-
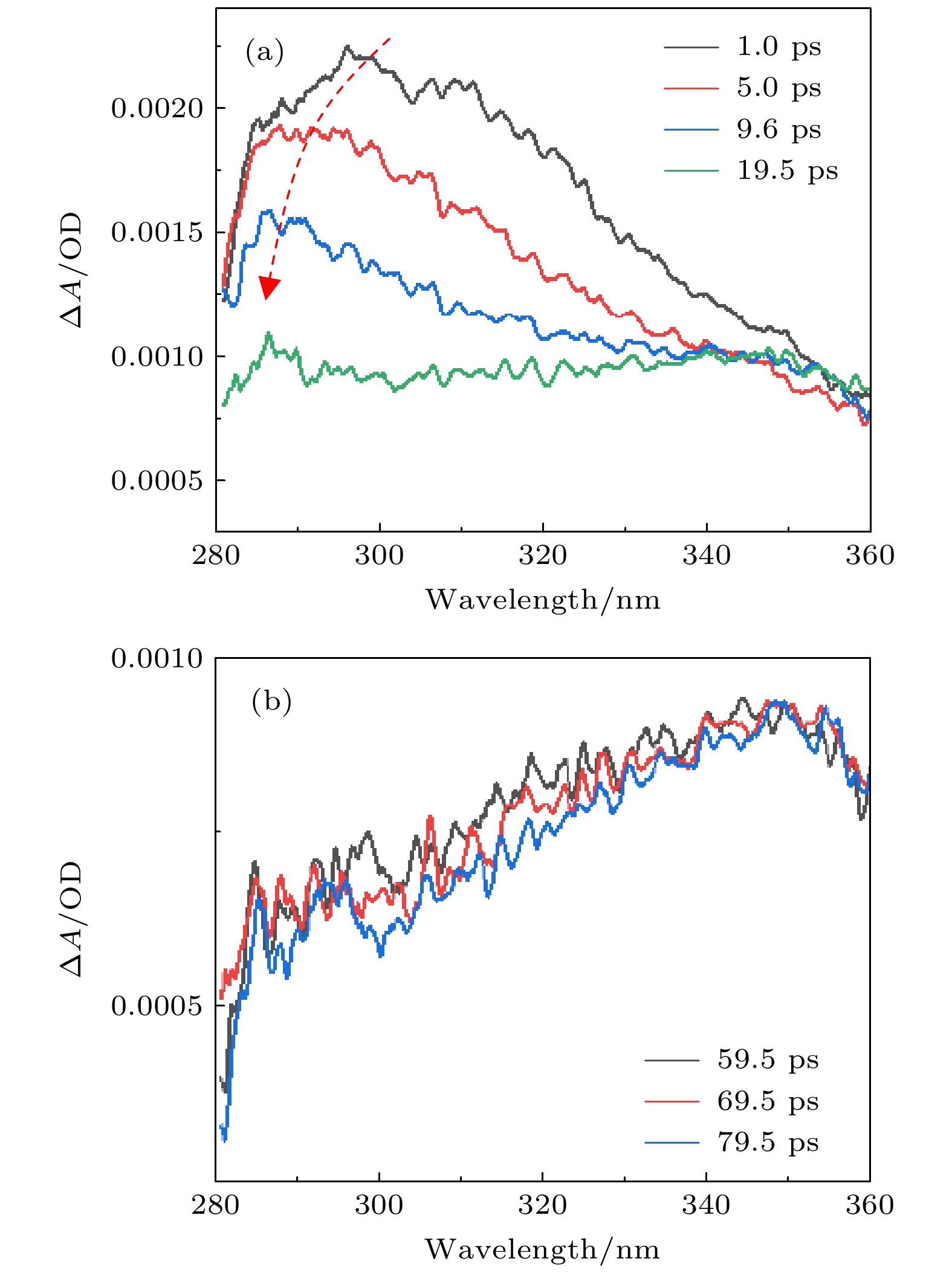
As one of the building blocks in RNA chain, uracil and its derivatives have attracted a great deal of interest since its ultrafast dynamics is closely related to mutagenic and carcinogenic effects. In this study, the solvent effect on the ultrafast decay of excited uracil is studied by femtosecond transient absorption spectroscopy in the UV region. The uracil molecule is populated to the 1(π, π*) state (i.e. S2 state) with a pump pulse at 264 nm. Broad-band white light continuum in the UV region from 280 to 360 nm is used as the probe. With a detail analysis of the measured transient spectra, two decay time constants, i.e. 9.8 ps and > 1000 ps, are directly obtained at 300 nm in the solvent of acetonitrile. Compared with our previous experiments, where no obvious triplet population is observed in water, triplet population is found to play an important role in acetonitrile. A comparison of excited-state dynamics among different solvents is also carried out. It reveals that the decay from the 1(n, π*) state (i.e., S1 state) to the T1 state shows a clear dependence on the H bonding of the solvents. With stronger H bonding, the 1(n, π*) excited state decays faster and has less chance to transfer to the triplet state. These results suggest that only when the 1(n, π*) state has excess vibrational energy can it transit to the triplet state through the intersystem crossing process. With this new information obtained in the present measurement, the decay dynamics of uracil on the S2 excited state can be further understood.
-
Keywords:
- femtosecond transient absorption spectroscopy /
- uracil /
- excited-state dynamic /
- solvent effect
[1] Zierhut M, Roth W, Fischer I 2004 Phys. Chem. Chem. Phys. 6 5178
 Google Scholar
Google Scholar
[2] Pfeifer G P, You Y H, Besaratinia A 2005 Mutat. Res.- Fund. Mol. Mech. Mutagen 571 19
 Google Scholar
Google Scholar
[3] de Gruijl F R 1999 Eur. J. Cancer 35 2003
 Google Scholar
Google Scholar
[4] Gustavsson T, Sarkar N, Lazzarotto E, Markovitsi D, Improta R 2006 Chem. Phys. Lett 429 551
 Google Scholar
Google Scholar
[5] Hare P M, Crespo-Hernandez C E, Kohler B 2007 Proc. Natl. Acad. Sci. U. S. A 104 435
 Google Scholar
Google Scholar
[6] Gustavsson T, Banyasz A, Lazzarotto E, Markovitsi D, Scalmani G, Frisch M J, Barone V, Improta R 2006 J. Am. Chem. Soc 128 607
 Google Scholar
Google Scholar
[7] Matsika S 2004 J. Phys. Chem. A 108 7584
 Google Scholar
Google Scholar
[8] Barbatti M, Aquino A J A, Szymczak J J, Nachtigallova D, ́Hobza P, Lischka H 2010 Proc. Natl. Acad. Sci. U. S. A 107 21453
 Google Scholar
Google Scholar
[9] Hare P M, Crespo-Hernandez C E, Kohler B 2006 J. Phys. Chem. B 110 18641
 Google Scholar
Google Scholar
[10] Gustavsson T, Sarkar N, Lazzarotto E, Markovitsi D, Barone V, Improta R 2006 J. Phys. Chem. B 110 12843
 Google Scholar
Google Scholar
[11] Gustavsson T, Sarkar N, Banyasz A, Markovitsi D, Improta R 2007 Photochem. Photobiol 83 595
 Google Scholar
Google Scholar
[12] Hua X, Hua L, Liu X 2015 J. Phys. Chem. A 119 12985
 Google Scholar
Google Scholar
[13] Hua X, Hua L, Liu X 2016 Phys. Chem. Chem. Phys 18 13904
 Google Scholar
Google Scholar
[14] Li P, Xue J, Zheng X 2019 J. Raman. Spectrosc 50 345
 Google Scholar
Google Scholar
[15] Santoro F, Barone V, Gustavsson T, Improta R 2006 J. Am. Chem. Soc 128 16312
 Google Scholar
Google Scholar
[16] Improta R, Barone V 2008 Theor. Chem. Acc 120 491
 Google Scholar
Google Scholar
[17] Improta R, Barone V, Lami A, Santoro F 2009 J. Phys. Chem. B 113 14491
 Google Scholar
Google Scholar
[18] Etinski M, Marian C M 2010 Phys. Chem. Chem. Phys 12 15665
 Google Scholar
Google Scholar
[19] Danillo V, Adalberto V S A, Antonio C B 2021 Molecules 26 5191
 Google Scholar
Google Scholar
[20] Salet C, Bensasson R 1975 Photochem. Photobiol 22 231
 Google Scholar
Google Scholar
[21] Gorner H 1990 Photochem. Photobiol 52 935
 Google Scholar
Google Scholar
[22] Charvat A, Assmann J, Abel B, Schwarzer D, Henning K, Luther K, Troe J 2001 Phys. Chem. Chem. Phys 3 2230
 Google Scholar
Google Scholar
[23] Schwarzer D, Hanisch C, Kutne P, Troe J 2002 J. Phys. Chem. A 106 8019
 Google Scholar
Google Scholar
[24] Schwarzer D, Kutne P, Schroder C, Troe J 2004 J. Chem. Phys 121 1754
 Google Scholar
Google Scholar
[25] Schwarzer D, Troe J, Votsmeier M, Zerezke M 1996 J. Chem. Phys 105 3121
 Google Scholar
Google Scholar
[26] Schwarzer D, Troe J, Votsmeier M, Zerezke M 1997 Ber. Bunsen. Phys. Chem. 101 595
 Google Scholar
Google Scholar
-
表 1 在不同溶剂中观察到的时间常数
Table 1. Observed time constants in different solvents.
乙腈 乙酸乙酯 甲醇 乙二醇 水 a τ1/ps 9.8 6.8 3.5 3.1 1.1 b τ2/ps >1000 >1000 >1000 >1000 >1000 a 以300 nm处的衰减曲线为例; b τ2 在之前的研究中未能被精确测量. -
[1] Zierhut M, Roth W, Fischer I 2004 Phys. Chem. Chem. Phys. 6 5178
 Google Scholar
Google Scholar
[2] Pfeifer G P, You Y H, Besaratinia A 2005 Mutat. Res.- Fund. Mol. Mech. Mutagen 571 19
 Google Scholar
Google Scholar
[3] de Gruijl F R 1999 Eur. J. Cancer 35 2003
 Google Scholar
Google Scholar
[4] Gustavsson T, Sarkar N, Lazzarotto E, Markovitsi D, Improta R 2006 Chem. Phys. Lett 429 551
 Google Scholar
Google Scholar
[5] Hare P M, Crespo-Hernandez C E, Kohler B 2007 Proc. Natl. Acad. Sci. U. S. A 104 435
 Google Scholar
Google Scholar
[6] Gustavsson T, Banyasz A, Lazzarotto E, Markovitsi D, Scalmani G, Frisch M J, Barone V, Improta R 2006 J. Am. Chem. Soc 128 607
 Google Scholar
Google Scholar
[7] Matsika S 2004 J. Phys. Chem. A 108 7584
 Google Scholar
Google Scholar
[8] Barbatti M, Aquino A J A, Szymczak J J, Nachtigallova D, ́Hobza P, Lischka H 2010 Proc. Natl. Acad. Sci. U. S. A 107 21453
 Google Scholar
Google Scholar
[9] Hare P M, Crespo-Hernandez C E, Kohler B 2006 J. Phys. Chem. B 110 18641
 Google Scholar
Google Scholar
[10] Gustavsson T, Sarkar N, Lazzarotto E, Markovitsi D, Barone V, Improta R 2006 J. Phys. Chem. B 110 12843
 Google Scholar
Google Scholar
[11] Gustavsson T, Sarkar N, Banyasz A, Markovitsi D, Improta R 2007 Photochem. Photobiol 83 595
 Google Scholar
Google Scholar
[12] Hua X, Hua L, Liu X 2015 J. Phys. Chem. A 119 12985
 Google Scholar
Google Scholar
[13] Hua X, Hua L, Liu X 2016 Phys. Chem. Chem. Phys 18 13904
 Google Scholar
Google Scholar
[14] Li P, Xue J, Zheng X 2019 J. Raman. Spectrosc 50 345
 Google Scholar
Google Scholar
[15] Santoro F, Barone V, Gustavsson T, Improta R 2006 J. Am. Chem. Soc 128 16312
 Google Scholar
Google Scholar
[16] Improta R, Barone V 2008 Theor. Chem. Acc 120 491
 Google Scholar
Google Scholar
[17] Improta R, Barone V, Lami A, Santoro F 2009 J. Phys. Chem. B 113 14491
 Google Scholar
Google Scholar
[18] Etinski M, Marian C M 2010 Phys. Chem. Chem. Phys 12 15665
 Google Scholar
Google Scholar
[19] Danillo V, Adalberto V S A, Antonio C B 2021 Molecules 26 5191
 Google Scholar
Google Scholar
[20] Salet C, Bensasson R 1975 Photochem. Photobiol 22 231
 Google Scholar
Google Scholar
[21] Gorner H 1990 Photochem. Photobiol 52 935
 Google Scholar
Google Scholar
[22] Charvat A, Assmann J, Abel B, Schwarzer D, Henning K, Luther K, Troe J 2001 Phys. Chem. Chem. Phys 3 2230
 Google Scholar
Google Scholar
[23] Schwarzer D, Hanisch C, Kutne P, Troe J 2002 J. Phys. Chem. A 106 8019
 Google Scholar
Google Scholar
[24] Schwarzer D, Kutne P, Schroder C, Troe J 2004 J. Chem. Phys 121 1754
 Google Scholar
Google Scholar
[25] Schwarzer D, Troe J, Votsmeier M, Zerezke M 1996 J. Chem. Phys 105 3121
 Google Scholar
Google Scholar
[26] Schwarzer D, Troe J, Votsmeier M, Zerezke M 1997 Ber. Bunsen. Phys. Chem. 101 595
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 5633
- PDF Downloads: 108
- Cited By: 0














 DownLoad:
DownLoad:



