-
The development of supercritical fluid technology is becoming more and more mature. The research on the adsorption behaviors of supercritical fluid in nanoporous materials has theoretical significance and application value for the development and application of supercritical fluid technology. In this paper, the grand canonical Monte Carlo (GCMC) method is used to simulate the adsorption behaviors of N2 in single-walled carbon nanotubes under supercritical condition and subcritical condition, and the isosteric heat of adsorption and integral molar enthalpy change in different adsorption systems are discussed. The results show that the adsorption isotherms of N2 in SWCNT do not strictly follow the layered adsorption mechanism under supercritical condition, as a result of the increase of molecular thermal motion, the degree of free mobility becomes higher, and it is easier for nitrogen molecule to intersperse and jump between different molecular layers. The nitrogen adsorption isotherm has a peak, which decreases gradually with the increase of temperature, while the critical pressure at peak increases with the temperature increasing. Around the critical point temperature (126 K), a small change in pressure can cause large fluctuations in the bulk gas phase density, resulting in a sharp drop in the adsorption isotherm after peaking. What is different from the subcritical condition is that the adsorption peak of local density distribution curve of the fluid under supercritical condition cannot represent the increase of excess adsorption capacity, and the influence of gas phase density on the adsorption process cannot be ignored. By studying the adsorption integral molar enthalpy under the supercritical condition, it is found that the excess integral molar enthalpy decreases with the pore size increasing; the adsorption integral molar enthalpy decreases with the augment of pore size at lower pressure, but due to a fact that the proportion of gas phase fluid in large-pore SWCNTs increases at higher pressure, on the contrary, it increases with the pore size at higher pressure increasing.
-
Keywords:
- supercritical /
- adsorption /
- Monte Carlo /
- integral molar enthalpy
[1] Jessop P G, Ikariya T, Noyori R 1995 Science 269 1065
 Google Scholar
Google Scholar
[2] Carolina D A A, Juliane V, Gabriela d S A A, Baião D A L, Dupas H M, Julian M 2022 Food Chem. X 13
[3] 王琳, 许大壮, 代奇轩, 楚成超, 李东, 刘刚 2021 科学通报 66 1187
 Google Scholar
Google Scholar
Wang L, Xu D Z, Dai Q X, Chu C C, Li D, Liu G 2021 Chin. Sci Bull. 66 1187
 Google Scholar
Google Scholar
[4] Jair K E, Nunes C P I, Grazielle N N, Renata V, A. M M A, Antônio d S E, Adeodato V M G 2021 J. Supercrit. Fluids
[5] 王博, 冯东 2021 化工进展 40 3270
Wang B, Feng D 2021 Chem. Ind. Eng. Prog. 40 3270
[6] Gusev V Y, O'Brien J A, Seaton N A 1997 Langmuir 13 2815
 Google Scholar
Google Scholar
[7] Zhou L, Zhou Y, Li M, Chen P, Wang Y 2000 Langmuir 16 5955
 Google Scholar
Google Scholar
[8] Ansari H, Joss L, Hwang J, Trusler J P M, Maitland G, Pini R 2020 Microporous Mesoporous Mater. 308 110537
 Google Scholar
Google Scholar
[9] Pini R, Ansari H, Hwang J 2021 Adsorpt. J. Int. Adsorpt. Soc. 27 659
 Google Scholar
Google Scholar
[10] Yang Q L, Xue J H, Li W, Hu B A, Ma Q, Zhan K L, Du X H, Chen Z H 2022 Chem. Eng. J. 433 133492
 Google Scholar
Google Scholar
[11] Tan S J, Liu L, Chew J W 2021 Langmuir 37 6754
 Google Scholar
Google Scholar
[12] Ravikovitch P I, Vishnyakov A, Neimark A V 2001 Phys. Rev. E 64 011602
 Google Scholar
Google Scholar
[13] Dickinson E 1982 Computer Simulation and the Statistical Mechanics of Adsorption (New York: Academic Press) p416
[14] Tjatjopoulos G J, Feke D L, Mann J A 1988 J. Phys. Chem. 92 4006
 Google Scholar
Google Scholar
[15] Fan C Y, Birkett G, Do D D 2010 J. Colloid Interface Sci. 342 485
 Google Scholar
Google Scholar
[16] Fan C Y, Do D D, Li Z L, Nicholson D 2010 Langmuir 26 15852
 Google Scholar
Google Scholar
[17] 范春艳 2010 博士学位论文 (青岛: 中国石油大学 (华东)
Fan C Y 2010 Ph. D. Dissertation (Qingdao: China University of Petroleum (East China) (in Chinese)
[18] Do D D, Do H D 2006 J. Phys. Chem. B 110 17531
 Google Scholar
Google Scholar
[19] Do D D, Do H D 2007 J. Colloid Interface Sci. 316 317
 Google Scholar
Google Scholar
[20] Do D D, Do H D 2005 J. Chem. Phys. 123 084701
 Google Scholar
Google Scholar
[21] Do D D, Nicholson D, Do H D 2008 J. Colloid Interface Sci. 325 7
 Google Scholar
Google Scholar
[22] 刘忠军 2011 博士学位论文 (沈阳: 东北大学)
Liu Z J 2011 Ph. D. Dissertation (Shenyang: Northeastern University) (in Chinese)
-
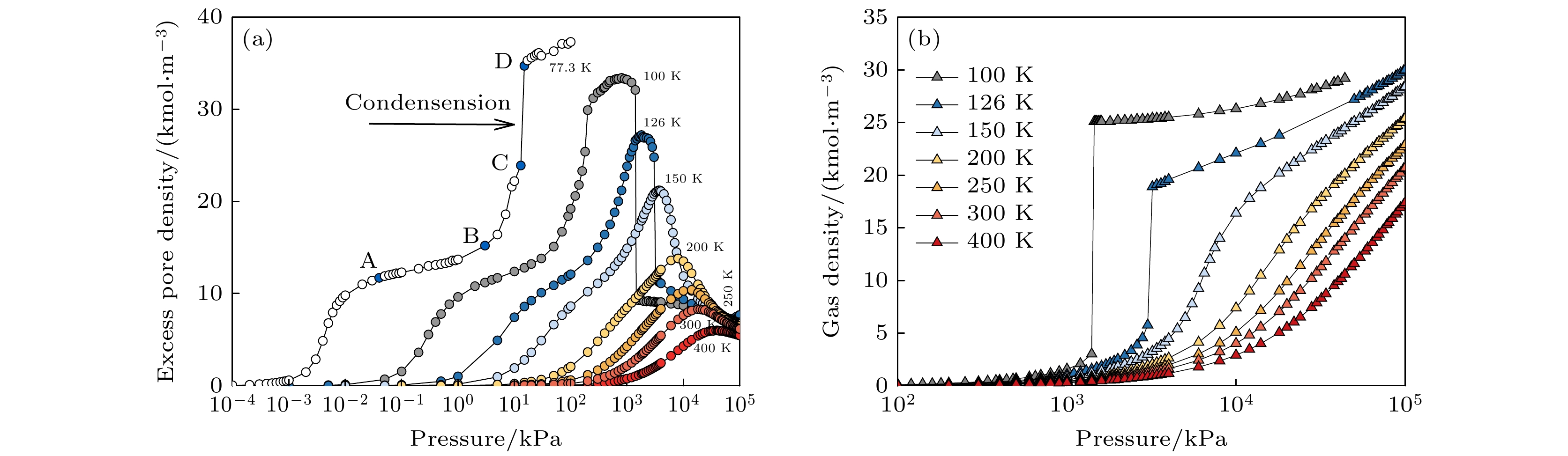
图 2 (a) 不同温度下(77.3, 100, 126, 150, 200, 250, 300和400 K)SWCNT (R = 1.831 nm)内的氮气吸附等温线; (b) 超临界状态下本体气相密度随压力变化曲线
Figure 2. (a) Adsorption isotherms of N2 in SWCNT (R = 1.831 nm) at different temperatures (77.3, 100, 126, 150, 200, 250, 300 and 400 K); (b) variation curve of bulk gas density with pressure under supercritical conditions.
图 5 SWCNT内氮气吸附过程瞬间构型图 (a) 77.3 K温度下, 压力分别为0.04, 3, 13和15 kPa时氮气分子的瞬间构型, 压力点分别对应于图2中标示点A, B, C和D; (b) 300 K温度下, 压力分别为1, 3, 18和100 MPa时氮气分子的瞬间构型
Figure 5. The snapshots of N2 adsorption process in SWCNT: (a) N2 molecules at the temperature of 77.3 K, and the pressure points correspond to the marked points A, B, C and D in the Fig. 2; (b) N2 molecules at the temperature of 300 K under the pressure of 1, 3, 18 and 100 MPa, respectively.
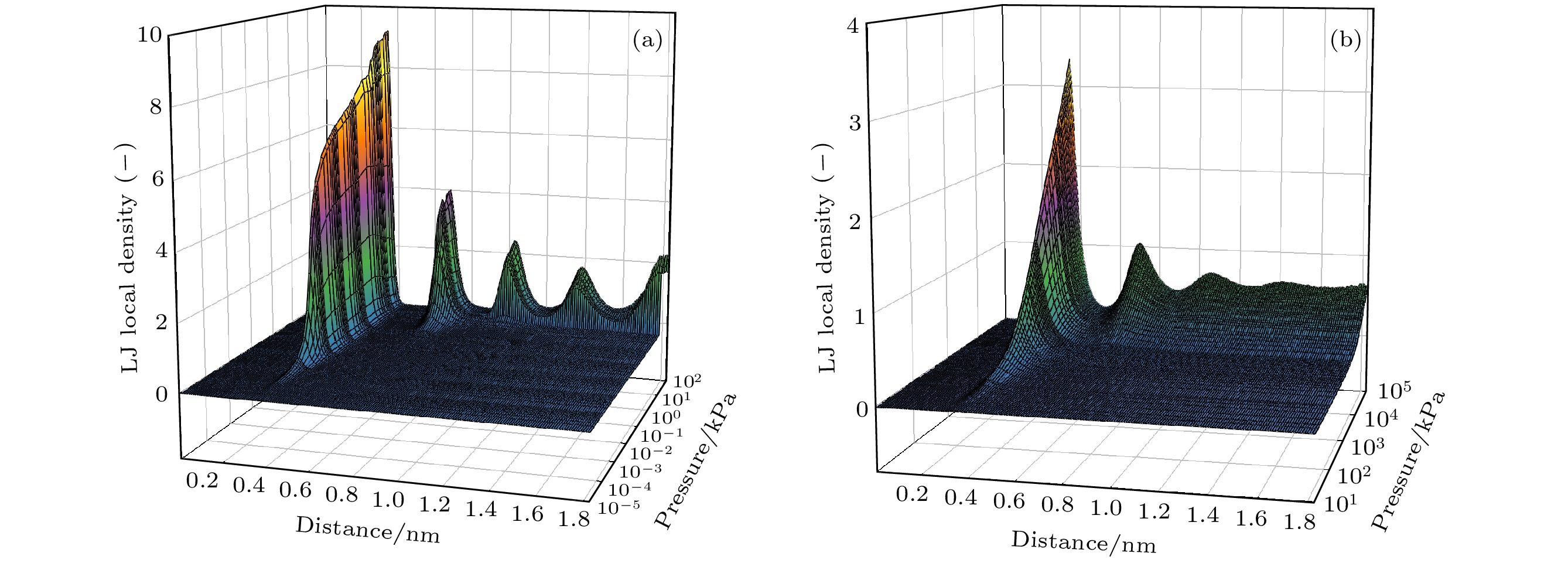
图 6 77.3 (a)和300 K (b)温度下孔径方向上特殊压力点下的局部密度分布. 图(a)中A, B, C和D分别对应于图2中的标示压力点; 图(b)中平行虚线为对应压力下本体流体气相密度
Figure 6. Local density distribution at special pressure points in the pore radii direction at 77.3 (a) and 300 K (b); A, B, C and D in Figure (a)correspond to the marked pressure points in the Fig.2 respectively; the dash-dotted horizontal line in Figure (b) refers to the bulk gas density at the corresponding pressure.
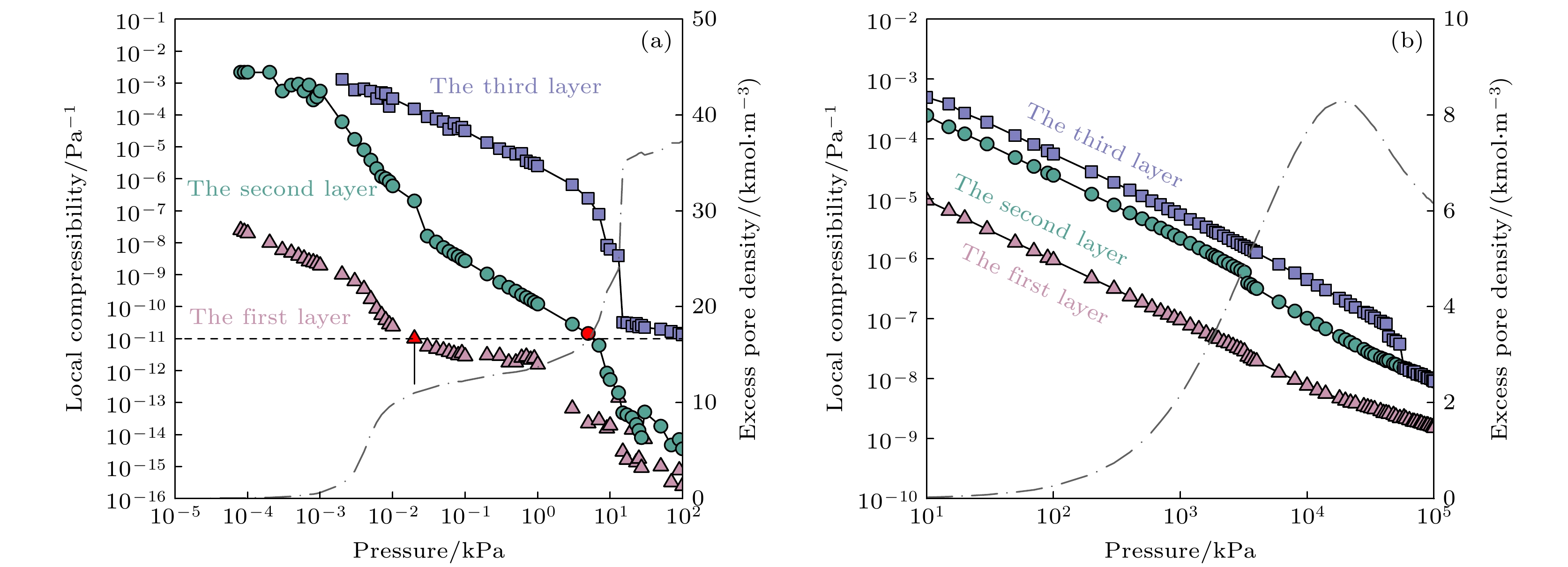
图 9 氮气在77.3, 150, 300和400 K时在SWCNT内吸附的积分摩尔焓, 灰色虚线为等量吸附热, 绿色点划线与右边Y轴对应为相对应温度下的过剩吸附量
Figure 9. Integral molar enthalpy of nitrogen adsorption in SWCNT at 77.3, 150, 300 and 400 K, The gray dotted line indicates the Isosteric heat of adsorption, and the green dash-dotted line corresponding to the right Y axis is the adsorption isotherm at the corresponding temperature.
图 11 (a) 77 K温度下氮气在不同孔径SWCNT内的等量吸附热随过剩吸附量的变化关系; (b) 150 K温度下过剩积分摩尔焓随压力的变化关系曲线, 插入图为吸附积分摩尔焓随压力的变化关系
Figure 11. (a) Isosteric heat of N2 adsorption versus excess adsorption at 77 K, in SWCNTs with different radii 0.7464, 1.153, 1.56, 2 nm; (b) the integral molar enthalpy versus pressure at 150 K, and the insertion diagram shows the variation relationship of adsorption integral molar enthalpy with pressure.
-
[1] Jessop P G, Ikariya T, Noyori R 1995 Science 269 1065
 Google Scholar
Google Scholar
[2] Carolina D A A, Juliane V, Gabriela d S A A, Baião D A L, Dupas H M, Julian M 2022 Food Chem. X 13
[3] 王琳, 许大壮, 代奇轩, 楚成超, 李东, 刘刚 2021 科学通报 66 1187
 Google Scholar
Google Scholar
Wang L, Xu D Z, Dai Q X, Chu C C, Li D, Liu G 2021 Chin. Sci Bull. 66 1187
 Google Scholar
Google Scholar
[4] Jair K E, Nunes C P I, Grazielle N N, Renata V, A. M M A, Antônio d S E, Adeodato V M G 2021 J. Supercrit. Fluids
[5] 王博, 冯东 2021 化工进展 40 3270
Wang B, Feng D 2021 Chem. Ind. Eng. Prog. 40 3270
[6] Gusev V Y, O'Brien J A, Seaton N A 1997 Langmuir 13 2815
 Google Scholar
Google Scholar
[7] Zhou L, Zhou Y, Li M, Chen P, Wang Y 2000 Langmuir 16 5955
 Google Scholar
Google Scholar
[8] Ansari H, Joss L, Hwang J, Trusler J P M, Maitland G, Pini R 2020 Microporous Mesoporous Mater. 308 110537
 Google Scholar
Google Scholar
[9] Pini R, Ansari H, Hwang J 2021 Adsorpt. J. Int. Adsorpt. Soc. 27 659
 Google Scholar
Google Scholar
[10] Yang Q L, Xue J H, Li W, Hu B A, Ma Q, Zhan K L, Du X H, Chen Z H 2022 Chem. Eng. J. 433 133492
 Google Scholar
Google Scholar
[11] Tan S J, Liu L, Chew J W 2021 Langmuir 37 6754
 Google Scholar
Google Scholar
[12] Ravikovitch P I, Vishnyakov A, Neimark A V 2001 Phys. Rev. E 64 011602
 Google Scholar
Google Scholar
[13] Dickinson E 1982 Computer Simulation and the Statistical Mechanics of Adsorption (New York: Academic Press) p416
[14] Tjatjopoulos G J, Feke D L, Mann J A 1988 J. Phys. Chem. 92 4006
 Google Scholar
Google Scholar
[15] Fan C Y, Birkett G, Do D D 2010 J. Colloid Interface Sci. 342 485
 Google Scholar
Google Scholar
[16] Fan C Y, Do D D, Li Z L, Nicholson D 2010 Langmuir 26 15852
 Google Scholar
Google Scholar
[17] 范春艳 2010 博士学位论文 (青岛: 中国石油大学 (华东)
Fan C Y 2010 Ph. D. Dissertation (Qingdao: China University of Petroleum (East China) (in Chinese)
[18] Do D D, Do H D 2006 J. Phys. Chem. B 110 17531
 Google Scholar
Google Scholar
[19] Do D D, Do H D 2007 J. Colloid Interface Sci. 316 317
 Google Scholar
Google Scholar
[20] Do D D, Do H D 2005 J. Chem. Phys. 123 084701
 Google Scholar
Google Scholar
[21] Do D D, Nicholson D, Do H D 2008 J. Colloid Interface Sci. 325 7
 Google Scholar
Google Scholar
[22] 刘忠军 2011 博士学位论文 (沈阳: 东北大学)
Liu Z J 2011 Ph. D. Dissertation (Shenyang: Northeastern University) (in Chinese)
Catalog
Metrics
- Abstract views: 7006
- PDF Downloads: 78
- Cited By: 0















 DownLoad:
DownLoad: