-
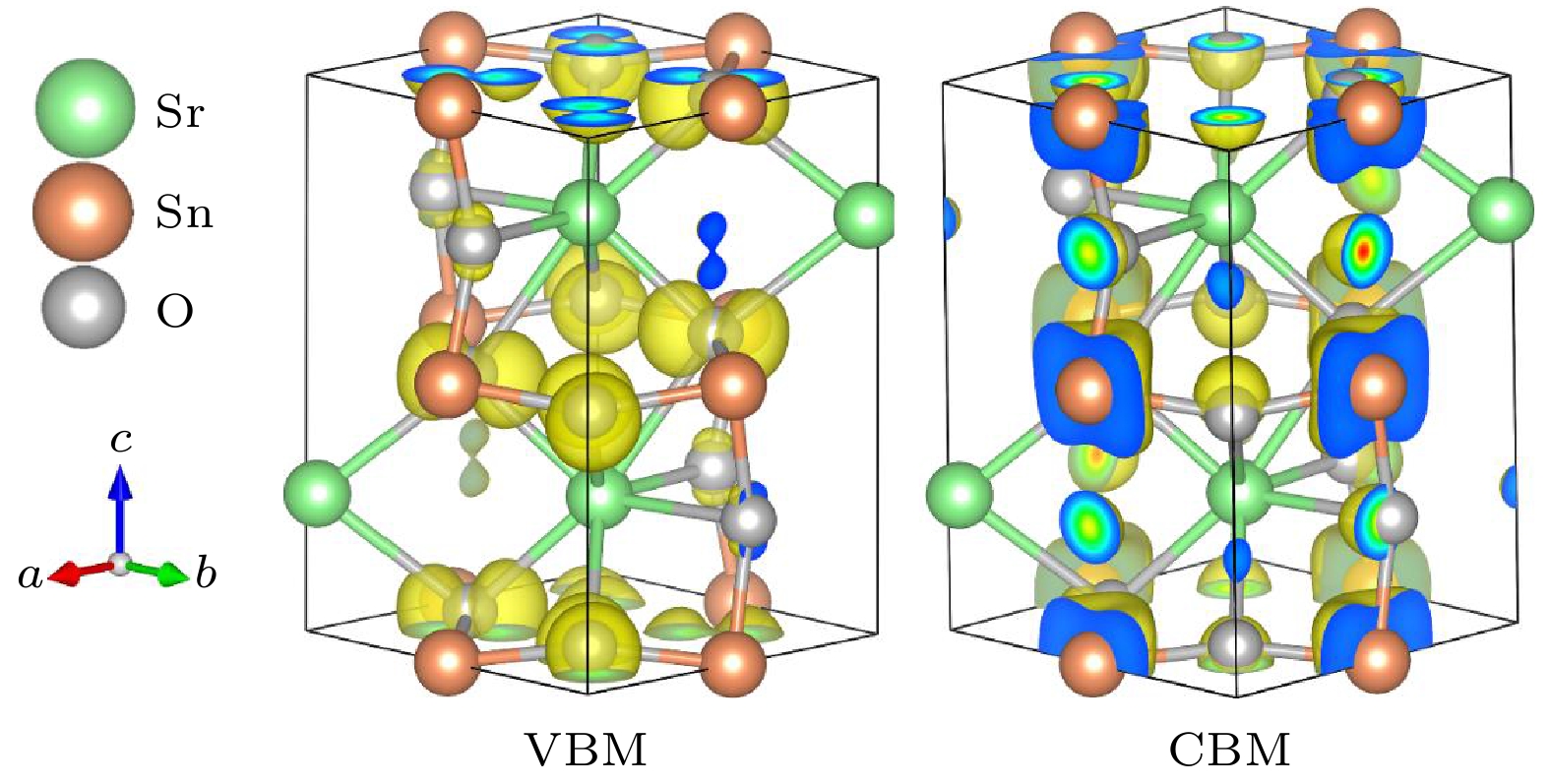
SrSnO3是一种钙钛矿结构的宽带隙半导体, 透明性高、无毒且价格低廉, 是一种有前景的透明导电氧化物的候选者. 本文通过第一性原理计算, 获得了SrSnO3的电子结构, 着重讨论了SrSnO3的本征缺陷、外界元素掺杂的缺陷形成能及过渡能级, 筛选出适宜的掺杂元素并指出了对应的实验制备环境, 进一步根据带边能量位置对其电导性能机制进行了探讨. 计算结果表明, SrSnO3是一种基础带隙为3.55 eV、光学带隙为4.10 eV的间接带隙半导体, 具有良好的透明性, 电子的有效质量轻, 利于n型电导. 在富金属贫氧条件下, As, Sb掺杂SrSnO3可以提升n型电导率; SrSnO3的价带顶位于–7.5 eV处, 导带底位于–4.0 eV处, 其价带顶和导带底的能量位置均相对较低, 解释了其易于n型掺杂而难于p型掺杂, 符合宽带隙半导体材料的掺杂规律. 最后, Sb掺杂SrSnO3被提出为有前景的廉价n型透明导电材料.
-
关键词:
- 第一性原理计算 /
- n型透明导电氧化物SrSnO3 /
- 缺陷形成能 /
- 带边能量位置
As a wide band gap semiconductor with perovskite structure, SnSnO3 is regarded as a promising candidate of transparent conductive oxides due to its superior properties like high transparency, non-toxicity and low price. In this work, the electronic structure of SrSnO3 is obtained through first-principles calculations based on HSE06 hybrid functional. Especially, we investigate the defect formation energy and transition levels of the intrinsic and external defects in SrSnO3. The intrinsic defects including the anti-site defects (SrSn and SnSr), the vacancy defects (VSr, VSn, and VO), and the interstitial defects (Sri, Sni and Oi) are considered while the external doping defects are taken into account, including the substitution of Li, Na, K, Al, Ga, In for Sr site, Al, Ga, In, P, As, Sb for Sn site, and N, P at O site. Subsequently, the suitable doping elements and the corresponding experimental preparation environments are pointed out. Furthermore, we discuss the mechanism of its conductance according to the energy positions of the band edges. Our calculation results demonstrate that SrSnO3 is an indirect-type semiconductor with a fundamental band gap of 3.55 eV and an optical band gap of 4.10 eV and then has a good visible light transmittance. Its valence band maximum (VBM) comes from O-2p state while its conduction band minimum (CBM) mainly originates from Sn-5s state. In consistent with the delocalized Sn-5s state at CBM, the electron effective mass is light and isotropic, which is beneficial to n-type conductance. The n-type intrinsic defects SnSr and Vo have lower defect formation energy than the p-type intrinsic defects under O-poor condition while the n-type and p-type defects with low defect formation energy are almost equal under O-rich condition. Moreover, the transition levels of SnSr and VO are both deep. Therefore, SrSnO3 cannot have a good conductance without external doping. Our calculations also demonstrate that it is hard to produce an efficient p-type external doping due to the compensation effect by VO. On the other hand, substitution of As or Sb for Sn site can result in an effective n-type external doping due to their low defect formation energy and shallow transition levels. According to the low energy positions of VBM (–7.5 eV) and CBM (–4.0 eV) of SrSnO3, we explain the reason why it is easy to realize an n-type conductance but hard to produce a high-performance p-type conductance, which follows the doping rules for wide band gap semiconductors. Finally, Sb-doped SrSnO3 is proposed as a promising candidate for n-type transparent conductive materials.-
Keywords:
- first-principles calculations /
- n-type transparent conductive oxide SrSnO3 /
- defect formation energy /
- band-edge energy position
[1] Bitla Y, Chu Y H 2020 Nanoscale 12 18523
 Google Scholar
Google Scholar
[2] Stadler A 2012 Materials 5 661
 Google Scholar
Google Scholar
[3] Cho H, Jeong S H, Park M H, Kim Y H, Wolf C, Lee C L, Heo Jin H, Sadhanala A, Myoung N, Yoo S, Im Sang H, Friend Richard H, Lee T W 2015 Science 350 1222
 Google Scholar
Google Scholar
[4] Tan Z K, Moghaddam R S, Lai M L, Docampo P, Higler R, Deschler F, Price M, Sadhanala A, Pazos L M, Credgington D, Hanusch F, Bein T, Snaith H J, Friend R H 2014 Nat. Nanotechnol. 9 687
 Google Scholar
Google Scholar
[5] Pan Z W, Dai Z R, Wang Z L 2001 Science 291 1947
 Google Scholar
Google Scholar
[6] Comini E, Faglia G, Sberveglieri G, Pan Z, Wang Z L 2002 Appl. Phys. Lett. 81 1869
 Google Scholar
Google Scholar
[7] Batzill M, Diebold U 2005 Prog. Surf. Sci. 79 47
 Google Scholar
Google Scholar
[8] Dou L, Yang Y, You J, Hong Z, Chang W H, Li G, Yang Y 2014 Nat. Commun. 5 5404
 Google Scholar
Google Scholar
[9] Lee Michael M, Teuscher J, Miyasaka T, Murakami Takurou N, Snaith Henry J 2012 Science 338 643
 Google Scholar
Google Scholar
[10] Baedeker K 1907 Ann. Phys. 327 749
 Google Scholar
Google Scholar
[11] Minami T 2008 Thin Solid Films 516 1314
 Google Scholar
Google Scholar
[12] Minami T 2008 Thin Solid Films 516 5822
 Google Scholar
Google Scholar
[13] Chen M J, Yang J R, Shiojiri M 2012 Semicond. Sci. Technol. 27 074005
 Google Scholar
Google Scholar
[14] Du X, Mei Z, Liu Z, Guo Y, Zhang T, Hou Y, Zhang Z, Xue Q, Kuznetsov A Y 2009 Adv. Mater. 21 4625
 Google Scholar
Google Scholar
[15] 王延峰, 谢希成, 刘晓洁, 韩冰, 武晗晗, 连宁宁, 杨富, 宋庆功, 裴海林, 李俊杰 2020 69 197801
 Google Scholar
Google Scholar
Wang Y F, Xie X C, Liu X J, Han B, Wu H H, Lian N N, Yang F, Song Q G, Pei H L, Li J J 2020 Acta. Phys. Sin. 69 197801
 Google Scholar
Google Scholar
[16] Wu F, Tong X, Zhao Z, Gao J, Zhou Y, Kelly P 2017 J. Alloys Compd. 695 765
 Google Scholar
Google Scholar
[17] Fleischer K, Norton E, Mullarkey D, Caffrey D, Shvets I V 2017 Materials 10 1019
 Google Scholar
Google Scholar
[18] Zhang K H L, Xi K, Blamire M G, Egdell R G 2016 J. Phys. Condens. Mater. 28 383002
 Google Scholar
Google Scholar
[19] Cao R, Deng H X, Luo J W 2019 ACS Appl. Mater. Interfaces 11 24837
 Google Scholar
Google Scholar
[20] Dixon S C, Scanlon D O, Carmalt C J, Parkin I P 2016 J. Mater. Chem. C 4 6946
 Google Scholar
Google Scholar
[21] Bel Hadj Tahar R, Ban T, Ohya Y, Takahashi Y 1998 J. Appl. Phys. 83 2631
 Google Scholar
Google Scholar
[22] Selopal G S, Milan R, Ortolani L, Morandi V, Rizzoli R, Sberveglieri G, Veronese G P, Vomiero A, Concina I 2015 Sol. Energy Mater. Sol. Cells 135 99
 Google Scholar
Google Scholar
[23] 王延峰, 张晓丹, 黄茜, 杨富, 孟旭东, 宋庆功, 赵颖 2013 62 247802
 Google Scholar
Google Scholar
Wang Y F, Zhang X D, Huang Q, Yang F, Meng X D, Song Q G, Zhao Y 2013 Acta Phys. Sin. 62 247802
 Google Scholar
Google Scholar
[24] 王延峰, 孟旭东, 郑伟, 宋庆功, 翟昌鑫, 郭兵, 张越, 杨富, 南景宇 2016 65 087802
 Google Scholar
Google Scholar
Wang Y F, Meng X D, Zheng W, Song Q G, Zhai C X, Guo B, Zhang Y, Yang F, Nan J Y 2016 Acta Phys. Sin. 65 087802
 Google Scholar
Google Scholar
[25] Ong K P, Fan X, Subedi A, Sullivan M B, Singh D J 2015 APL Mater. 3 062505
 Google Scholar
Google Scholar
[26] Riza M A, Ibrahim M A, Ahamefula U C, Mat Teridi M A, Ahmad Ludin N, Sepeai S, Sopian K 2016 Sol. Energy 137 371
 Google Scholar
Google Scholar
[27] Liu Q, Dai J, Zhang X, Zhu G, Liu Z, Ding G 2011 Thin Solid Films 519 6059
 Google Scholar
Google Scholar
[28] Liu Q, Jin F, Gao G, Wang W 2017 J. Alloys Compd. 717 62
 Google Scholar
Google Scholar
[29] Kumar Y, Kumar R, Asokan K, Choudhary R J, Phase D M, Singh A P 2021 J. Mater. Sci. -Mater. Electron. 32 11835
 Google Scholar
Google Scholar
[30] Wei M, Sanchela A V, Feng B, Ikuhara Y, Cho H J, Ohta H 2020 Appl. Phys. Lett. 116 022103
 Google Scholar
Google Scholar
[31] Liu Y, Zhou Y, Jia D, Zhao J, Wang B, Cui Y, Li Q, Liu B 2020 J. Mater. Sci. Technol. 42 212
 Google Scholar
Google Scholar
[32] Rahman A B A, Sarjadi M S, Alias A, Ibrahim M A 2019 J. Phys:Conf. Ser. 1358 012043
 Google Scholar
Google Scholar
[33] Kumar Y, Kumar R, Choudhary R J, Thakur A, Singh A P 2020 Ceram. Int. 46 17569
 Google Scholar
Google Scholar
[34] Hohenberg P, Kohn W 1964 Phys. Rev. 136 B864
 Google Scholar
Google Scholar
[35] Kohn W, Sham L J 1965 Phys. Rev. 140 A1133
 Google Scholar
Google Scholar
[36] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[37] Heyd J, Scuseria G E 2004 J. Chem. Phys. 121 1187
 Google Scholar
Google Scholar
[38] Green M A, Prassides K, Day P, Neumann D A 2000 J. Inorg. Mater. 2 35
 Google Scholar
Google Scholar
[39] Schumann T, Raghavan S, Ahadi K, Kim H, Stemmer S 2016 J. Vac. Sci. Technol., A 34 050601
[40] Mizoguchi H, Eng H W, Woodward P M 2004 Inorg. Chem. 43 1667
 Google Scholar
Google Scholar
[41] Zhang S B, Wei S H, Zunger A 2001 Phys. Rev. B 63 075205
 Google Scholar
Google Scholar
[42] Lany S, Zunger A 2008 Phys. Rev. B 78 235104
 Google Scholar
Google Scholar
[43] Singh M K, Hong J W, Karan N K, Jang H M, Katiyar R S, Redfern S A T, Scott J F 2010 J. Phys. Condens. Matter. 22 095901
 Google Scholar
Google Scholar
[44] Gao Q, Chen H, Li K, Liu Q 2018 ACS Appl. Mater. Interfaces 10 27503
 Google Scholar
Google Scholar
[45] KC S, Rowberg A J E, Weston L, Van de Walle C G 2019 J. Appl. Phys. 126 195701
 Google Scholar
Google Scholar
[46] Putz M V, Russo N, Sicilia E 2005 Theor. Chem. Acc. 114 38
 Google Scholar
Google Scholar
[47] Huang D, Xu J P, Jiang J W, Zhao Y J, Peng B L, Zhou W Z, Guo J 2017 Phys. Lett. A 381 2743
 Google Scholar
Google Scholar
[48] Hu S, Xia B, Yan Y, Xiao Z 2020 Phys. Rev. Mater. 4 115201
 Google Scholar
Google Scholar
[49] Schein F L, von Wenckstern H, Grundmann M 2013 Appl. Phys. Lett. 102 092109
 Google Scholar
Google Scholar
[50] Arai T, Iimura S, Kim J, Toda Y, Ueda S, Hosono H 2017 J. Am. Chem. Soc. 139 17175
 Google Scholar
Google Scholar
[51] Zhang Z, Guo Y, Robertson J 2022 Chem. Mater. 34 643
 Google Scholar
Google Scholar
[52] Yan Y, Wei S H 2008 Phys. Status Solidi B 245 641
 Google Scholar
Google Scholar
-
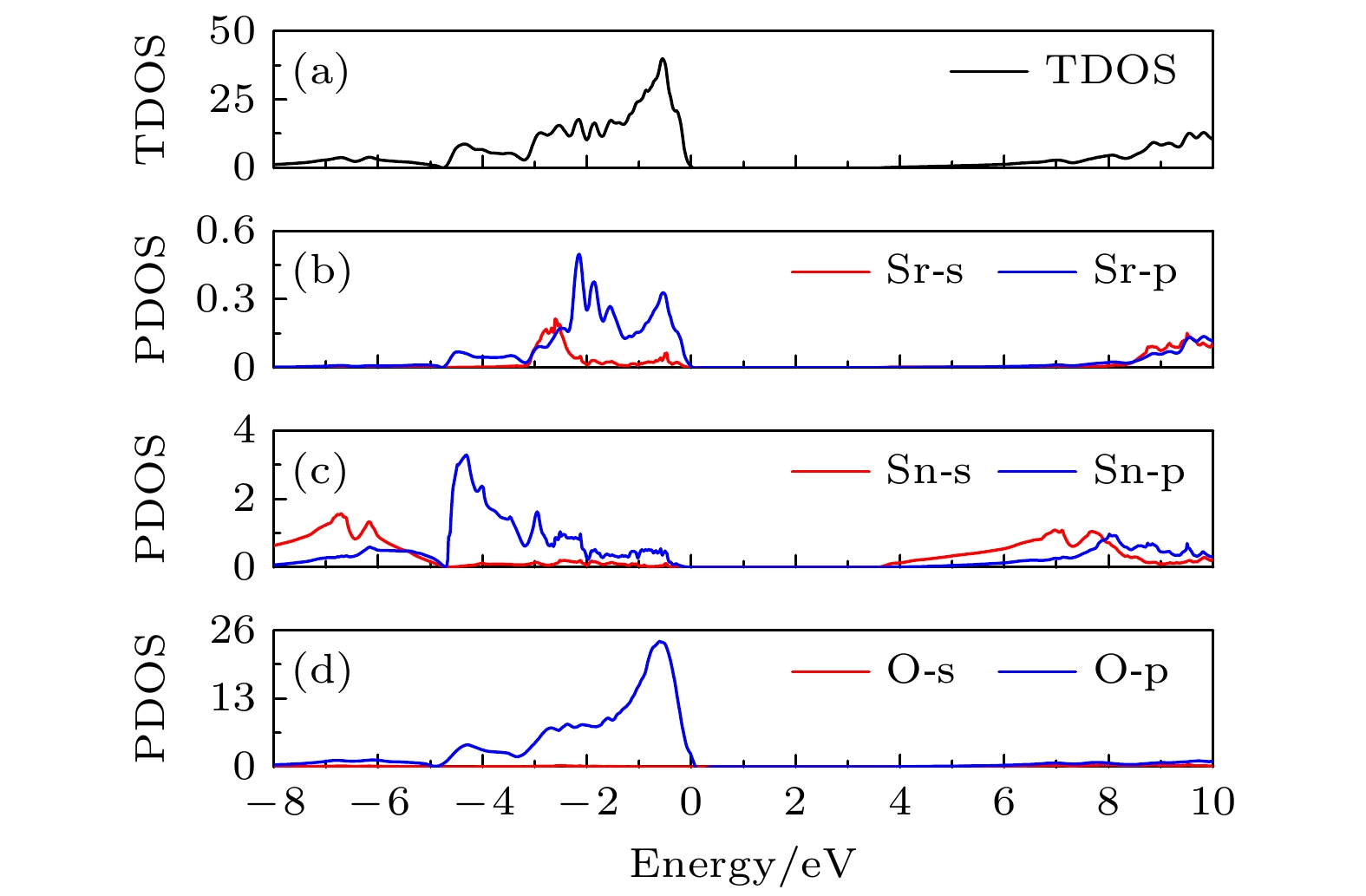
图 4 SrSnO3的能带结构(a)和光吸收系数(b). 图(b)中彩色区域可见光谱范围, SrSnO3在可见光谱基本无光吸收, 说明其具有较好的透明性
Fig. 4. The band structure (a) and the absorption coefficients (b) of SrSnO3. Colorful regions in figure b are the range of visible light spectrum. SrSnO3 cannot absorb light at the range of visible light spectrum, which stands for it has a good transparency.
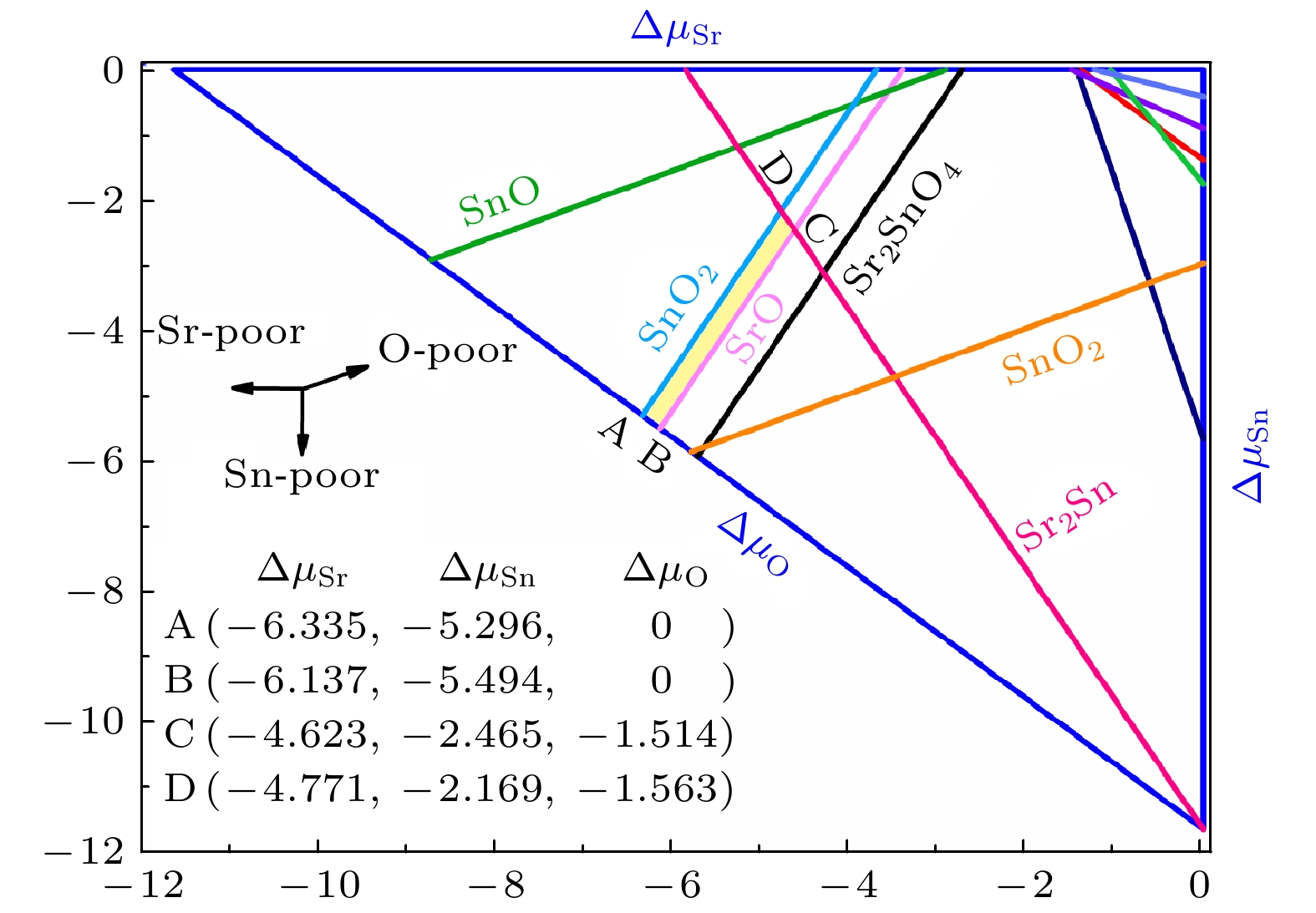
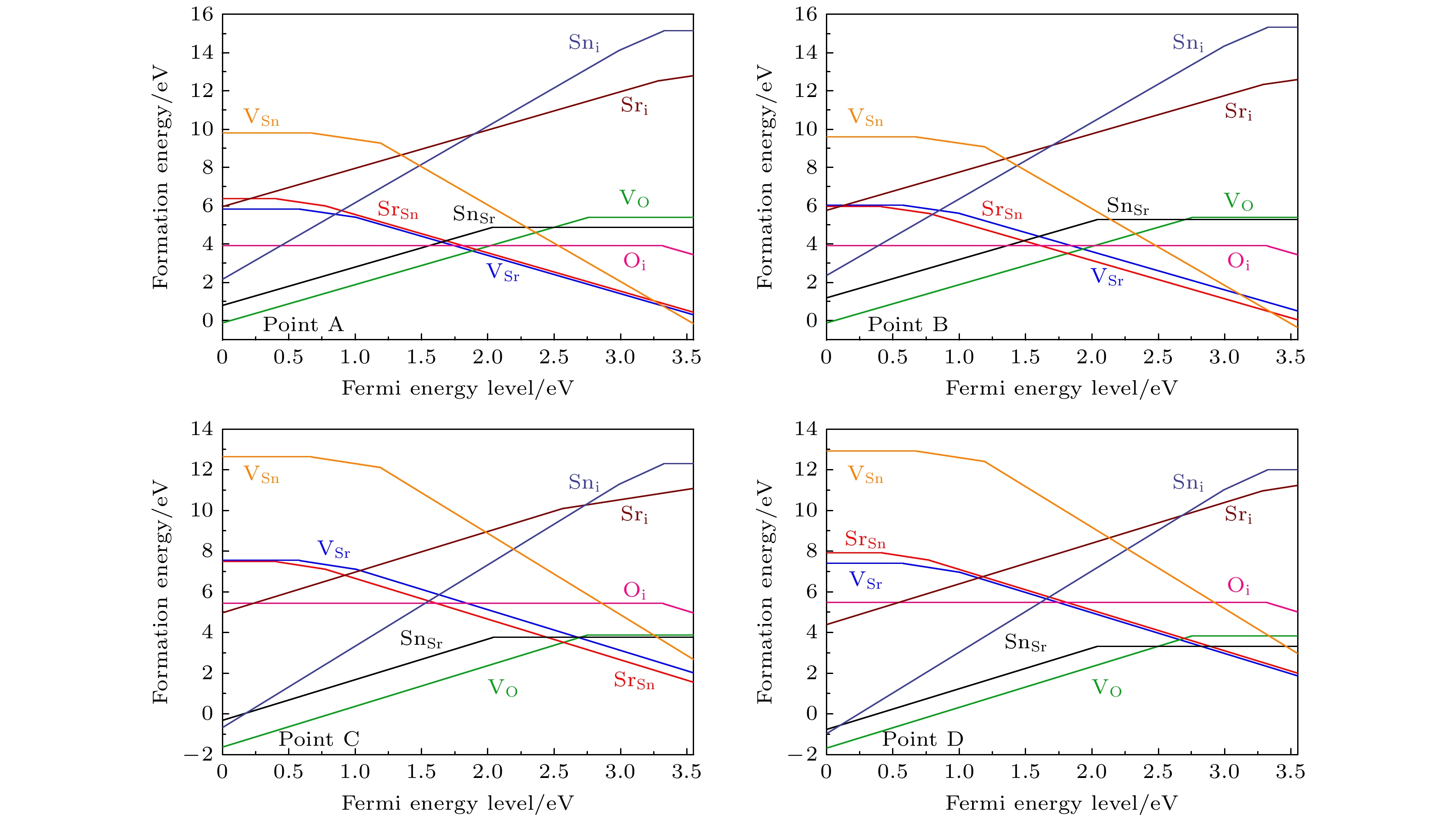
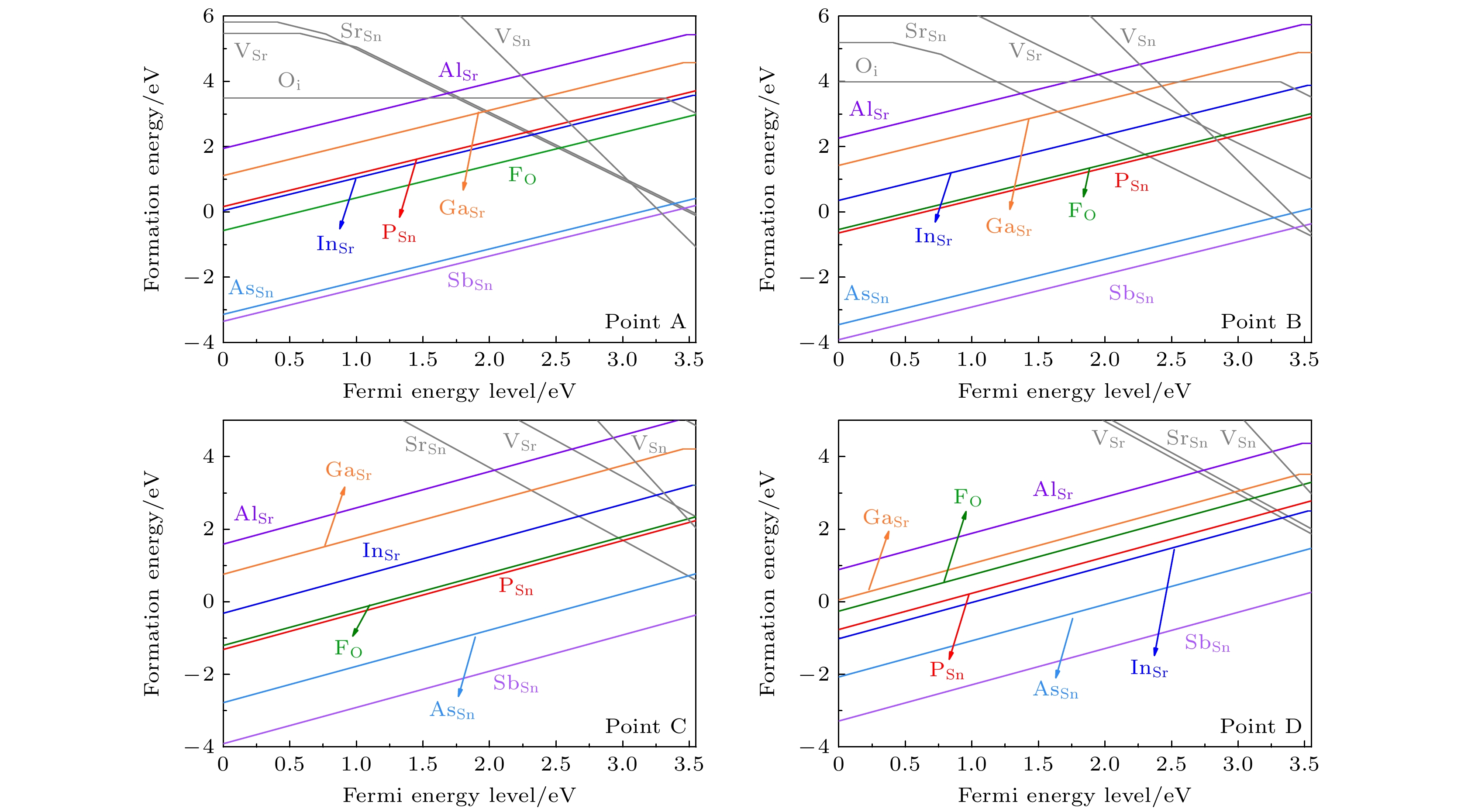
图 7 在不同相对化学势条件下, 各外界掺杂施主型缺陷的缺陷形成能. 灰色的线条表示为可能产生补偿作用的p型本征缺陷的缺陷形成能
Fig. 7. The defect formation energies of external donor defects under different relative chemical potential conditions. The grey lines represent the defect formation energies of p-type intrinsic defects which may lead to a carrier compensation effect.
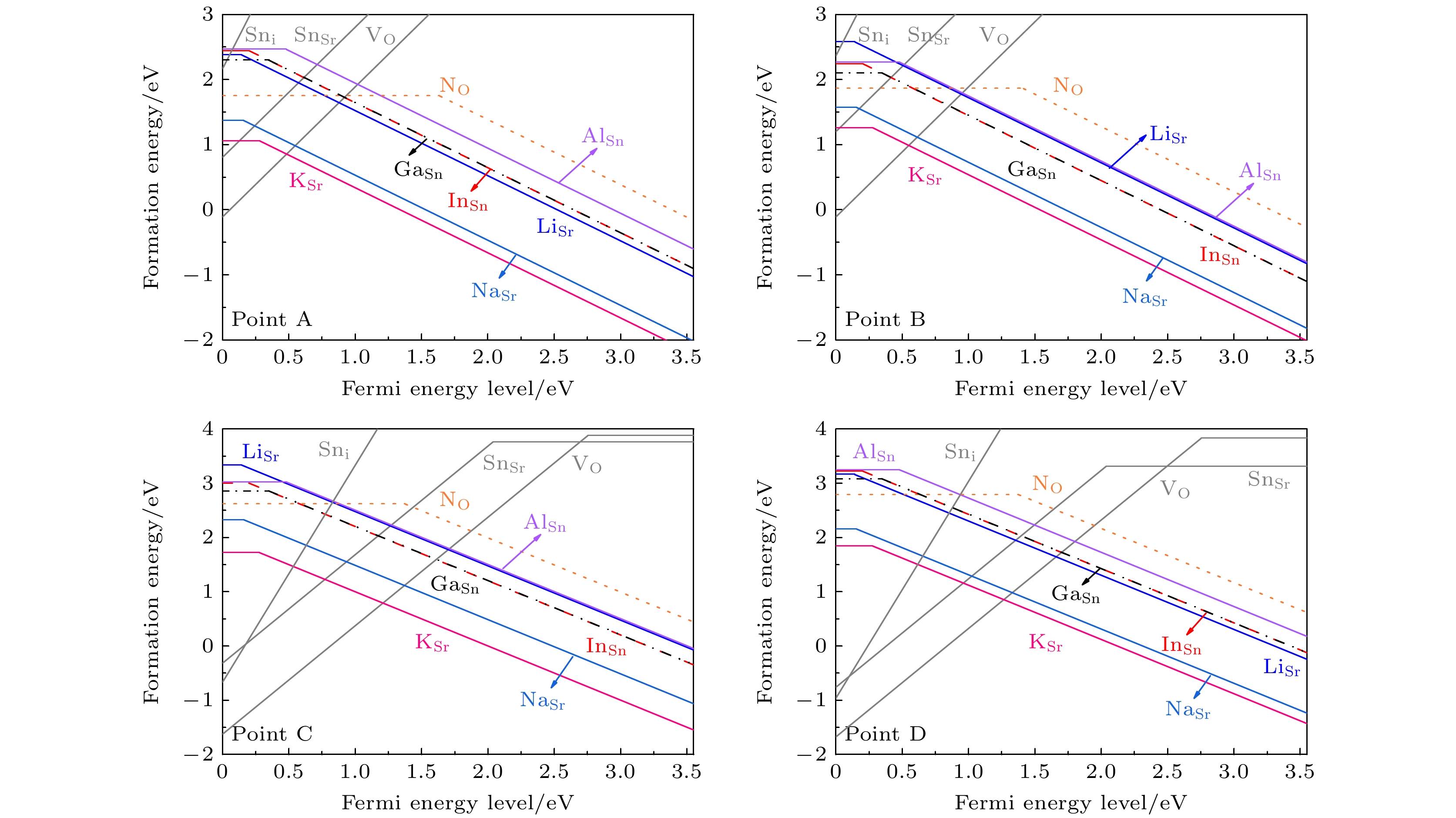
图 8 在不同相对化学势条件下, 各外界掺杂受主型缺陷的缺陷形成能. 灰色的线条表示为可能产生补偿作用的n型本征缺陷的缺陷形成能
Fig. 8. The defect formation energies of external acceptor defects under different relative chemical potential conditions. The grey lines represent the defect formation energies of n-type intrinsic defects which may lead to a carrier compensation effect.
图 9 一系列宽禁带半导体材料SnO2, In2O3, SrSnO3, CuI和Cu2O的带边能量位置对比, 带隙宽度分别为3.52 eV, 3.73 eV, 3.55 eV, 3.1 eV以及2.02 eV
Fig. 9. The band-edge energy positions among a series of wide band gap semiconductors: SnO2, In2O3, SrSnO3, CuI and Cu2O. The band gaps of them are 3.52 eV, 3.73 eV, 3.55 eV, 3.1 eV and 2.02 eV, respectively.
表 1 SrSnO3中电子和空穴的有效质量(单位: m0)
Table 1. Effective masses of electrons and holes in SrSnO3 (in: m0).
有效质量 电子 空穴 $ {m}_{001}^{*} $ 0.36 1.72 $ {m}_{010}^{*} $ 0.32 0.44 $ {m}_{100}^{*} $ 0.36 0.48 -
[1] Bitla Y, Chu Y H 2020 Nanoscale 12 18523
 Google Scholar
Google Scholar
[2] Stadler A 2012 Materials 5 661
 Google Scholar
Google Scholar
[3] Cho H, Jeong S H, Park M H, Kim Y H, Wolf C, Lee C L, Heo Jin H, Sadhanala A, Myoung N, Yoo S, Im Sang H, Friend Richard H, Lee T W 2015 Science 350 1222
 Google Scholar
Google Scholar
[4] Tan Z K, Moghaddam R S, Lai M L, Docampo P, Higler R, Deschler F, Price M, Sadhanala A, Pazos L M, Credgington D, Hanusch F, Bein T, Snaith H J, Friend R H 2014 Nat. Nanotechnol. 9 687
 Google Scholar
Google Scholar
[5] Pan Z W, Dai Z R, Wang Z L 2001 Science 291 1947
 Google Scholar
Google Scholar
[6] Comini E, Faglia G, Sberveglieri G, Pan Z, Wang Z L 2002 Appl. Phys. Lett. 81 1869
 Google Scholar
Google Scholar
[7] Batzill M, Diebold U 2005 Prog. Surf. Sci. 79 47
 Google Scholar
Google Scholar
[8] Dou L, Yang Y, You J, Hong Z, Chang W H, Li G, Yang Y 2014 Nat. Commun. 5 5404
 Google Scholar
Google Scholar
[9] Lee Michael M, Teuscher J, Miyasaka T, Murakami Takurou N, Snaith Henry J 2012 Science 338 643
 Google Scholar
Google Scholar
[10] Baedeker K 1907 Ann. Phys. 327 749
 Google Scholar
Google Scholar
[11] Minami T 2008 Thin Solid Films 516 1314
 Google Scholar
Google Scholar
[12] Minami T 2008 Thin Solid Films 516 5822
 Google Scholar
Google Scholar
[13] Chen M J, Yang J R, Shiojiri M 2012 Semicond. Sci. Technol. 27 074005
 Google Scholar
Google Scholar
[14] Du X, Mei Z, Liu Z, Guo Y, Zhang T, Hou Y, Zhang Z, Xue Q, Kuznetsov A Y 2009 Adv. Mater. 21 4625
 Google Scholar
Google Scholar
[15] 王延峰, 谢希成, 刘晓洁, 韩冰, 武晗晗, 连宁宁, 杨富, 宋庆功, 裴海林, 李俊杰 2020 69 197801
 Google Scholar
Google Scholar
Wang Y F, Xie X C, Liu X J, Han B, Wu H H, Lian N N, Yang F, Song Q G, Pei H L, Li J J 2020 Acta. Phys. Sin. 69 197801
 Google Scholar
Google Scholar
[16] Wu F, Tong X, Zhao Z, Gao J, Zhou Y, Kelly P 2017 J. Alloys Compd. 695 765
 Google Scholar
Google Scholar
[17] Fleischer K, Norton E, Mullarkey D, Caffrey D, Shvets I V 2017 Materials 10 1019
 Google Scholar
Google Scholar
[18] Zhang K H L, Xi K, Blamire M G, Egdell R G 2016 J. Phys. Condens. Mater. 28 383002
 Google Scholar
Google Scholar
[19] Cao R, Deng H X, Luo J W 2019 ACS Appl. Mater. Interfaces 11 24837
 Google Scholar
Google Scholar
[20] Dixon S C, Scanlon D O, Carmalt C J, Parkin I P 2016 J. Mater. Chem. C 4 6946
 Google Scholar
Google Scholar
[21] Bel Hadj Tahar R, Ban T, Ohya Y, Takahashi Y 1998 J. Appl. Phys. 83 2631
 Google Scholar
Google Scholar
[22] Selopal G S, Milan R, Ortolani L, Morandi V, Rizzoli R, Sberveglieri G, Veronese G P, Vomiero A, Concina I 2015 Sol. Energy Mater. Sol. Cells 135 99
 Google Scholar
Google Scholar
[23] 王延峰, 张晓丹, 黄茜, 杨富, 孟旭东, 宋庆功, 赵颖 2013 62 247802
 Google Scholar
Google Scholar
Wang Y F, Zhang X D, Huang Q, Yang F, Meng X D, Song Q G, Zhao Y 2013 Acta Phys. Sin. 62 247802
 Google Scholar
Google Scholar
[24] 王延峰, 孟旭东, 郑伟, 宋庆功, 翟昌鑫, 郭兵, 张越, 杨富, 南景宇 2016 65 087802
 Google Scholar
Google Scholar
Wang Y F, Meng X D, Zheng W, Song Q G, Zhai C X, Guo B, Zhang Y, Yang F, Nan J Y 2016 Acta Phys. Sin. 65 087802
 Google Scholar
Google Scholar
[25] Ong K P, Fan X, Subedi A, Sullivan M B, Singh D J 2015 APL Mater. 3 062505
 Google Scholar
Google Scholar
[26] Riza M A, Ibrahim M A, Ahamefula U C, Mat Teridi M A, Ahmad Ludin N, Sepeai S, Sopian K 2016 Sol. Energy 137 371
 Google Scholar
Google Scholar
[27] Liu Q, Dai J, Zhang X, Zhu G, Liu Z, Ding G 2011 Thin Solid Films 519 6059
 Google Scholar
Google Scholar
[28] Liu Q, Jin F, Gao G, Wang W 2017 J. Alloys Compd. 717 62
 Google Scholar
Google Scholar
[29] Kumar Y, Kumar R, Asokan K, Choudhary R J, Phase D M, Singh A P 2021 J. Mater. Sci. -Mater. Electron. 32 11835
 Google Scholar
Google Scholar
[30] Wei M, Sanchela A V, Feng B, Ikuhara Y, Cho H J, Ohta H 2020 Appl. Phys. Lett. 116 022103
 Google Scholar
Google Scholar
[31] Liu Y, Zhou Y, Jia D, Zhao J, Wang B, Cui Y, Li Q, Liu B 2020 J. Mater. Sci. Technol. 42 212
 Google Scholar
Google Scholar
[32] Rahman A B A, Sarjadi M S, Alias A, Ibrahim M A 2019 J. Phys:Conf. Ser. 1358 012043
 Google Scholar
Google Scholar
[33] Kumar Y, Kumar R, Choudhary R J, Thakur A, Singh A P 2020 Ceram. Int. 46 17569
 Google Scholar
Google Scholar
[34] Hohenberg P, Kohn W 1964 Phys. Rev. 136 B864
 Google Scholar
Google Scholar
[35] Kohn W, Sham L J 1965 Phys. Rev. 140 A1133
 Google Scholar
Google Scholar
[36] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[37] Heyd J, Scuseria G E 2004 J. Chem. Phys. 121 1187
 Google Scholar
Google Scholar
[38] Green M A, Prassides K, Day P, Neumann D A 2000 J. Inorg. Mater. 2 35
 Google Scholar
Google Scholar
[39] Schumann T, Raghavan S, Ahadi K, Kim H, Stemmer S 2016 J. Vac. Sci. Technol., A 34 050601
[40] Mizoguchi H, Eng H W, Woodward P M 2004 Inorg. Chem. 43 1667
 Google Scholar
Google Scholar
[41] Zhang S B, Wei S H, Zunger A 2001 Phys. Rev. B 63 075205
 Google Scholar
Google Scholar
[42] Lany S, Zunger A 2008 Phys. Rev. B 78 235104
 Google Scholar
Google Scholar
[43] Singh M K, Hong J W, Karan N K, Jang H M, Katiyar R S, Redfern S A T, Scott J F 2010 J. Phys. Condens. Matter. 22 095901
 Google Scholar
Google Scholar
[44] Gao Q, Chen H, Li K, Liu Q 2018 ACS Appl. Mater. Interfaces 10 27503
 Google Scholar
Google Scholar
[45] KC S, Rowberg A J E, Weston L, Van de Walle C G 2019 J. Appl. Phys. 126 195701
 Google Scholar
Google Scholar
[46] Putz M V, Russo N, Sicilia E 2005 Theor. Chem. Acc. 114 38
 Google Scholar
Google Scholar
[47] Huang D, Xu J P, Jiang J W, Zhao Y J, Peng B L, Zhou W Z, Guo J 2017 Phys. Lett. A 381 2743
 Google Scholar
Google Scholar
[48] Hu S, Xia B, Yan Y, Xiao Z 2020 Phys. Rev. Mater. 4 115201
 Google Scholar
Google Scholar
[49] Schein F L, von Wenckstern H, Grundmann M 2013 Appl. Phys. Lett. 102 092109
 Google Scholar
Google Scholar
[50] Arai T, Iimura S, Kim J, Toda Y, Ueda S, Hosono H 2017 J. Am. Chem. Soc. 139 17175
 Google Scholar
Google Scholar
[51] Zhang Z, Guo Y, Robertson J 2022 Chem. Mater. 34 643
 Google Scholar
Google Scholar
[52] Yan Y, Wei S H 2008 Phys. Status Solidi B 245 641
 Google Scholar
Google Scholar
计量
- 文章访问数: 7092
- PDF下载量: 214
- 被引次数: 0














 下载:
下载: