-
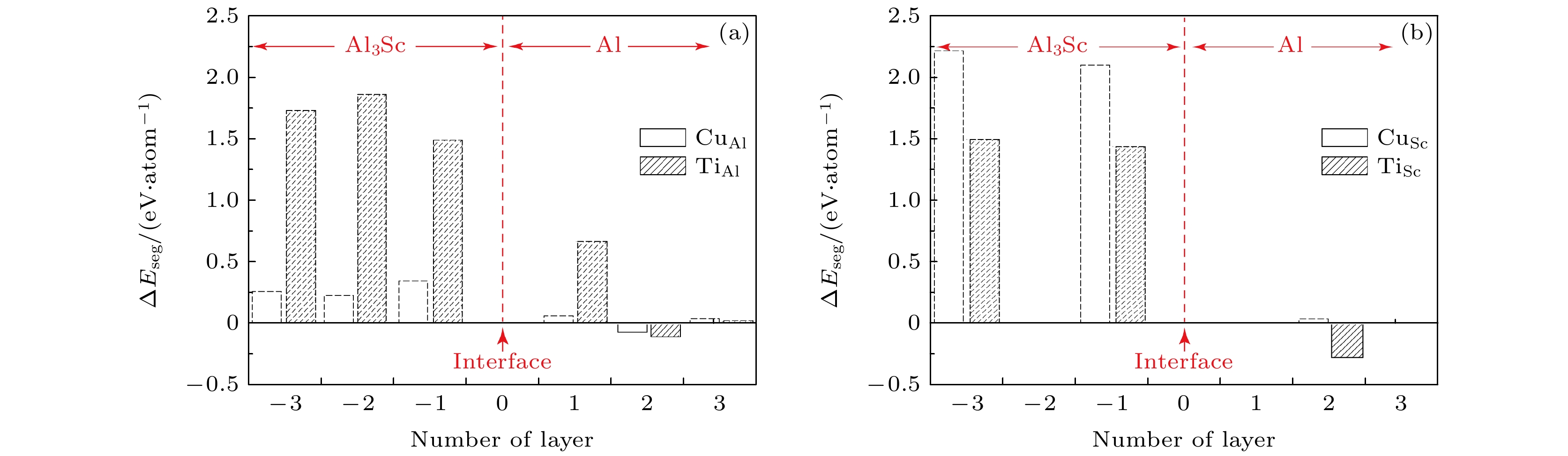
Thermal stabilities of L12-Al3Sc nano-precipitates are critical for the thermotolerance of Al-Sc based alloys. Previous experiments have suggested that different alloying elements may have different segregation behaviors at the L12-Al3Sc/Al interface, which can exert different influences on the thermal stability of L12-Al3Sc nano-precipitates. To clarify the responsible mechanism from a quantitative approach, first-principles calculations of energetics are performed in this work, to investigate the segregation behaviors of transition-metal elements Cu and Ti at the L12-Al3Sc/Al interface. The results suggest that both Cu and Ti can segregate to the interface, and substitute Al or Sc sites on its Al side with different thermodynamic driving forces. Given a temperature, segregation amount is largely determined by the initial elemental concentration in the Al matrix. The higher the segregation driving force and the initial matrix concentration are, the higher the equilibrium segregation amount (or the maximum interfacial coverage) could be. With an initial matrix atomic concentration of 1%, the maximum interfacial coverage of Ti can reach up to 80% (0.8 monolayer layer (ML)) while that of Cu is less than 4% (0.04 ML) at T = 600 K.
-
Keywords:
- Al-Sc alloy /
- alloying elements /
- Al3Sc /
- interface segregation /
- first-principles
[1] Royset J, Ryum N 2005 Mater. Sci. Eng., A 396 409
 Google Scholar
Google Scholar
[2] Kanresky R A, Dunand D C, Seidman D N 2009 Acta Mater. 57 4022
 Google Scholar
Google Scholar
[3] Krug M E, Dunand D C, Seidman D N 2011 Acta Mater. 59 1700
 Google Scholar
Google Scholar
[4] Fuller C B, Seidman D N 2005 Acta Mater. 53 5415
 Google Scholar
Google Scholar
[5] Yoon K E, Noebe R D, Seidman D N 2007 Acta Mater. 55 1159
 Google Scholar
Google Scholar
[6] Deschamps A, Lae L, Guyot P 2007 Acta Mater. 55 2775
 Google Scholar
Google Scholar
[7] Jia Q, Rometsch P, Cao S, et al. 2018 Scr. Mater. 151 42
 Google Scholar
Google Scholar
[8] Booth-Morrison C, Mao Z, Diaz M, et al. 2012 Acta Mater. 60 4740
 Google Scholar
Google Scholar
[9] Vo N Q, Dunand D C, Seidman D N 2014 Acta Mater. 63 73
 Google Scholar
Google Scholar
[10] Zhang C M, Xie P, Jiang Y, Zhan S, Ming W Q, Chen J H, Song K X, Zhang H 2021 Acta Metall. Sinica-Eng. Lett. 34 1277
 Google Scholar
Google Scholar
[11] Zhang C M, Jiang Y, Cao F, Hu T, Wang Y, Yin D 2019 J. Mater. Sci. Technol. 35 930
 Google Scholar
Google Scholar
[12] Marsha E D, David N, Seidman D N, David C D 2008 Acta Mater. 56 4369
 Google Scholar
Google Scholar
[13] Van Dalen M E, Dunand D C, Seidman D N 2005 Acta Mater. 53 4225
 Google Scholar
Google Scholar
[14] Gao Y H, Cao L F, Kuang J, Zhang J Y, Liu G, Sun J 2020 J. Mater. Sci. Technol. 37 38
 Google Scholar
Google Scholar
[15] Dong W, Kresse G, Furthmüller J, et al. 1996 Phys. Rev. B 54 2157
 Google Scholar
Google Scholar
[16] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[17] Leese J, Lord A E 1968 J. Appl. Phys. 39 3986
 Google Scholar
Google Scholar
[18] Clouet E, Sanchez J M, Sigli C 2002 Phys. Rev. B 65 094105
 Google Scholar
Google Scholar
[19] Jiang Y, Smith J R, Evans A G 2008 Appl. Phys. Lett. 92 141918
 Google Scholar
Google Scholar
[20] Jiang Y, Xu C H, Lan G Q 2013 Trans. Nonferrous Met. Soc. China 23 180
 Google Scholar
Google Scholar
[21] Chen Z G, Ringer S P, Zheng Z Q, Zhong J 2007 Mater. Sci. Forum. 546-549 629
 Google Scholar
Google Scholar
[22] Kairy S K, Rouxel B, Dumbre J, Lamb J, Langan T J, Dorin T, Birbilis N 2019 Corrs. Sci. 158 108095
 Google Scholar
Google Scholar
[23] McLean D 1957 Grain Boundaries in Metals (London: Oxford University Press) p116
-
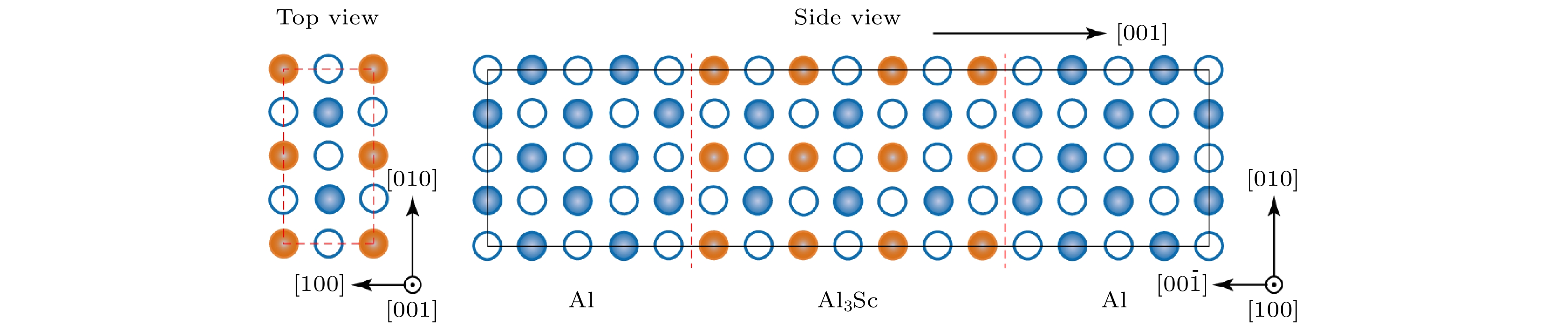
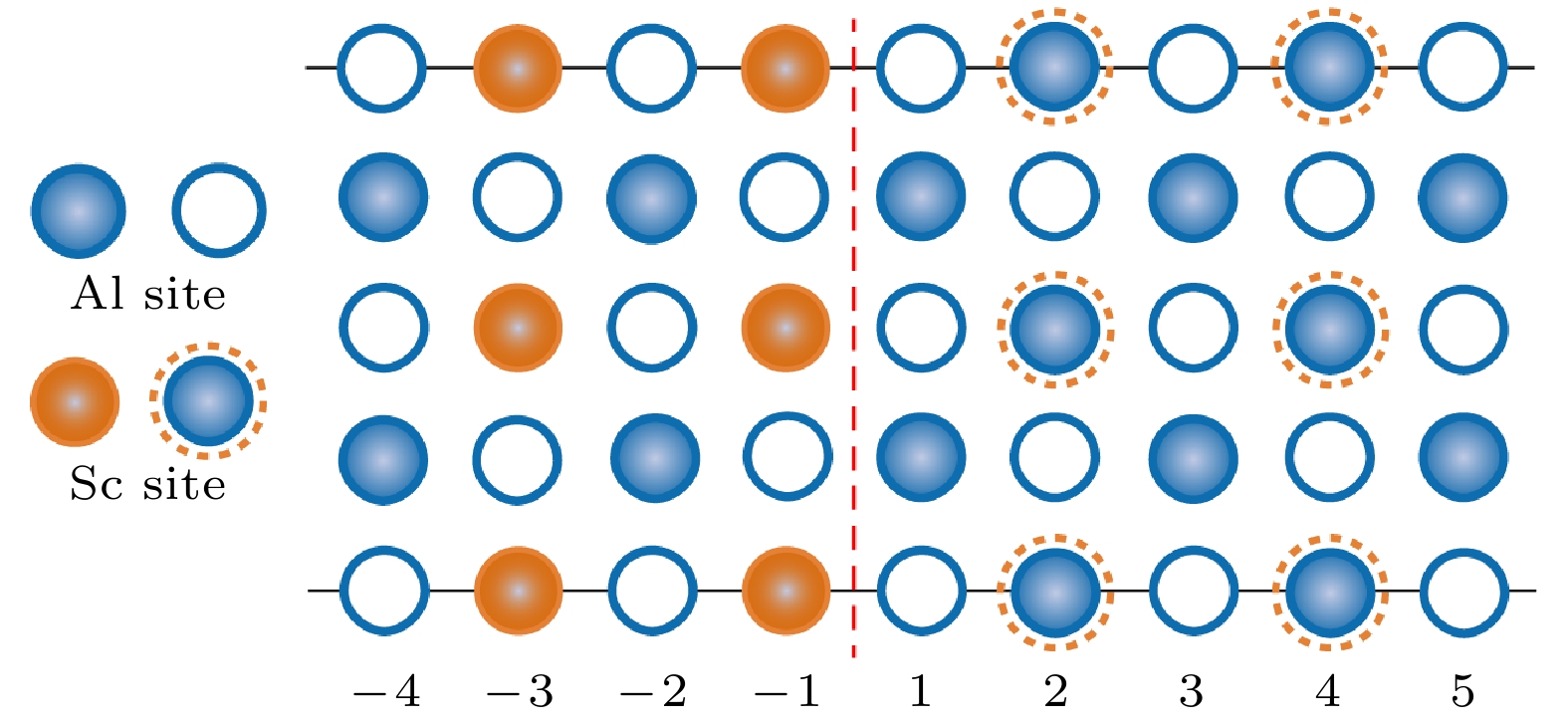
图 2 基于实验位向关系, 弛豫计算确定的L12-Al3Sc/Al界面的能量最低结构. 其中红色虚线标记界面位置. 俯视图显示界面两侧最近邻原子层的配位关系为on-hollow型. 实心与空心球分别代表不同层(z = 0, 0.5)上的原子
Figure 2. The lowest energy structure of L12-Al3Sc/Al obtained by relaxation calculation using the experimental interfacial orientation relation. Dashed red lines locate the interface. The on-hollow type of interfacial coordination is manifested in the top view. The solid and open balls denote atoms at different layers z = 0 , 0.5, respectively.
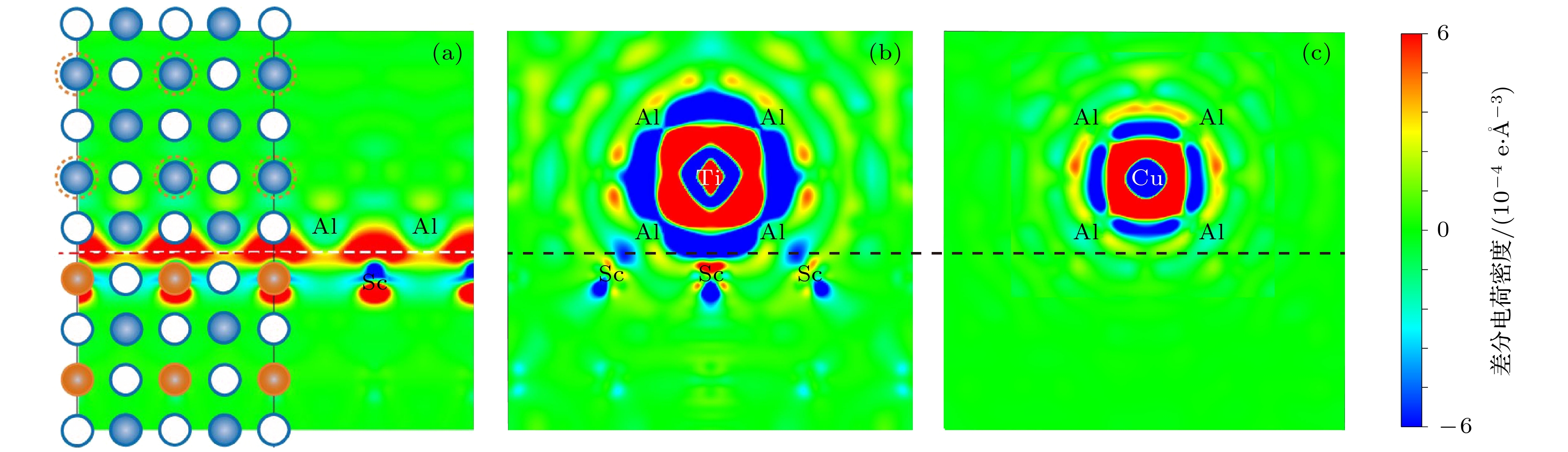
表 1 界面偏析前后Cu 和Ti原子的Bader电荷分析(单位: e/atom)
Table 1. The Bader charge analysis of Cu and Ti before and after interface segregation (unit: e/atom)
Inside bulk At interface Cu Ti Cu-Al Cu-Sc Ti-Al Ti-Sc Bader 11.73 3.378 11.773 11.687 3.328 3.288 Net change –0.730 +0.622 –0.773 –0.687 +0.627 +0.712 -
[1] Royset J, Ryum N 2005 Mater. Sci. Eng., A 396 409
 Google Scholar
Google Scholar
[2] Kanresky R A, Dunand D C, Seidman D N 2009 Acta Mater. 57 4022
 Google Scholar
Google Scholar
[3] Krug M E, Dunand D C, Seidman D N 2011 Acta Mater. 59 1700
 Google Scholar
Google Scholar
[4] Fuller C B, Seidman D N 2005 Acta Mater. 53 5415
 Google Scholar
Google Scholar
[5] Yoon K E, Noebe R D, Seidman D N 2007 Acta Mater. 55 1159
 Google Scholar
Google Scholar
[6] Deschamps A, Lae L, Guyot P 2007 Acta Mater. 55 2775
 Google Scholar
Google Scholar
[7] Jia Q, Rometsch P, Cao S, et al. 2018 Scr. Mater. 151 42
 Google Scholar
Google Scholar
[8] Booth-Morrison C, Mao Z, Diaz M, et al. 2012 Acta Mater. 60 4740
 Google Scholar
Google Scholar
[9] Vo N Q, Dunand D C, Seidman D N 2014 Acta Mater. 63 73
 Google Scholar
Google Scholar
[10] Zhang C M, Xie P, Jiang Y, Zhan S, Ming W Q, Chen J H, Song K X, Zhang H 2021 Acta Metall. Sinica-Eng. Lett. 34 1277
 Google Scholar
Google Scholar
[11] Zhang C M, Jiang Y, Cao F, Hu T, Wang Y, Yin D 2019 J. Mater. Sci. Technol. 35 930
 Google Scholar
Google Scholar
[12] Marsha E D, David N, Seidman D N, David C D 2008 Acta Mater. 56 4369
 Google Scholar
Google Scholar
[13] Van Dalen M E, Dunand D C, Seidman D N 2005 Acta Mater. 53 4225
 Google Scholar
Google Scholar
[14] Gao Y H, Cao L F, Kuang J, Zhang J Y, Liu G, Sun J 2020 J. Mater. Sci. Technol. 37 38
 Google Scholar
Google Scholar
[15] Dong W, Kresse G, Furthmüller J, et al. 1996 Phys. Rev. B 54 2157
 Google Scholar
Google Scholar
[16] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[17] Leese J, Lord A E 1968 J. Appl. Phys. 39 3986
 Google Scholar
Google Scholar
[18] Clouet E, Sanchez J M, Sigli C 2002 Phys. Rev. B 65 094105
 Google Scholar
Google Scholar
[19] Jiang Y, Smith J R, Evans A G 2008 Appl. Phys. Lett. 92 141918
 Google Scholar
Google Scholar
[20] Jiang Y, Xu C H, Lan G Q 2013 Trans. Nonferrous Met. Soc. China 23 180
 Google Scholar
Google Scholar
[21] Chen Z G, Ringer S P, Zheng Z Q, Zhong J 2007 Mater. Sci. Forum. 546-549 629
 Google Scholar
Google Scholar
[22] Kairy S K, Rouxel B, Dumbre J, Lamb J, Langan T J, Dorin T, Birbilis N 2019 Corrs. Sci. 158 108095
 Google Scholar
Google Scholar
[23] McLean D 1957 Grain Boundaries in Metals (London: Oxford University Press) p116
Catalog
Metrics
- Abstract views: 6208
- PDF Downloads: 111
- Cited By: 0














 DownLoad:
DownLoad: