-
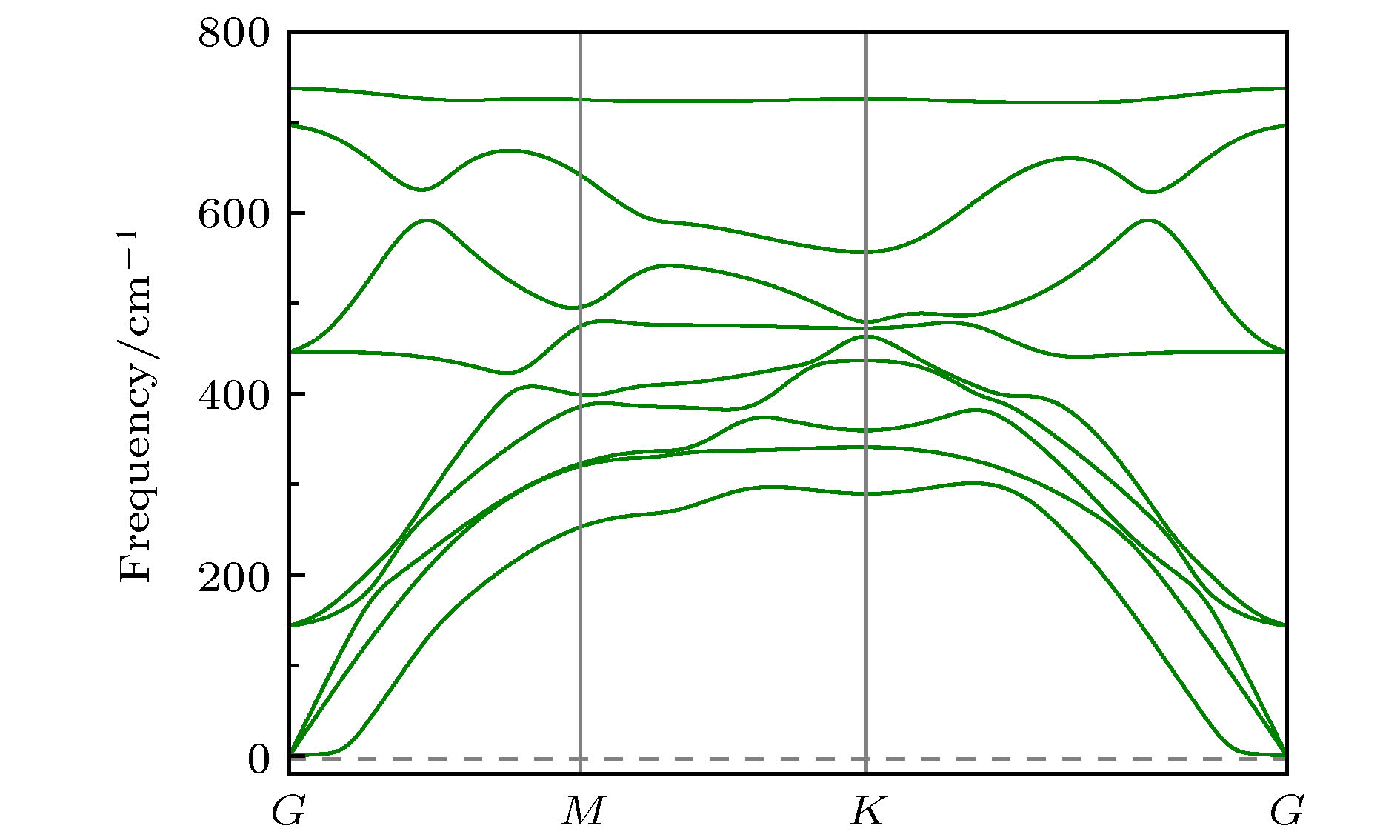
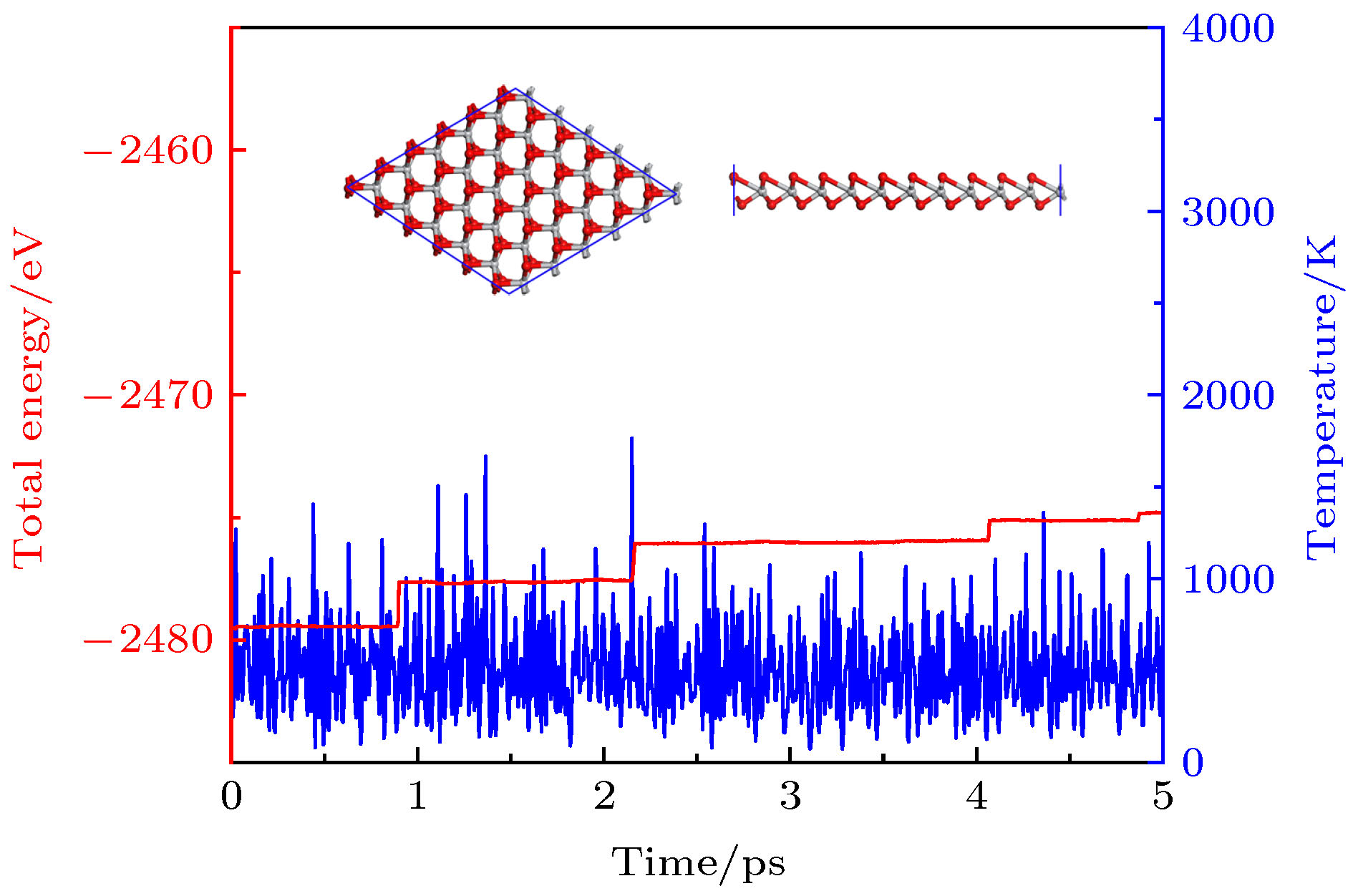
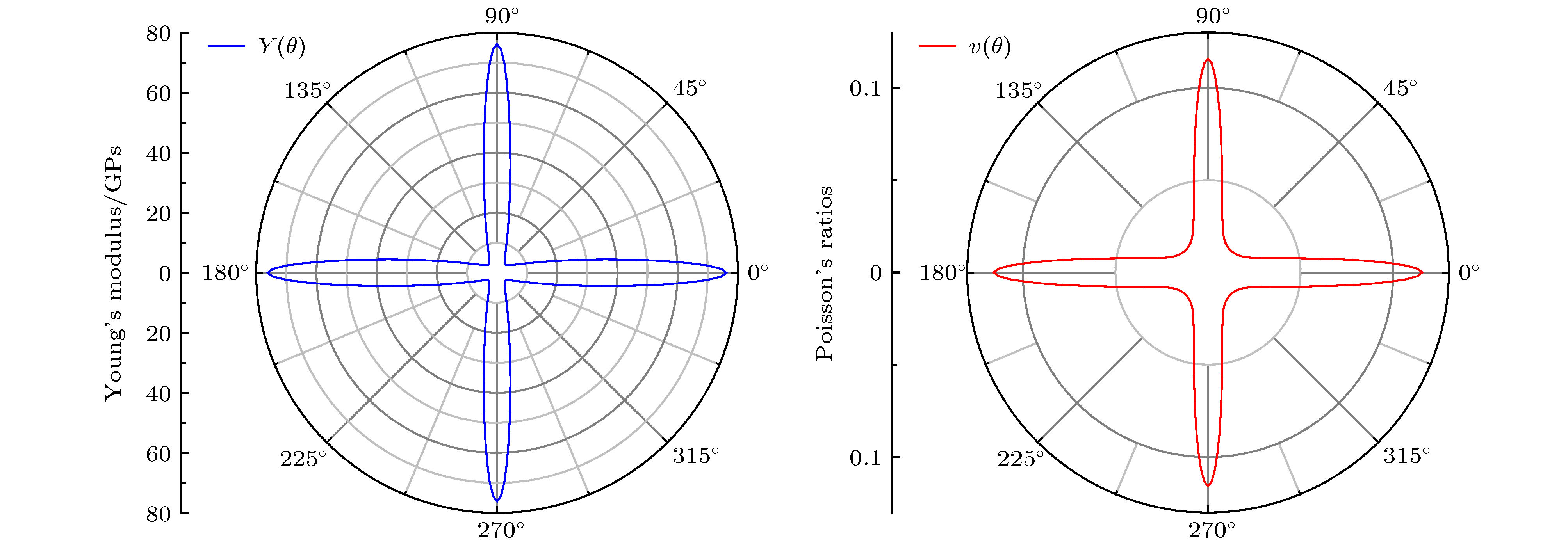
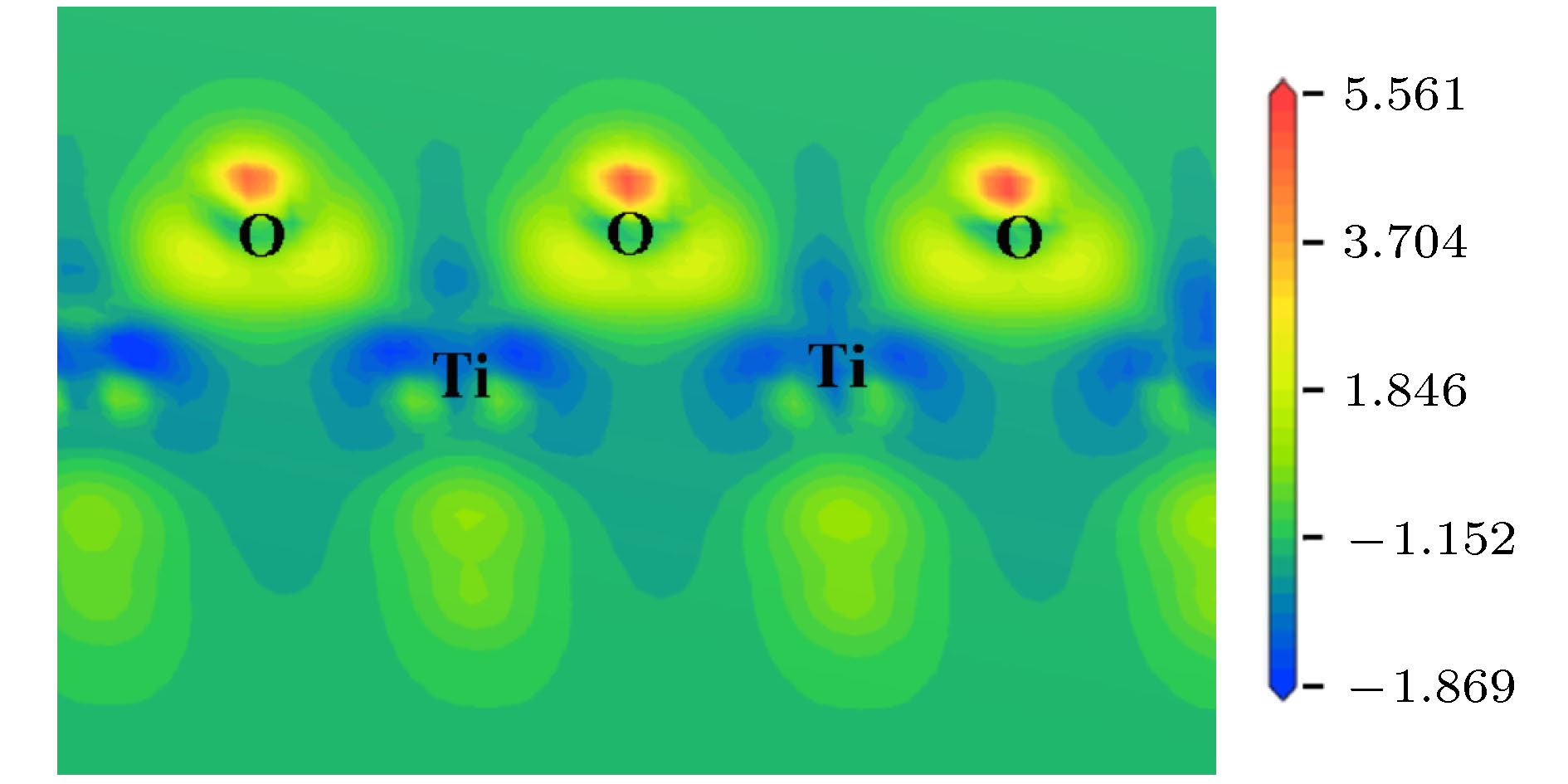
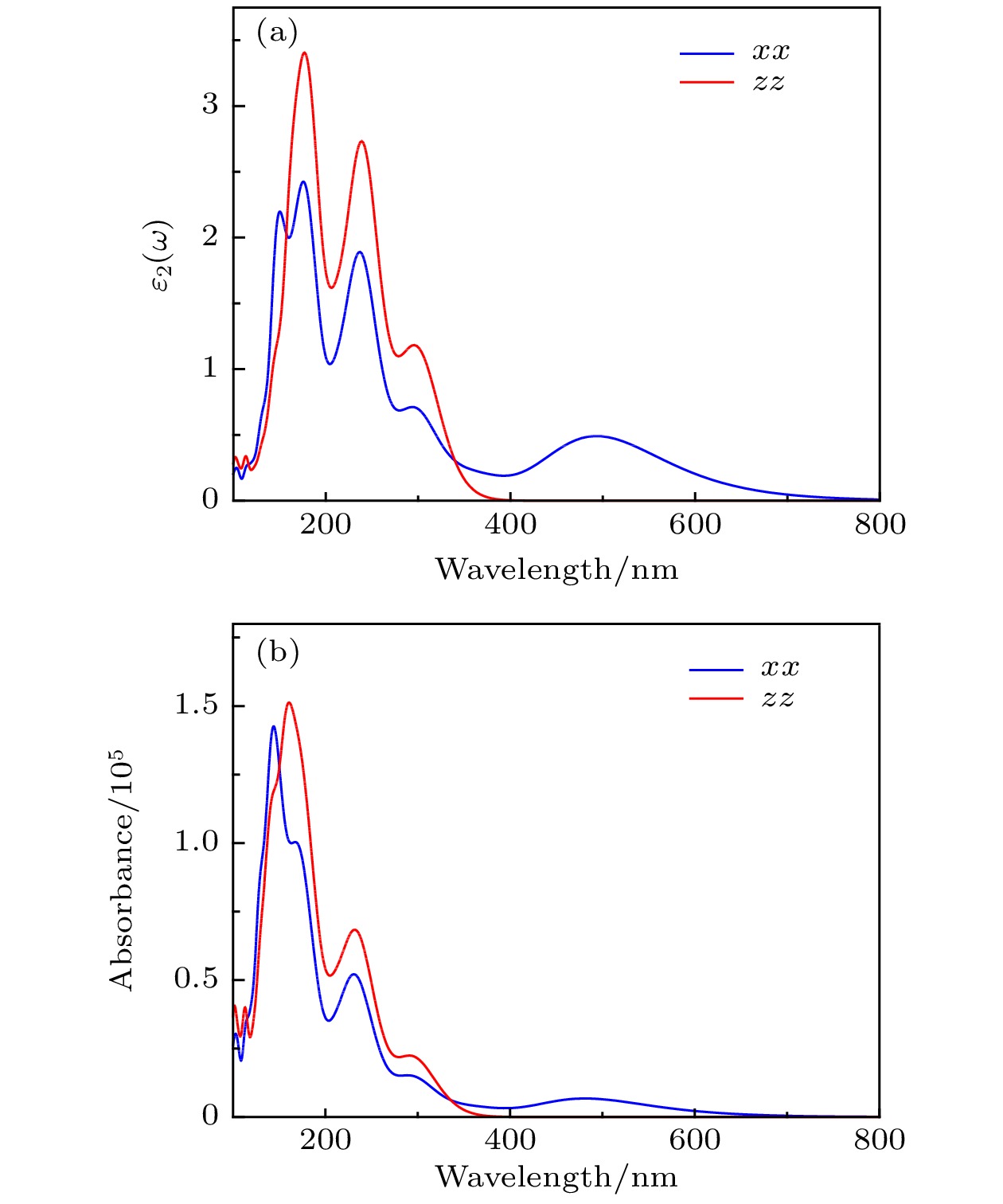
By means of state-of-the-art density functional theory (DFT) computations, We designed a new two-dimensional material TiO2. We further investigated the stability, electronic structure, carrier mobility, and optical properties of monolayer TiO2. Our results show that monolayer TiO2 has good kinetic, thermodynamic and mechanical stability and can exist stably at room temperature. The results were demonstrated using the binding energy, phonon spectrum, molecular dynamics simulation, and elastic constant calculation. The band structure indicates that the monolayer TiO2 is an indirect bandgap semiconductor with energy gaps of 1.19 eV (GGA+PBE) and 2.76 eV (HSE06), respectively. The results of state density show that the Ti-3d state electrons constitute the top of the valence band and Ti-4s state electrons constitute the bottom of the conduction band. The electron states of O atoms contribute very little near the Fermi energy level and are mainly distributed in the deep energy level. In addition, the carrier mobility of monolayer TiO2 is smaller than monolayer MoS2, and the electron and hole mobility can reach 31.09 cm2·V–1·s–1 and 36.29 cm2·V–1·s–1, respectively. Due to the anisotropy of hole mobility and electron mobility, the composite rate of electrons and holes is relatively low. This ensures longer service life and better photocatalytic activity of monolayer TiO2. Furthermore, under the condition of uniaxial strain and biaxial strain, the energy gap of monolayer TiO2 has a clear response. The energy gap is more sensitive to biaxial strain than uniaxial strain, indicating that monolayer TiO2 can be applied to various semiconductor devices. The band-edge potential and optical properties of semiconductors indicate that two-dimensional TiO2 is capable of photo-splitting water production, H2 at –5~2% single/biaxial strain, and O2, H2O2, O3, etc. at –5~5% single/biaxial strain. Moreover, the monolayer TiO2 has a high absorption coefficient for visible and ultraviolet light. In conclusion, the monolayer TiO2 has a potential application prospect in the field of optoelectronic devices and photocatalytic materials in the future.
-
Keywords:
- two-dimensional TiO2 /
- first principles /
- electronic structures /
- optical properties
[1] Hashimoto A, Suenaga K, Gloter A, Urita K, Iijima S 2004 Nature 430 870
 Google Scholar
Google Scholar
[2] Liu L, Zhang J, Zhao J, Liu F 2012 Nanoscale 4 5910
 Google Scholar
Google Scholar
[3] Kvashnin D G, Bellucci S, Chernozatonskii L A 2015 Phys. Chem. Chem. Phys. 17 4354
 Google Scholar
Google Scholar
[4] Liu Y, Duan X, Huang Y, Duan X 2018 Chem. Soc. Rev. 47 6388
 Google Scholar
Google Scholar
[5] Gupta S, Kutana A, Yakobson B I 2018 J. Phys. Chem. Lett. 9 2757
 Google Scholar
Google Scholar
[6] Yuan J, Yu N, Xue K, Miao X 2017 Appl. Surf. Sci. 409 85
 Google Scholar
Google Scholar
[7] Lee C, Wei X, Kysar J W, Hone J 2008 Science 321 385
 Google Scholar
Google Scholar
[8] Wang H, Chan T L, Xie Z 2018 Chem. Commun. (Camb) 54 385
 Google Scholar
Google Scholar
[9] Hartman T, Sofer Z 2019 ACS Nano 13 8566
 Google Scholar
Google Scholar
[10] Li P 2019 Phys. Chem. Chem. Phys. 21 11150
 Google Scholar
Google Scholar
[11] Li L, Yang J 2017 Nanotechnology 28 475701
 Google Scholar
Google Scholar
[12] Yuan J, Xie Q, Yu N, Wang J 2017 Appl. Surf. Sci. 394 625
 Google Scholar
Google Scholar
[13] Zhang S, Yan Z, Li Y, Chen Z, Zeng H 2015 Angew. Chem. Int. Ed. Engl. 54 3112
 Google Scholar
Google Scholar
[14] Zhang D, Xiong Y, Cheng J, Chai J, Liu T, Ba X, Ullah S, Zheng G, Yan M, Cao M S 2020 Sci. Bull. 65 138
 Google Scholar
Google Scholar
[15] Cao M S, Shu J C, Wang X X, Wang X, Zhang M, Yang H J, Fang X Y, Yuan J 2019 Ann. Phys.-Berlin 531 1800390
 Google Scholar
Google Scholar
[16] Cao M S, Wang X X, Zhang M, Shu J C, Cao W Q, Yang H J, Fang X Y, Yuan J 2019 Adv. Funct. Mater. 29 1807398
 Google Scholar
Google Scholar
[17] Matta S K, Zhang C, Jiao Y, O'mullane A, Du A 2018 Nanoscale 10 6369
 Google Scholar
Google Scholar
[18] Zhu Y L, Yuan J H, Song Y Q, Wang S, Xue K H, Xu M, Cheng X M, Miao X S 2019 J. Mater. Sci. 54 11485
 Google Scholar
Google Scholar
[19] Sun Y, Cheng H, Gao S, Sun Z, Liu Q, Liu Q, Lei F, Yao T, He J, Wei S, Xie Y 2012 Angew. Chem. Int. Ed. Engl. 51 8727
 Google Scholar
Google Scholar
[20] 方文玉, 张鹏程, 赵军, 康文斌 2020 69 056301
 Google Scholar
Google Scholar
Fang W Y, Zhang P C, Zhao J, Kang W B 2020 Acta Phys. Sin. 69 056301
 Google Scholar
Google Scholar
[21] Chen Y, Wang L, Wang W, Cao M S 2017 Appl. Catal., B Environ. 209 110
 Google Scholar
Google Scholar
[22] Zhang Y, Kuwahara Y, Mori K, Yamashita H 2019 Chem. Asian J. 14 278
 Google Scholar
Google Scholar
[23] Liang Z, Sun B, Xu X, Cui H, Tian J 2019 Nanoscale 11 12266
 Google Scholar
Google Scholar
[24] Zeng H, Cui X 2015 Chem. Soc. Rev. 44 2629
 Google Scholar
Google Scholar
[25] Guo Z, Zhou J, Zhu L, Sun Z 2016 J. Mater. Chem. A 4 11446
 Google Scholar
Google Scholar
[26] Zhuang H L, Hennig R G 2013 Chem. Mater. 25 3232
 Google Scholar
Google Scholar
[27] Zhang X, Zhao X, Wu D, Jing Y, Zhou Z 2016 Adv. Sci. 3 1600062
 Google Scholar
Google Scholar
[28] Lacerda A M, Larrosa I, Dunn S 2015 Nanoscale 7 12331
 Google Scholar
Google Scholar
[29] Ishida Y, Motokane Y, Tokunaga T, Yonezawa T 2015 Phys. Chem. Chem. Phys. 17 24556
 Google Scholar
Google Scholar
[30] Yoshida T, Niimi S, Yamamoto M, Nomoto T, Yagi S 2015 J. Colloid Interface Sci. 447 278
 Google Scholar
Google Scholar
[31] Liu X, Chen Z, Cao M S 2019 ACS Appl. Energy Mater. 2 5960
 Google Scholar
Google Scholar
[32] Yang J, Jiang Y L, Li L J, Muhire E, Gao M Z 2016 Nanoscale 8 8170
 Google Scholar
Google Scholar
[33] Song S S, Xia B Y, Chen J, Yang J, Shen X, Fan S J, Guo M L, Sun Y M, Zhang X D 2014 RSC Adv. 4 42598
 Google Scholar
Google Scholar
[34] Liu X, Chen Z, Li W, Cao M S 2017 J. Phys. Chem. C 121 20605
 Google Scholar
Google Scholar
[35] Song X F, Hu L F, Li D H, Chen L, Sun Q Q, Zhou P, Zhang D W 2015 Sci Rep 5 15989
 Google Scholar
Google Scholar
[36] Tao J, Luttrell T, Batzill M 2011 Nat. Chem. 3 296
 Google Scholar
Google Scholar
[37] Xie Q, Yuan J, Yu N, Wang L, Wang J 2017 Comput. Mater. Sci. 135 160
 Google Scholar
Google Scholar
[38] Qiu G, Xiao Q, Hu Y, Qin W, Wang D 2004 J. Colloid Interface Sci. 270 127
 Google Scholar
Google Scholar
[39] Yuan J H, Song Y Q, Chen Q, Xue K H, Miao X S 2019 Appl. Surf. Sci. 469 456
 Google Scholar
Google Scholar
[40] Castellanos-Gomez A, Poot M, Steele G A, Van Der Zant H S, Agrait N, Rubio-Bollinger G 2012 Adv. Mater. 24 772
 Google Scholar
Google Scholar
[41] Yuan J, Yu N, Xue K, Miao X 2017 RSC Adv. 7 8654
 Google Scholar
Google Scholar
[42] Song Y Q, Yuan J H, Li L H, Xu M, Wang J F, Xue K H, Miao X S 2019 Nanoscale 11 1131
 Google Scholar
Google Scholar
[43] Xu L C, Du A, Kou L 2016 Phys. Chem. Chem. Phys. 18 27284
 Google Scholar
Google Scholar
[44] Peng R, Ma Y, He Z, Huang B, Kou L, Dai Y 2019 Nano Lett. 19 1227
 Google Scholar
Google Scholar
[45] Li P, You Z, Haugstad G, Cui T 2011 Appl. Phys. Lett. 98 253105
 Google Scholar
Google Scholar
[46] Wang Y, Ma R, Hu K, Kim S, Fang G, Shao Z, Tsukruk V V 2016 ACS Appl. Mater. Interfaces 8 24962
 Google Scholar
Google Scholar
[47] Li Y, Yu C, Gan Y, Kong Y, Jiang P, Zou D F, Li P, Yu X F, Wu R, Zhao H, Gao C F, Li J 2019 Nanotechnology 30 335703
 Google Scholar
Google Scholar
[48] Liu F, Ming P, Li J 2007 Phys. Rev. B 76 064120
 Google Scholar
Google Scholar
[49] Kudin K N, Scuseria G E, Yakobson B I 2001 Phys. Rev. B 64 235406
 Google Scholar
Google Scholar
[50] Kang J, Sahin H, Peeters F M 2015 Phys. Chem. Chem. Phys. 17 27742
 Google Scholar
Google Scholar
[51] Zhao J, Li Y, Ma J 2016 Nanoscale 8 9657
 Google Scholar
Google Scholar
[52] Yuan J, Yu N, Wang J, Xue K-H, Miao X 2018 Appl. Surf. Sci. 436 919
 Google Scholar
Google Scholar
[53] El Mragui A, Logvina Y, Pinto Da Silva L, Zegaoui O, Esteves Da Silva J C G 2019 Materials 1 2
 Google Scholar
Google Scholar
[54] An X, Hu C, Liu H, Qu J 2018 Langmuir 34 1883
 Google Scholar
Google Scholar
[55] Luican-Mayer A, Zhang Y, Dilullo A, Li Y, Fisher B, Ulloa S E, Hla S W 2019 Nanoscale 11 22351
 Google Scholar
Google Scholar
[56] Yu W, Zhang J, Peng T 2016 Appl. Catal., B Environ. 181 220
 Google Scholar
Google Scholar
[57] Zhong L, Chen X, Qi J 2017 Phys. Chem. Chem. Phys. 19 15388
 Google Scholar
Google Scholar
[58] Hua C, Sheng F, Hu Q, Xu Z A, Lu Y, Zheng Y 2018 J. Phys. Chem. Lett. 9 6695
 Google Scholar
Google Scholar
[59] Sarker H P, Rao P M, Huda M N 2019 ChemPhysChem 20 773
 Google Scholar
Google Scholar
[60] Fang W Y, Li P A, Yuan J H, Xue K H, Wang J F 2019 J. Electron. Mater. 49 959
 Google Scholar
Google Scholar
[61] Liu X, Wang Y, Li F, Li Y 2016 Phys. Chem. Chem. Phys. 18 14638
 Google Scholar
Google Scholar
[62] Xiao J, Long M, Li M, Li X, Xu H, Chan K 2015 Phys. Chem. Chem. Phys. 17 6865
 Google Scholar
Google Scholar
[63] Zhang J, Wageh S, Al-Ghamdi A, Yu J 2016 Appl. Catal., B Environ. 192 101
 Google Scholar
Google Scholar
[64] Mogulkoc A, Mogulkoc Y, Kecik D, Durgun E 2018 Phys. Chem. Chem. Phys. 20 21043
 Google Scholar
Google Scholar
-
图 7 (a) 二维TiO2沿a/b方向的总能量与应变量
$\Delta l/l$ 的关系, 采用二次数据拟合二维结构的平面刚度, 黑色和红色曲线表示沿a和b方向的面内刚度; (b), (c)单层TiO2的VBM和CBM随应变量相对真空能级的变化, 采取线性拟合计算形变势Figure 7. (a) The relation between total energy and the applied strain
$\Delta l/l$ along the a/b directions of monolayer TiO2. The quadratic data fitting gives the in-plane stiffness of 2D structures. Black and red curves show the in-plane stiffness along the a and b directions of monolayer TiO2. The shift of VBMs and CBMs for (b-c) monolayer TiO2 with respect to the vacuum energy, as a function of the applied strain along either the a and b direction. The linear fit of the data yields the deformation potential constant.表 1 二维TiO2的结构常数和结合能
Table 1. Structure constants and binding energy of monolayer TiO2.
Material a/b/Å ${\theta _1}$/($^ \circ $) ${\theta _2}$/($^ \circ $) l/Å $\sigma $/Å Ef/eV TiO2 2.89 91.66 68.16 2.01 2.26 –8.11 MoS2 3.18 82.58 80.74 2.41 3.14 –7.35 表 2 二维TiO2有效质量
${m^ * }$ , 形变势常数$E_{_{\rm{d}}}^i$ , 弹性常数${C^{{\rm{2 D}}}}$ 和载流子迁移率${\mu _{{\rm{2 D}}}}$ Table 2. Calculated effective mass
${m^ * }$ , deformation potential constant$E_{_{\rm{d}}}^i$ , elastic modulus${C^{{\rm{2 D}}}}$ , and carrier mobility${\mu _{{\rm{2 D}}}}$ for monolayer TiO2 along the a ($\zeta \to K$ ) and b ($\zeta \to G$ ) directions, where$\zeta $ represents the position of the valence band top and the conduction band bottom.Carrier type $m_a^ * $/${m_{\rm{e}}}$ $m_b^ * $/${m_{\rm{e}}}$ $m_l^ * $/${m_{\rm{e}}}$ $\left| {{E_{la}}} \right|$/ eV $\left| {{E_{lb}}} \right|$/ eV $C_a^{2{\rm{D}}}$/ N·m–1 $C_b^{{\rm{2 D}}}$/ N·m–1 $\mu _a^{2{\rm{D}}}$/ cm2·V–1·s–1 $\mu _b^{2{\rm{D}}}$/ cm2·V–1·s–1 Electrons 3.21 1.39 2.11 3.43 3.38 21.27 21.28 12.92 30.75 Holes 4.73 4.12 4.41 1.26 1.25 21.27 21.28 31.09 36.29 -
[1] Hashimoto A, Suenaga K, Gloter A, Urita K, Iijima S 2004 Nature 430 870
 Google Scholar
Google Scholar
[2] Liu L, Zhang J, Zhao J, Liu F 2012 Nanoscale 4 5910
 Google Scholar
Google Scholar
[3] Kvashnin D G, Bellucci S, Chernozatonskii L A 2015 Phys. Chem. Chem. Phys. 17 4354
 Google Scholar
Google Scholar
[4] Liu Y, Duan X, Huang Y, Duan X 2018 Chem. Soc. Rev. 47 6388
 Google Scholar
Google Scholar
[5] Gupta S, Kutana A, Yakobson B I 2018 J. Phys. Chem. Lett. 9 2757
 Google Scholar
Google Scholar
[6] Yuan J, Yu N, Xue K, Miao X 2017 Appl. Surf. Sci. 409 85
 Google Scholar
Google Scholar
[7] Lee C, Wei X, Kysar J W, Hone J 2008 Science 321 385
 Google Scholar
Google Scholar
[8] Wang H, Chan T L, Xie Z 2018 Chem. Commun. (Camb) 54 385
 Google Scholar
Google Scholar
[9] Hartman T, Sofer Z 2019 ACS Nano 13 8566
 Google Scholar
Google Scholar
[10] Li P 2019 Phys. Chem. Chem. Phys. 21 11150
 Google Scholar
Google Scholar
[11] Li L, Yang J 2017 Nanotechnology 28 475701
 Google Scholar
Google Scholar
[12] Yuan J, Xie Q, Yu N, Wang J 2017 Appl. Surf. Sci. 394 625
 Google Scholar
Google Scholar
[13] Zhang S, Yan Z, Li Y, Chen Z, Zeng H 2015 Angew. Chem. Int. Ed. Engl. 54 3112
 Google Scholar
Google Scholar
[14] Zhang D, Xiong Y, Cheng J, Chai J, Liu T, Ba X, Ullah S, Zheng G, Yan M, Cao M S 2020 Sci. Bull. 65 138
 Google Scholar
Google Scholar
[15] Cao M S, Shu J C, Wang X X, Wang X, Zhang M, Yang H J, Fang X Y, Yuan J 2019 Ann. Phys.-Berlin 531 1800390
 Google Scholar
Google Scholar
[16] Cao M S, Wang X X, Zhang M, Shu J C, Cao W Q, Yang H J, Fang X Y, Yuan J 2019 Adv. Funct. Mater. 29 1807398
 Google Scholar
Google Scholar
[17] Matta S K, Zhang C, Jiao Y, O'mullane A, Du A 2018 Nanoscale 10 6369
 Google Scholar
Google Scholar
[18] Zhu Y L, Yuan J H, Song Y Q, Wang S, Xue K H, Xu M, Cheng X M, Miao X S 2019 J. Mater. Sci. 54 11485
 Google Scholar
Google Scholar
[19] Sun Y, Cheng H, Gao S, Sun Z, Liu Q, Liu Q, Lei F, Yao T, He J, Wei S, Xie Y 2012 Angew. Chem. Int. Ed. Engl. 51 8727
 Google Scholar
Google Scholar
[20] 方文玉, 张鹏程, 赵军, 康文斌 2020 69 056301
 Google Scholar
Google Scholar
Fang W Y, Zhang P C, Zhao J, Kang W B 2020 Acta Phys. Sin. 69 056301
 Google Scholar
Google Scholar
[21] Chen Y, Wang L, Wang W, Cao M S 2017 Appl. Catal., B Environ. 209 110
 Google Scholar
Google Scholar
[22] Zhang Y, Kuwahara Y, Mori K, Yamashita H 2019 Chem. Asian J. 14 278
 Google Scholar
Google Scholar
[23] Liang Z, Sun B, Xu X, Cui H, Tian J 2019 Nanoscale 11 12266
 Google Scholar
Google Scholar
[24] Zeng H, Cui X 2015 Chem. Soc. Rev. 44 2629
 Google Scholar
Google Scholar
[25] Guo Z, Zhou J, Zhu L, Sun Z 2016 J. Mater. Chem. A 4 11446
 Google Scholar
Google Scholar
[26] Zhuang H L, Hennig R G 2013 Chem. Mater. 25 3232
 Google Scholar
Google Scholar
[27] Zhang X, Zhao X, Wu D, Jing Y, Zhou Z 2016 Adv. Sci. 3 1600062
 Google Scholar
Google Scholar
[28] Lacerda A M, Larrosa I, Dunn S 2015 Nanoscale 7 12331
 Google Scholar
Google Scholar
[29] Ishida Y, Motokane Y, Tokunaga T, Yonezawa T 2015 Phys. Chem. Chem. Phys. 17 24556
 Google Scholar
Google Scholar
[30] Yoshida T, Niimi S, Yamamoto M, Nomoto T, Yagi S 2015 J. Colloid Interface Sci. 447 278
 Google Scholar
Google Scholar
[31] Liu X, Chen Z, Cao M S 2019 ACS Appl. Energy Mater. 2 5960
 Google Scholar
Google Scholar
[32] Yang J, Jiang Y L, Li L J, Muhire E, Gao M Z 2016 Nanoscale 8 8170
 Google Scholar
Google Scholar
[33] Song S S, Xia B Y, Chen J, Yang J, Shen X, Fan S J, Guo M L, Sun Y M, Zhang X D 2014 RSC Adv. 4 42598
 Google Scholar
Google Scholar
[34] Liu X, Chen Z, Li W, Cao M S 2017 J. Phys. Chem. C 121 20605
 Google Scholar
Google Scholar
[35] Song X F, Hu L F, Li D H, Chen L, Sun Q Q, Zhou P, Zhang D W 2015 Sci Rep 5 15989
 Google Scholar
Google Scholar
[36] Tao J, Luttrell T, Batzill M 2011 Nat. Chem. 3 296
 Google Scholar
Google Scholar
[37] Xie Q, Yuan J, Yu N, Wang L, Wang J 2017 Comput. Mater. Sci. 135 160
 Google Scholar
Google Scholar
[38] Qiu G, Xiao Q, Hu Y, Qin W, Wang D 2004 J. Colloid Interface Sci. 270 127
 Google Scholar
Google Scholar
[39] Yuan J H, Song Y Q, Chen Q, Xue K H, Miao X S 2019 Appl. Surf. Sci. 469 456
 Google Scholar
Google Scholar
[40] Castellanos-Gomez A, Poot M, Steele G A, Van Der Zant H S, Agrait N, Rubio-Bollinger G 2012 Adv. Mater. 24 772
 Google Scholar
Google Scholar
[41] Yuan J, Yu N, Xue K, Miao X 2017 RSC Adv. 7 8654
 Google Scholar
Google Scholar
[42] Song Y Q, Yuan J H, Li L H, Xu M, Wang J F, Xue K H, Miao X S 2019 Nanoscale 11 1131
 Google Scholar
Google Scholar
[43] Xu L C, Du A, Kou L 2016 Phys. Chem. Chem. Phys. 18 27284
 Google Scholar
Google Scholar
[44] Peng R, Ma Y, He Z, Huang B, Kou L, Dai Y 2019 Nano Lett. 19 1227
 Google Scholar
Google Scholar
[45] Li P, You Z, Haugstad G, Cui T 2011 Appl. Phys. Lett. 98 253105
 Google Scholar
Google Scholar
[46] Wang Y, Ma R, Hu K, Kim S, Fang G, Shao Z, Tsukruk V V 2016 ACS Appl. Mater. Interfaces 8 24962
 Google Scholar
Google Scholar
[47] Li Y, Yu C, Gan Y, Kong Y, Jiang P, Zou D F, Li P, Yu X F, Wu R, Zhao H, Gao C F, Li J 2019 Nanotechnology 30 335703
 Google Scholar
Google Scholar
[48] Liu F, Ming P, Li J 2007 Phys. Rev. B 76 064120
 Google Scholar
Google Scholar
[49] Kudin K N, Scuseria G E, Yakobson B I 2001 Phys. Rev. B 64 235406
 Google Scholar
Google Scholar
[50] Kang J, Sahin H, Peeters F M 2015 Phys. Chem. Chem. Phys. 17 27742
 Google Scholar
Google Scholar
[51] Zhao J, Li Y, Ma J 2016 Nanoscale 8 9657
 Google Scholar
Google Scholar
[52] Yuan J, Yu N, Wang J, Xue K-H, Miao X 2018 Appl. Surf. Sci. 436 919
 Google Scholar
Google Scholar
[53] El Mragui A, Logvina Y, Pinto Da Silva L, Zegaoui O, Esteves Da Silva J C G 2019 Materials 1 2
 Google Scholar
Google Scholar
[54] An X, Hu C, Liu H, Qu J 2018 Langmuir 34 1883
 Google Scholar
Google Scholar
[55] Luican-Mayer A, Zhang Y, Dilullo A, Li Y, Fisher B, Ulloa S E, Hla S W 2019 Nanoscale 11 22351
 Google Scholar
Google Scholar
[56] Yu W, Zhang J, Peng T 2016 Appl. Catal., B Environ. 181 220
 Google Scholar
Google Scholar
[57] Zhong L, Chen X, Qi J 2017 Phys. Chem. Chem. Phys. 19 15388
 Google Scholar
Google Scholar
[58] Hua C, Sheng F, Hu Q, Xu Z A, Lu Y, Zheng Y 2018 J. Phys. Chem. Lett. 9 6695
 Google Scholar
Google Scholar
[59] Sarker H P, Rao P M, Huda M N 2019 ChemPhysChem 20 773
 Google Scholar
Google Scholar
[60] Fang W Y, Li P A, Yuan J H, Xue K H, Wang J F 2019 J. Electron. Mater. 49 959
 Google Scholar
Google Scholar
[61] Liu X, Wang Y, Li F, Li Y 2016 Phys. Chem. Chem. Phys. 18 14638
 Google Scholar
Google Scholar
[62] Xiao J, Long M, Li M, Li X, Xu H, Chan K 2015 Phys. Chem. Chem. Phys. 17 6865
 Google Scholar
Google Scholar
[63] Zhang J, Wageh S, Al-Ghamdi A, Yu J 2016 Appl. Catal., B Environ. 192 101
 Google Scholar
Google Scholar
[64] Mogulkoc A, Mogulkoc Y, Kecik D, Durgun E 2018 Phys. Chem. Chem. Phys. 20 21043
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 15672
- PDF Downloads: 444
- Cited By: 0















 DownLoad:
DownLoad: