scholar
Zhang G, Wang Q, Sha L T, Li Y J, Wang D, Shi S Q 2020 Acta Phys. Sin 69 226401
 Google Scholar
Google Scholar
[15] Chen L Q, Zhao Y 2022 Prog. Mater. Sci. 124 100868
 Google Scholar
Google Scholar
[16] Chen L Q 2002 Annu. Rev. Mater. Res. 32 113
 Google Scholar
Google Scholar
[17] 刘续希, 柳文波, 李博岩, 贺新福, 杨朝曦, 恽迪 2022 金属学报 58 943
Liu X X, Liu W B, Li B Y, He X F, Yang Z X, Yun D 2022 Acta Metal. Sin 58 943
[18] Mai W, Soghrati S 2017 Corros. Sci. 125 87
 Google Scholar
Google Scholar
[19] Fang X R, Pan Z C, Ma R J, Chen A R 2023 Comuput. Methods Appl. Mech. Engrg. 414 116196
 Google Scholar
Google Scholar
[20] 冯力, 王智平, 路阳, 朱昌盛 2008 57 1084
 Google Scholar
Google Scholar
Feng L, Wang Z P, Lu Y, Zhu C S 2008 Acta Phys. Sin 57 1084
 Google Scholar
Google Scholar
[21] Yang C, Huang H B, Liu W B, Wang J S, Wang J, Jafri H M, Liu Y, Han G M, Song H F, Chen L Q 2021 Adv. Theor. Simul. 4 2000162
 Google Scholar
Google Scholar
[22] Zhou F Y, Qiu K J, Bian D, Zheng Y F, Lin J P 2014 J. Mater. Sci. Technol. 30 299
 Google Scholar
Google Scholar
[23] Dinsdale A T 1991 CALPHAD 15 317
 Google Scholar
Google Scholar
[24] Aricó S F, Gribaudo L M, 1999 Scripta Mater. 41 159
 Google Scholar
Google Scholar
[25] Wang C, Zinkevich M, Aldinger F 2004 CALPHAD 28 281
 Google Scholar
Google Scholar
[26] Yin T, Lee J, Moosavi K E, Jung I H 2021 Ceram. Int. 47 29267
 Google Scholar
Google Scholar
[27] Isomäki I, Hämäläinen M, Gierlotka W, Onderka B, Fitzner K 2006 J. Alloys Compd. 422 173
 Google Scholar
Google Scholar
[28] Fang X R, Pan Z C, Chen A R, Tian H, Ma R J 2023 Eng. Fract. Mech. 281 109131
 Google Scholar
Google Scholar
[29] Allen S M, Cahn J W 1979 Acta Metall. 27 1085
 Google Scholar
Google Scholar
[30] Larché F C, Cahn J W 1985 Acta Metall. 33 331
 Google Scholar
Google Scholar
[31] Cahn J W 1961 Acta Metall. 9 795
 Google Scholar
Google Scholar
[32] Cox B, Pemsler J P 1968 J. Nucl. Mater. 28 73
 Google Scholar
Google Scholar
[33] Ritchie I G, Atrens A 1977 J. Nucl. Mater. 67 254
 Google Scholar
Google Scholar
[34] Keneshea F J, Douglass D L 1971 Oxid. Met. 3 1
 Google Scholar
Google Scholar
[35] 姜彦博, 柳文波, 孙志鹏, 喇永孝, 恽迪 2022 71 026103
 Google Scholar
Google Scholar
Jiang Y B, Liu W B, Sun Z P, La Y X, Yun D 2022 Acta Phys. Sin 71 026103
 Google Scholar
Google Scholar
[36] Qiu K, Wang R, Peng C, Lu X, Wang N 2015 CALPHAD 48 175
 Google Scholar
Google Scholar
[37] Fisher E, Renken C 1964 Phys. Rev. 135 A482
 Google Scholar
Google Scholar
[38] Fogaing E Y, Lorgouilloux Y, Huger M, Gault C P 2006 J. Mater. Sci. 41 7663
 Google Scholar
Google Scholar
[39] Mirgorodsky A, Smirnov M, Quintard P 1997 Phys. Rev. B 55 19
 Google Scholar
Google Scholar
[40] 刘建章 2007 核结构材料 (北京: 化学工业出版社) 第89页
Liu J Z 2007 Nuclear Structure Material (Beijing: Chemical Industry Press) p89
[41] Fan D, Chen L Q 1997 Acta Mater. 45 611
 Google Scholar
Google Scholar
[42] Bi Y B, Zhang X L, Lu L, Xu Z F, Xie Z G, Chen B B, Liang Z X, Sun Z Q, Luo Z 2023 J. Mater. Res. Technol. 26 5888
 Google Scholar
Google Scholar
[43] Gwinner B, Bataillon C, Chelagemdib R, Gruet N, Lorentz V, Puga B 2023 Electrochim. Acta 470 143334
 Google Scholar
Google Scholar
[44] Zhang M H, Liu S D, Jiang J Y, Wei W C 2023 T Nonferr Metal. Soc. 33 1963
 Google Scholar
Google Scholar
[45] Liu J L, Yu H B, Karamched P, Hu J, He G Z, Goran D, Hughes G M, Wilkinson A J, Sergio L P, Grovenor C R 2019 Acta Mater. 179 328
 Google Scholar
Google Scholar
计量
- 文章访问数: 5165
- PDF下载量: 100
- 被引次数: 0













 下载:
下载: