-
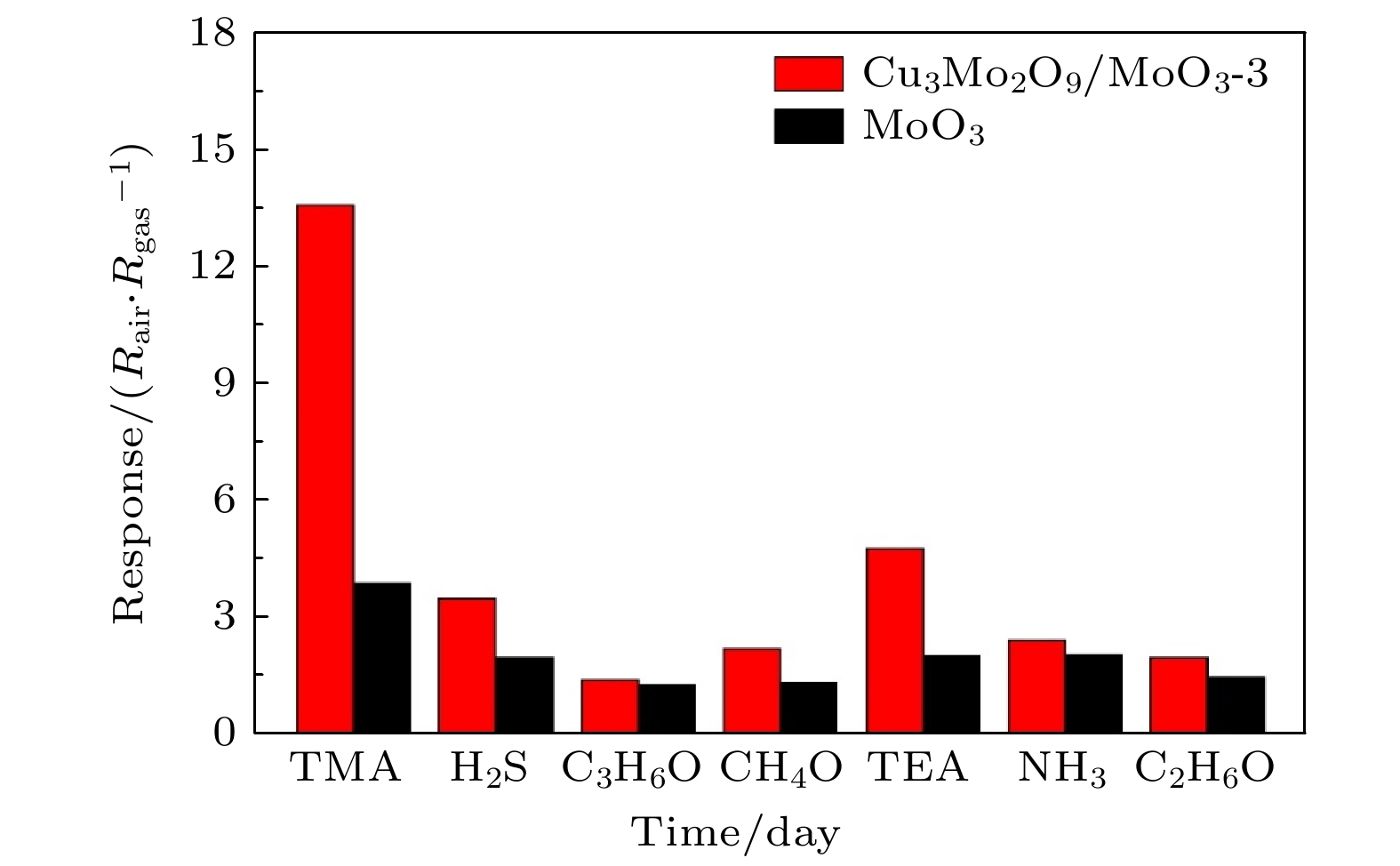
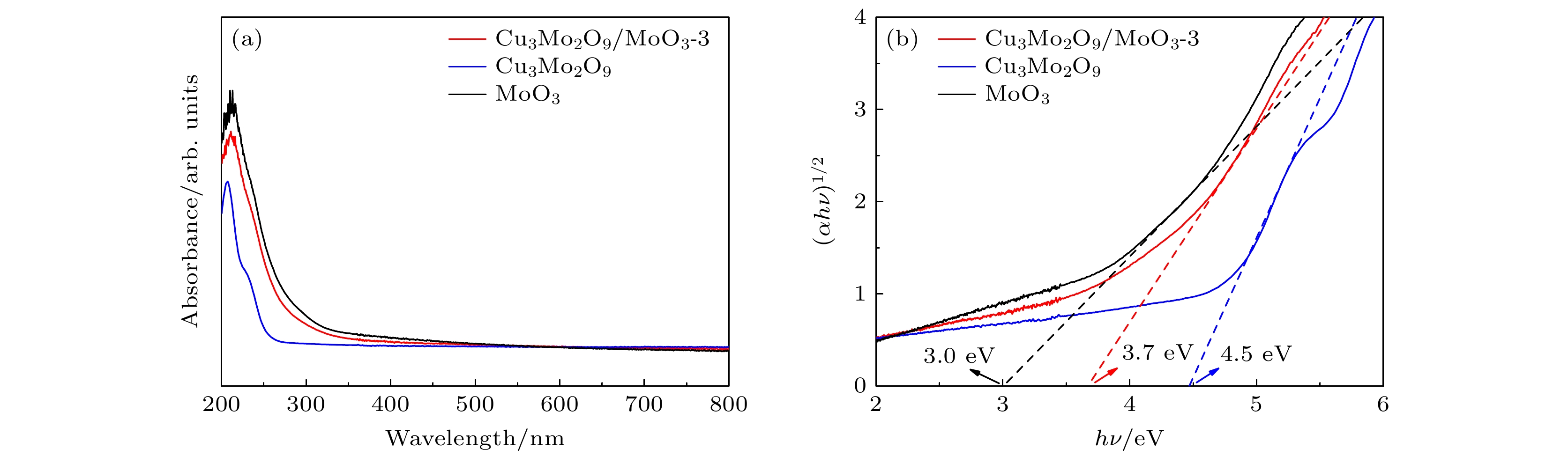
水产品的新鲜度极大地影响着人类的生命及身体健康, 水产品在存放过程中会释放出以三甲胺为代表的胺类气体, 通过检测这类气体的浓度可以监控水产品的新鲜度. 本文以具有优良气体敏感性能的MoO3纳米带作为基体, 通过引入Cu3Mo2O9纳米颗粒制备Cu3Mo2O9/MoO3复合材料, 具有非常好的三甲胺气体敏感性能、快速响应/恢复时间及长期稳定性. 结果表明, 采用这种复合材料制备的气敏元件在50—240 ℃, 质量分数为5×10–6时对三甲胺气体的响应可达到Rair/Rgas = 13.9, 最小检测极限的体积分数为2×10–7. 分布在MoO3纳米带表面的Cu3Mo2O9颗粒与基体形成异质结界面, 利用Cu3Mo2O9的强氧吸附能力与催化效应促进电子与空穴的分离, 显著改善了复合材料的电子输运性能和气敏特性, 为制备高性能MoO3基气敏材料提供了新的策略.
-
关键词:
- 气体传感器 /
- 三甲胺 /
- MoO3纳米带 /
- Cu3Mo2O9颗粒 /
- 异质结
Aquatic products contain an incredibly high nutritional value for the human body and gradually become indispensable ingredients on the Chinese table. Trimethylamine (TMA) from the deterioration of aquatic products can serve as an indicator to measure fish freshness. It is a challenge to develop an instant, fast, convenient, and efficient gas sensor for fish freshness. In this study, a novel Cu3Mo2O9/MoO3 composite gas sensing material is prepared by introducing Cu3Mo2O9 nanoparticles on the surface of MoO3 nanobelts. The results of SEM and TEM images show that the Cu3Mo2O9 nanoparticles are uniformly dispersed. Then, the TMA sensing performance of a resistance-type gas sensor based the prepared Cu3Mo2O9/MoO3 composite is tested at optimal operating temperature (240 °C). the results show that the sensor possesses good response (13.9) at low concentration (5×10–6), with excellent low detection limit (2×10–7). The response time is also significantly shortened. The high sensing performance of Cu3Mo2O9/MoO3 composite is attributed to the heterojunction interface, which promotes the separation of electrons from holes through its strong oxygen adsorption and catalytic effect. This significantly improves the electron transport properties and gas sensing characteristics of the composite material. Electrons flow from MoO3 nanoribbons to Cu3Mo2O9, and the Fermi level reaches equilibrium. This process results in the formation of an electron loss layer underneath MoO3, and the charge transfer channel narrows, which is consistent with previous result. When trimethylamine dissociates on the nanoribbons to release electrons, the balance of the fermi lever is disrupted, and electrons flow from MoO3 to Cu3Mo2O9. As a result, the charge transfer channel becomes thinner, resulting in resistance modulation and increased sensitivity. In addition, the enhancement of trimethylamine sensing performance of Cu3Mo2O9/MoO3 nanocomposite can be explained by the enhancement of gas adsorption and diffusion: MoO3 nanoribbons as a skeleton can effectively disperse Cu3Mo2O9 particles and increase the adsorption capacity of gas molecules. And the enhanced response of Cu3Mo2O9/MoO3 may be due to the good catalytic effect of Cu3Mo2O9, which is conducive to oxygen adsorption. This work provides a new strategy for preparing high-performance MoO3-based gas sensing materials.-
Keywords:
- gas sensors /
- trimethylamine /
- MoO3 nanoribbons /
- Cu3Mo2O9 particles /
- heterojunctions
[1] Wang T S, Zhang S F, Yu Q, Wang S P, Sun P, Lu H Y, Liu F M, Yan X, Lu G Y 2018 ACS Appl. Mater. Interfaces 10 38
 Google Scholar
Google Scholar
[2] Li C, Feng C H, Qu F D, Liu J, Zhu L H, Lin Y, Wang Y, Li F, Zhou J R, Ruan S P 2015 Sens. Actuators B 207 90
 Google Scholar
Google Scholar
[3] Yoon H C, Liang X S, Kang Y C, Lee J H 2015 Sens. Actuators A 207 330
 Google Scholar
Google Scholar
[4] Chu X, Liang S, Chen T 2010 Mater. Chem. Phys. 123 396
 Google Scholar
Google Scholar
[5] Chu X F, Liang S M, Sun W Q 2010 Sens. Actuators, A 148 399
 Google Scholar
Google Scholar
[6] Adamu B I, Falak A, Tian Y, Tan X H, Meng X M, Chen P P, Wang H F, Chu W G 2020 ACS Appl. Mater. Interfaces 12 8411
 Google Scholar
Google Scholar
[7] Xu K, Duan K L, Tang Q, Zhu Q, Zhao W, Yu W, Yang Y, Yu T, Yuan G L 2019 Cryst. Eng. Comm 21 5834
 Google Scholar
Google Scholar
[8] Li Z Q, Song P, Yang Z X, Wang Q 2017 Ceram. Int. 44 3364
 Google Scholar
Google Scholar
[9] Meng D, Li R X, Zhang L, Wang G S, Zhang Y, San X G, Wang X L 2022 Sens. Actuators. 4 87
 Google Scholar
Google Scholar
[10] Wang L P, Jin Z, Luo T, Ding Y, Liu J H, Wang X F, Li M Q 2019 New J. Chem. 43 3619
 Google Scholar
Google Scholar
[11] Ahn H, Noh H J, Kim S B, Overfelt R A, Yoon Y S, KimD J 2010 Mater. Chem. Phys. 124 563
 Google Scholar
Google Scholar
[12] Zhang S, Song P, Zhang J, Li Z Q, Yang Z X, Wang Q 2016 RSC Adv. 6 50423
 Google Scholar
Google Scholar
[13] Kathirvelan J, Vijayaraghavan R, Thomas A 2017 Sens. Rev. 37 147
 Google Scholar
Google Scholar
[14] Gou Y, Yang L, Liu Z, Asiri A M, Hu J, Sun X P 2018 Inorg. Nano-Met. Chem. 57 147
 Google Scholar
Google Scholar
[15] Wang W X, Jin W, Yang S, Jian Z L, Chen W 2021 Sens. Actuators B 15 129583
 Google Scholar
Google Scholar
[16] Dutta D P, Rathore A, Ballal A, Tyagi A K 2015 RSC Adv. 10 10389
 Google Scholar
Google Scholar
[17] Pan H, Jin L, Su H, Zhang B B, Zhang L, Zhang H T, Yang W Q 2017 J. Alloys Compd. 695 2965
 Google Scholar
Google Scholar
[18] Shen S K, Zhang X F, Cheng X L, Xu Y M, Gao S, Zhao H, Zhou X, Huo L H 2019 ACS Appl. Nano Mater. 2 8016
 Google Scholar
Google Scholar
[19] 薄小庆, 刘唱白, 李海英, 刘丽, 郭欣, 刘震, 刘丽丽, 苏畅 2014 63 176803
 Google Scholar
Google Scholar
Bo X Q, Liu C B, Li H Y, Liu L, Guo X, Liu Z, Liu L L, Su C 2014 Acta Phys. Sin. 63 176803
 Google Scholar
Google Scholar
[20] 韩丹, 刘志华, 刘琭琭, 韩晓美, 刘东明, 禚凯, 桑胜波 2022 71 010701
 Google Scholar
Google Scholar
Han D, Liu Z H, Liu L L, Han X M, Liu D M, Gao K, Sang S B 2022 Acta Phys. Sin. 71 010701
 Google Scholar
Google Scholar
[21] Wang J X, Zhou Q, Peng S D, Xu L N, Zeng W 2020 Front. Nanochem. 8 339
 Google Scholar
Google Scholar
[22] Sui L L, Xu Y M, Zhang X F, Cheng X L, Gao S, Zhao H, Cai Z, Huo L H 2015 Sens. Actuators, B 208 73
 Google Scholar
Google Scholar
[23] 秦玉香, 王飞, 沈万江, 胡明 2012 61 057301
 Google Scholar
Google Scholar
Qin Y X, Wang F, Shen W J, Hu M 2012 Acta Phys. Sin. 61 057301
 Google Scholar
Google Scholar
[24] 刘志福, 李培, 程铁栋, 黄文 2020 69 248101
 Google Scholar
Google Scholar
Liu Z F, Li P, Cheng T D, Huang W 2020 Acta Phys. Sin. 69 248101
 Google Scholar
Google Scholar
[25] 李东珂, 贺冰彦, 陈坤权, 皮明雨, 崔玉亭, 张丁可 2019 69 198101
 Google Scholar
Google Scholar
Li D K, He B Y, Chen K Q, Pi M Y, Cui Y T, Zhang D K 2019 Acta Phys. Sin. 69 198101
 Google Scholar
Google Scholar
[26] 艾雯, 胡小会, 潘林, 陈长春, 王一峰, 沈晓冬 2019 68 197101
 Google Scholar
Google Scholar
Ai W, Hu X H, Pan L, Chen C C, Wang Y F, Shen X D 2019 Acta Phys. Sin. 68 197101
 Google Scholar
Google Scholar
[27] Sakaushi K, Thomas J, Kaskel S, Eckert J 2013 Chem. Mater. 25 2557
 Google Scholar
Google Scholar
[28] Rothschild A, Komem Y 2004 Appl. Surf. Sci 95 6374
 Google Scholar
Google Scholar
[29] Yao M S, Tang W X, Wang G E, Nath B, Xu G 2016 Adv. Mater. 28 5229
 Google Scholar
Google Scholar
[30] Lupan O, Postica V, Hoppe M, Wolff N, Polonskyi O, Pauporté T, Viana B, Majérus O, Kienle L, Faupel F, Adelung R 2018 Nanoscale 10 14107
 Google Scholar
Google Scholar
[31] Majhi S M, Lee H J, Choi H N, Cho H Y, Kim J S, Lee C R, Yu Y T 2019 Cryst. Eng. Comm 21 5084
 Google Scholar
Google Scholar
[32] Gao X, Li Y Q, Zeng W, Zhang C F, Wei Y M 2017 J. Mater. Sci. Mater. Electron. 28 18781
 Google Scholar
Google Scholar
[33] Ji H C, Zeng W, Li Y Q 2019 Physica E 114 113646
 Google Scholar
Google Scholar
[34] Lü J X, Chen X L, Chen S S, Li H, Deng H 2019 J. Electroanal. Chem. 842 161
 Google Scholar
Google Scholar
[35] Li Z Q, Wang W J, Zhao Z C, Liu X R, Song P 2017 Mater. Sci. Semicond. Process. 66 33
 Google Scholar
Google Scholar
[36] Li Z Q, Wang W J, Zhao Z F, Liu X R, Song P 2017 RSC Adv. 7 28366
 Google Scholar
Google Scholar
[37] Yang S, Liu Y L, Chen W, Jin W, Zhou J, Zhang H, Galina S Z 2016 Sens. Actuators, B 226 478
 Google Scholar
Google Scholar
[38] Zhang F D, Dong X, Cheng X L, Xu Y M, Zhang X F, Huo L H 2019 ACS Appl. Mater. Interfaces 11 11755
 Google Scholar
Google Scholar
-
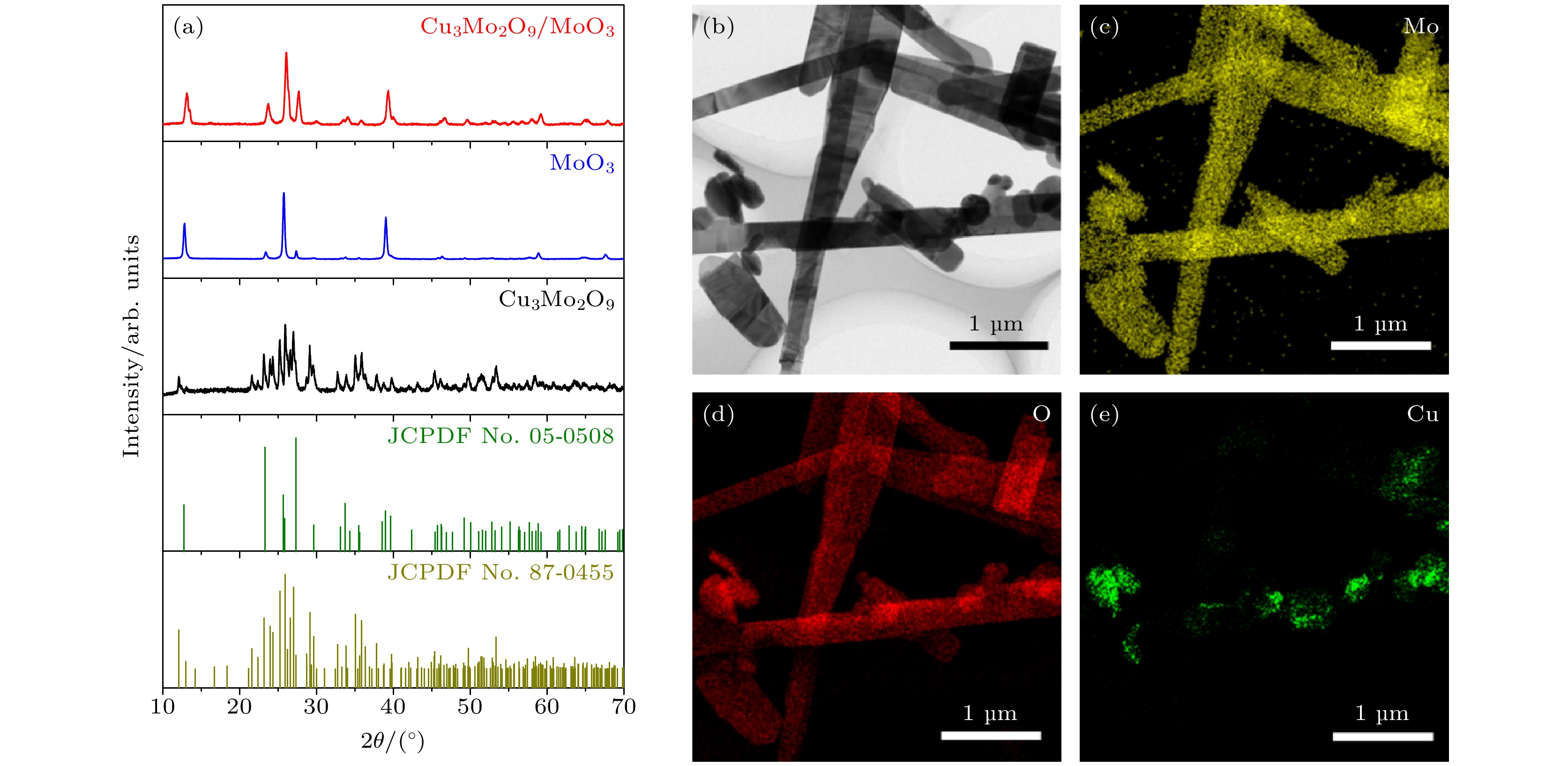
图 1 (a) MoO3纳米带、Cu3Mo2O9颗粒及Cu3Mo2O9/MoO3纳米复合材料的XRD图谱; (b) Cu3Mo2O9/MoO3纳米复合材料的TEM图像; (c)—(e) 元素映射图像: Cu3Mo2O9/MoO3纳米复合材料的Mo, O, Cu图像
Fig. 1. (a) XRD patterns of MoO3 nanobelts, Cu3Mo2O9 particle, and Cu3Mo2O9/MoO3 nanocomposites; (b) TEM image of Cu3Mo2O9/MoO3 nanocomposites; (c)–(e) Mo, O and Cu element mapping images of Cu3Mo2O9/MoO3 nanocomposites.
图 2 (a), (b) 纯MoO3纳米带和Cu3Mo2O9/MoO3纳米复合材料的SEM图像; (c) Cu3Mo2O9/MoO3纳米复合材料的TEM图像 (插图为MoO3纳米带和Cu3Mo2O9颗粒的HRTEM图)
Fig. 2. (a), (b) SEM image of pure MoO3 nanobelts and Cu3Mo2O9/MoO3 nanocomposites; (c) TEM image of Cu3Mo2O9/MoO3 nanocomposites (Inset shows HRTEM patten of the MoO3 nanobelts and Cu3Mo2O9 particle).
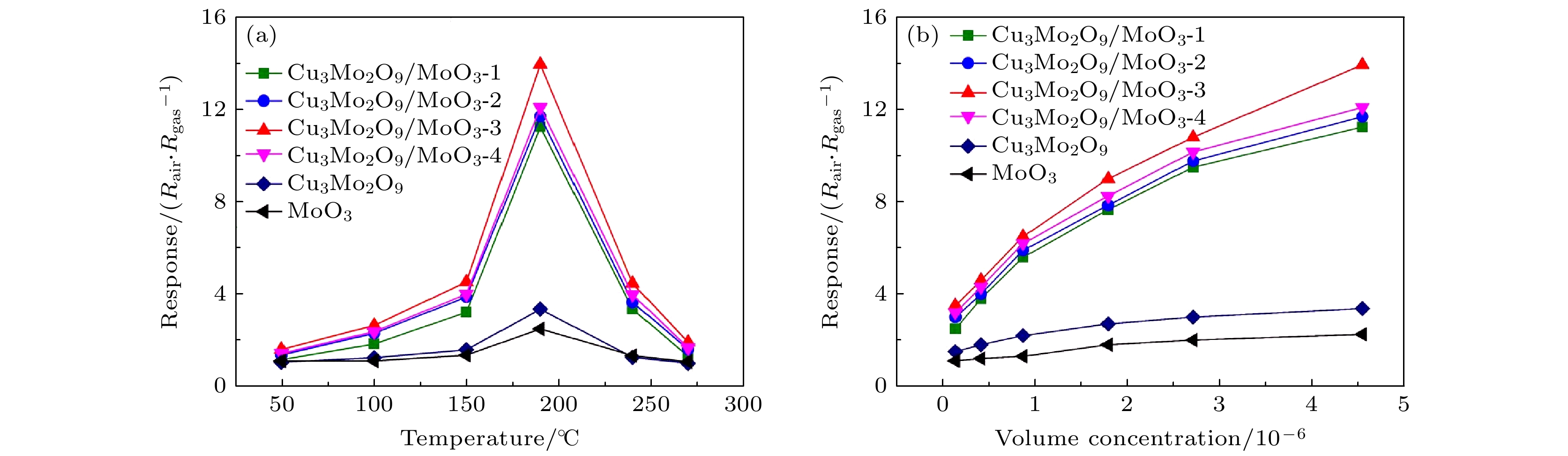
图 4 (a) 不同工作温度下MoO3纳米带、Cu3Mo2O9颗粒和Cu3Mo2O9/MoO3纳米复合材料对体积分数为5×10–6 的TMA的响应; (b) 190 ℃下MoO3纳米带、Cu3Mo2O9颗粒和Cu3Mo2O9/MoO3纳米复合材料对不同浓度TMA的响应折线图
Fig. 4. (a) Response of MoO3 nanobelts, Cu3Mo2O9 particle, and Cu3Mo2O9/MoO3 nanocomposites to TMA with a volume fraction of 5×10–6 at different working temperatures; (b) the corresponding line chart of MoO3 nanobelts, Cu3Mo2O9 particle, and Cu3Mo2O9/MoO3 nanocomposites to different concentrations of TMA at 190 ℃.
图 5 (a) MoO3纳米带、Cu3Mo2O9颗粒和Cu3Mo2O9/MoO3纳米复合材料在190 ℃下对不同浓度TMA的实时响应/恢复曲线; (b) Cu3Mo2O9/MoO3纳米复合材料对体积分数为5×10–6的TMA的响应/恢复时间
Fig. 5. (a) Real-time response/recovery curves of MoO3 nanobelts, Cu3Mo2O9 particle, and Cu3Mo2O9/MoO3 nanocomposites to different concentrations of TMA at 190 ℃; (b) response/recovery time of Cu3Mo2O9/MoO3 nanocomposites to TMA with a volume fraction of 5×10–6.
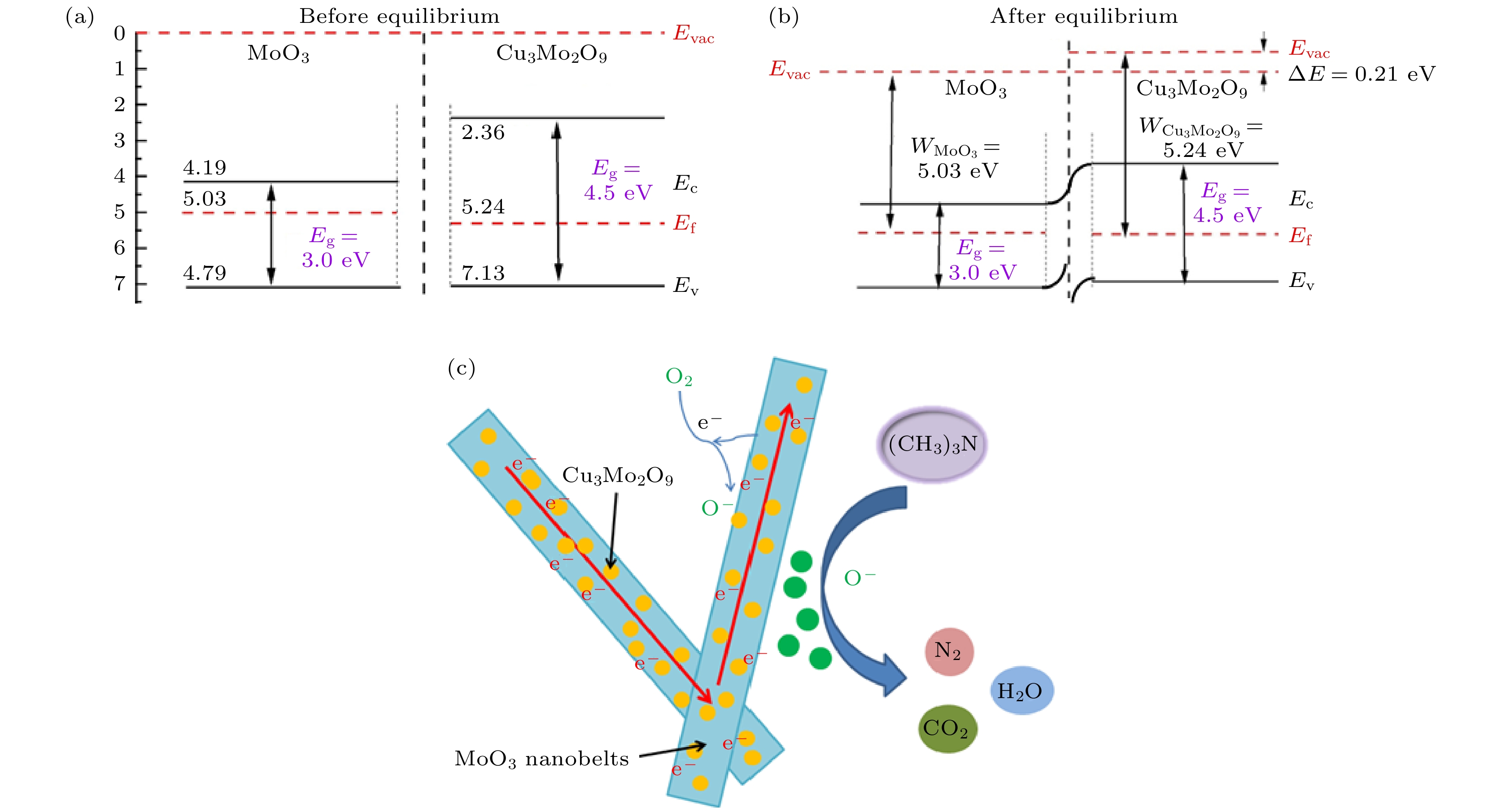
图 11 Cu3Mo2O9/MoO3纳米复合材料体系的能带图 (a) 平衡前 (b) 平衡后 (Evac, 真空水平; Ef, 费米能级; Ec, 导带底部; Ev, 价电子带顶部; Eg, 带隙). (c) Cu3Mo2O9/MoO3纳米复合材料暴露于TMA的示意图
Fig. 11. Energy band diagrams of Cu3Mo2O9/MoO3 nanocomposites system: (a) Before and (b) after equilibrium (Evac, the vacuum level; Ef, Fermi level; Ec, the bottom of conduction band; Ev, the top of valence band; Eg, band gap). (c) Schematic diagram of Cu3Mo2O9/MoO3 nanocomposites exposed to TMA.
表 1 不同材料对TMA的气敏性能对比
Table 1. Comparison of gas-sensing performance of gas towards TMA.
-
[1] Wang T S, Zhang S F, Yu Q, Wang S P, Sun P, Lu H Y, Liu F M, Yan X, Lu G Y 2018 ACS Appl. Mater. Interfaces 10 38
 Google Scholar
Google Scholar
[2] Li C, Feng C H, Qu F D, Liu J, Zhu L H, Lin Y, Wang Y, Li F, Zhou J R, Ruan S P 2015 Sens. Actuators B 207 90
 Google Scholar
Google Scholar
[3] Yoon H C, Liang X S, Kang Y C, Lee J H 2015 Sens. Actuators A 207 330
 Google Scholar
Google Scholar
[4] Chu X, Liang S, Chen T 2010 Mater. Chem. Phys. 123 396
 Google Scholar
Google Scholar
[5] Chu X F, Liang S M, Sun W Q 2010 Sens. Actuators, A 148 399
 Google Scholar
Google Scholar
[6] Adamu B I, Falak A, Tian Y, Tan X H, Meng X M, Chen P P, Wang H F, Chu W G 2020 ACS Appl. Mater. Interfaces 12 8411
 Google Scholar
Google Scholar
[7] Xu K, Duan K L, Tang Q, Zhu Q, Zhao W, Yu W, Yang Y, Yu T, Yuan G L 2019 Cryst. Eng. Comm 21 5834
 Google Scholar
Google Scholar
[8] Li Z Q, Song P, Yang Z X, Wang Q 2017 Ceram. Int. 44 3364
 Google Scholar
Google Scholar
[9] Meng D, Li R X, Zhang L, Wang G S, Zhang Y, San X G, Wang X L 2022 Sens. Actuators. 4 87
 Google Scholar
Google Scholar
[10] Wang L P, Jin Z, Luo T, Ding Y, Liu J H, Wang X F, Li M Q 2019 New J. Chem. 43 3619
 Google Scholar
Google Scholar
[11] Ahn H, Noh H J, Kim S B, Overfelt R A, Yoon Y S, KimD J 2010 Mater. Chem. Phys. 124 563
 Google Scholar
Google Scholar
[12] Zhang S, Song P, Zhang J, Li Z Q, Yang Z X, Wang Q 2016 RSC Adv. 6 50423
 Google Scholar
Google Scholar
[13] Kathirvelan J, Vijayaraghavan R, Thomas A 2017 Sens. Rev. 37 147
 Google Scholar
Google Scholar
[14] Gou Y, Yang L, Liu Z, Asiri A M, Hu J, Sun X P 2018 Inorg. Nano-Met. Chem. 57 147
 Google Scholar
Google Scholar
[15] Wang W X, Jin W, Yang S, Jian Z L, Chen W 2021 Sens. Actuators B 15 129583
 Google Scholar
Google Scholar
[16] Dutta D P, Rathore A, Ballal A, Tyagi A K 2015 RSC Adv. 10 10389
 Google Scholar
Google Scholar
[17] Pan H, Jin L, Su H, Zhang B B, Zhang L, Zhang H T, Yang W Q 2017 J. Alloys Compd. 695 2965
 Google Scholar
Google Scholar
[18] Shen S K, Zhang X F, Cheng X L, Xu Y M, Gao S, Zhao H, Zhou X, Huo L H 2019 ACS Appl. Nano Mater. 2 8016
 Google Scholar
Google Scholar
[19] 薄小庆, 刘唱白, 李海英, 刘丽, 郭欣, 刘震, 刘丽丽, 苏畅 2014 63 176803
 Google Scholar
Google Scholar
Bo X Q, Liu C B, Li H Y, Liu L, Guo X, Liu Z, Liu L L, Su C 2014 Acta Phys. Sin. 63 176803
 Google Scholar
Google Scholar
[20] 韩丹, 刘志华, 刘琭琭, 韩晓美, 刘东明, 禚凯, 桑胜波 2022 71 010701
 Google Scholar
Google Scholar
Han D, Liu Z H, Liu L L, Han X M, Liu D M, Gao K, Sang S B 2022 Acta Phys. Sin. 71 010701
 Google Scholar
Google Scholar
[21] Wang J X, Zhou Q, Peng S D, Xu L N, Zeng W 2020 Front. Nanochem. 8 339
 Google Scholar
Google Scholar
[22] Sui L L, Xu Y M, Zhang X F, Cheng X L, Gao S, Zhao H, Cai Z, Huo L H 2015 Sens. Actuators, B 208 73
 Google Scholar
Google Scholar
[23] 秦玉香, 王飞, 沈万江, 胡明 2012 61 057301
 Google Scholar
Google Scholar
Qin Y X, Wang F, Shen W J, Hu M 2012 Acta Phys. Sin. 61 057301
 Google Scholar
Google Scholar
[24] 刘志福, 李培, 程铁栋, 黄文 2020 69 248101
 Google Scholar
Google Scholar
Liu Z F, Li P, Cheng T D, Huang W 2020 Acta Phys. Sin. 69 248101
 Google Scholar
Google Scholar
[25] 李东珂, 贺冰彦, 陈坤权, 皮明雨, 崔玉亭, 张丁可 2019 69 198101
 Google Scholar
Google Scholar
Li D K, He B Y, Chen K Q, Pi M Y, Cui Y T, Zhang D K 2019 Acta Phys. Sin. 69 198101
 Google Scholar
Google Scholar
[26] 艾雯, 胡小会, 潘林, 陈长春, 王一峰, 沈晓冬 2019 68 197101
 Google Scholar
Google Scholar
Ai W, Hu X H, Pan L, Chen C C, Wang Y F, Shen X D 2019 Acta Phys. Sin. 68 197101
 Google Scholar
Google Scholar
[27] Sakaushi K, Thomas J, Kaskel S, Eckert J 2013 Chem. Mater. 25 2557
 Google Scholar
Google Scholar
[28] Rothschild A, Komem Y 2004 Appl. Surf. Sci 95 6374
 Google Scholar
Google Scholar
[29] Yao M S, Tang W X, Wang G E, Nath B, Xu G 2016 Adv. Mater. 28 5229
 Google Scholar
Google Scholar
[30] Lupan O, Postica V, Hoppe M, Wolff N, Polonskyi O, Pauporté T, Viana B, Majérus O, Kienle L, Faupel F, Adelung R 2018 Nanoscale 10 14107
 Google Scholar
Google Scholar
[31] Majhi S M, Lee H J, Choi H N, Cho H Y, Kim J S, Lee C R, Yu Y T 2019 Cryst. Eng. Comm 21 5084
 Google Scholar
Google Scholar
[32] Gao X, Li Y Q, Zeng W, Zhang C F, Wei Y M 2017 J. Mater. Sci. Mater. Electron. 28 18781
 Google Scholar
Google Scholar
[33] Ji H C, Zeng W, Li Y Q 2019 Physica E 114 113646
 Google Scholar
Google Scholar
[34] Lü J X, Chen X L, Chen S S, Li H, Deng H 2019 J. Electroanal. Chem. 842 161
 Google Scholar
Google Scholar
[35] Li Z Q, Wang W J, Zhao Z C, Liu X R, Song P 2017 Mater. Sci. Semicond. Process. 66 33
 Google Scholar
Google Scholar
[36] Li Z Q, Wang W J, Zhao Z F, Liu X R, Song P 2017 RSC Adv. 7 28366
 Google Scholar
Google Scholar
[37] Yang S, Liu Y L, Chen W, Jin W, Zhou J, Zhang H, Galina S Z 2016 Sens. Actuators, B 226 478
 Google Scholar
Google Scholar
[38] Zhang F D, Dong X, Cheng X L, Xu Y M, Zhang X F, Huo L H 2019 ACS Appl. Mater. Interfaces 11 11755
 Google Scholar
Google Scholar
计量
- 文章访问数: 4845
- PDF下载量: 85
- 被引次数: 0













 下载:
下载: