-
本文基于最新构建的
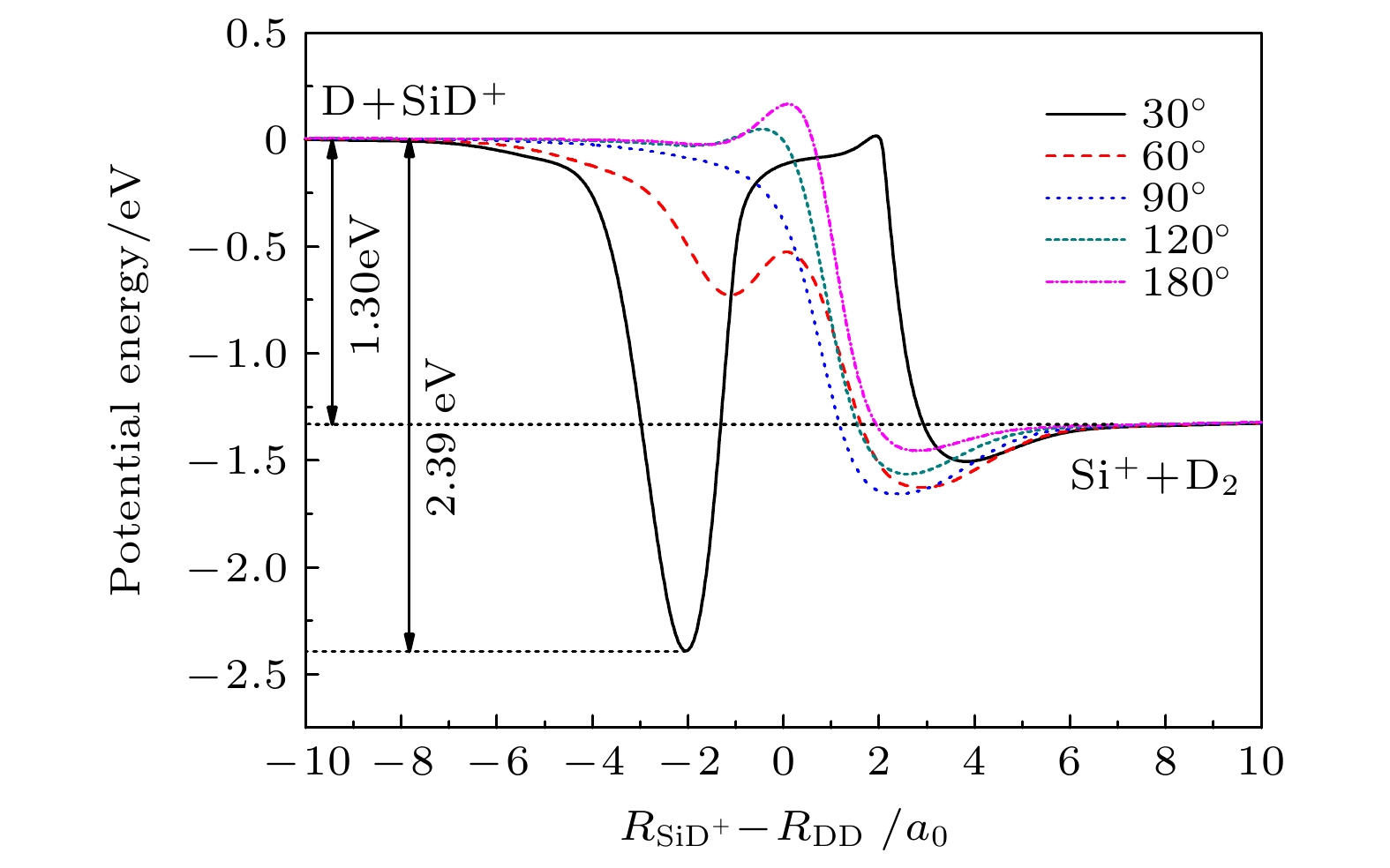
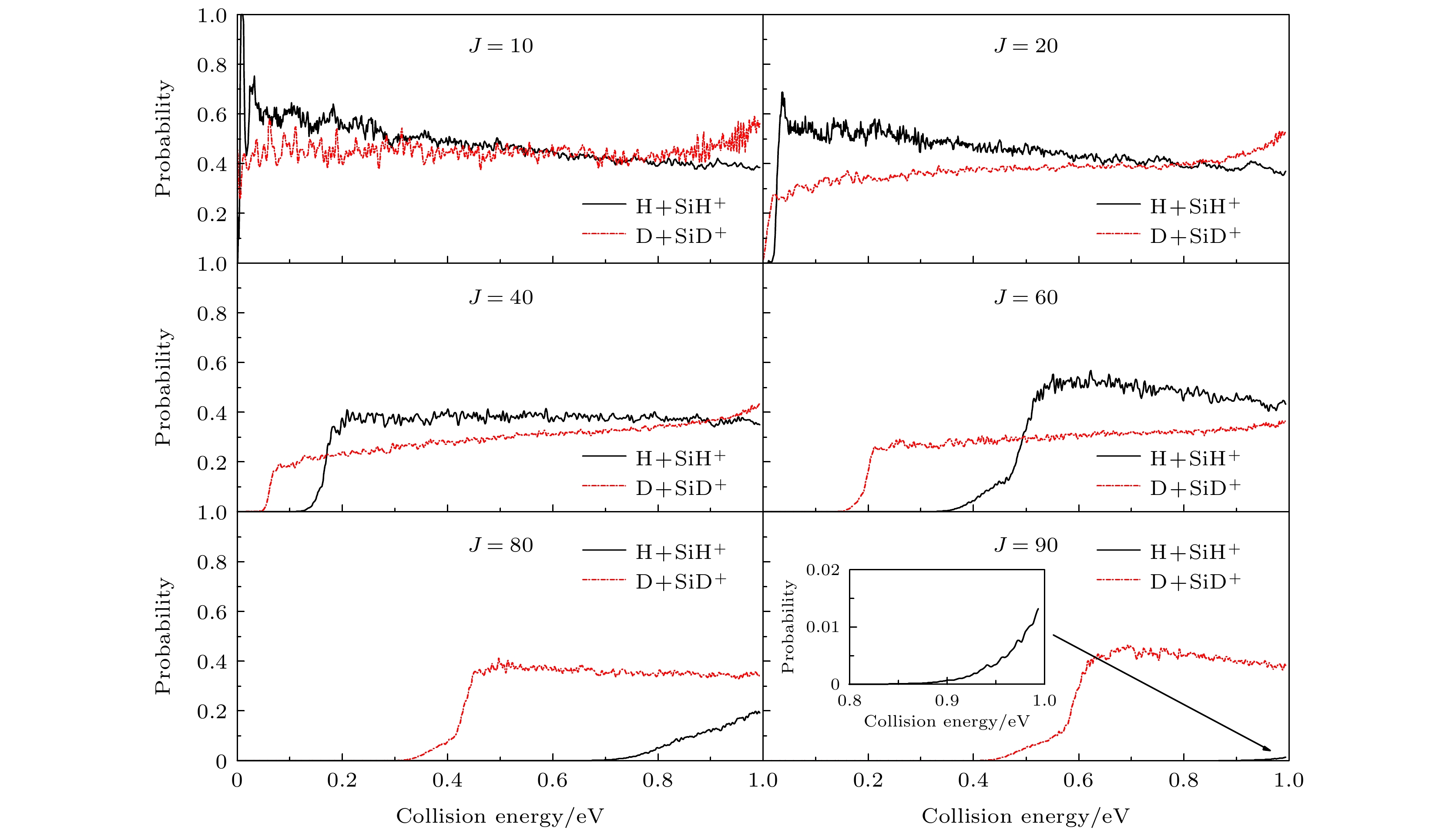
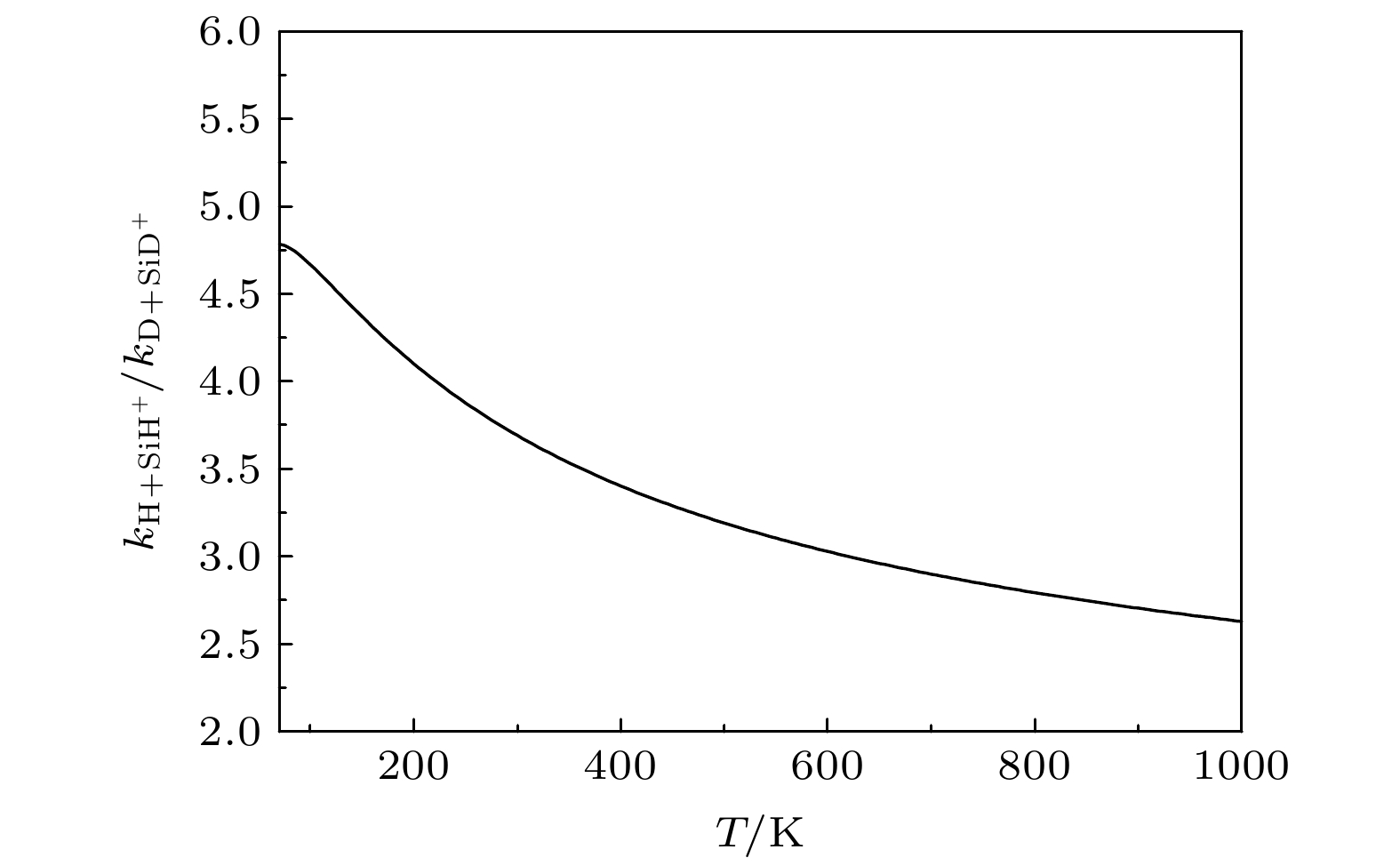
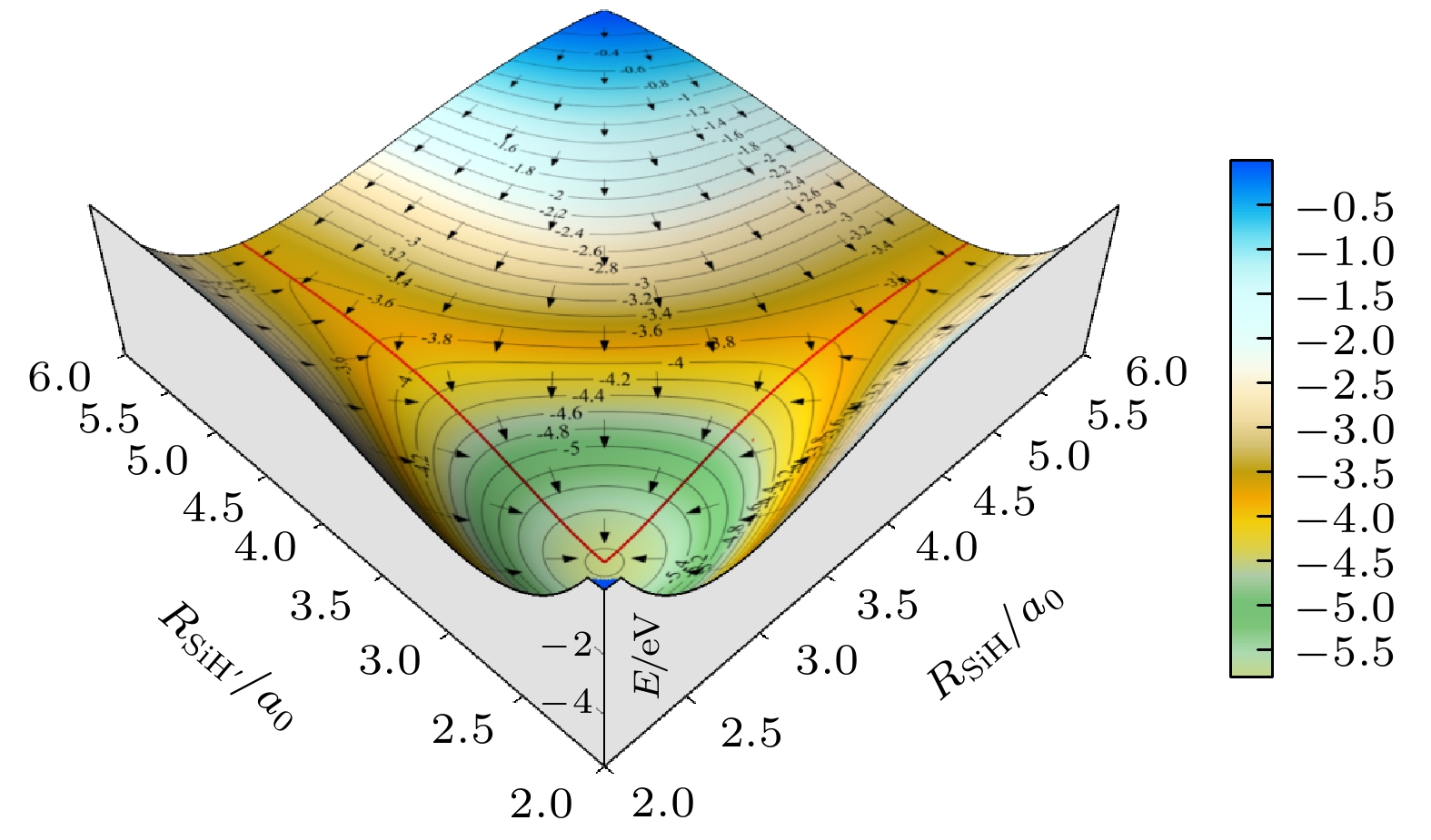
${{\rm{SiH}}_2^+}({\tilde{\rm{X}}}{}^{3}{\rm{A}}'')$ 势能面, 运用切比雪夫波包方法, 对初始态为$ \nu = 0, j = 0 $ 的${\text{D}} + {\text{Si}}{{\text{D}}^ + } $ 反应体系在$ 1.0 \times {10^{ - 3}} $ —1.0 eV的碰撞能量范围内进行动力学研究. 通过对角动量量子数$ J \leqslant 110 $ 的所有分波进行计算, 运用耦合态近似和考虑科里奥利耦合效应的精确量子力学两种方法, 得到该反应的反应概率、积分散射截面和速率常数. 文章详细分析了碰撞能量和科里奥利耦合效应对该反应的动力学性质的影响, 并与H+SiH+反应做了对比. 结果表明, 忽略科里奥利耦合效应会使D +SiD+反应积分散射截面和速率常数增大. 与H+SiH+反应动力学相比较, D +SiD+反应的积分反应截面的值较小且随着能量的衰减非常缓慢, 在低能区没有H+SiH+随能量增大而剧烈衰减的情况. 计算发现, 相同温度下, D +SiD+反应速率常数小于H +SiH+反应速率常数, 随着温度的升高, 二者的差距减小. 这表明, 同位素替代对反应的动力学性质有明显的影响.The quantum dynamics calculations are carried out for the title reaction D +SiD+→D2+Si+ to obtain the initial ($ \nu = 0{\text{ }},j = 0 $ )reaction probability, integral cross section (ICS) and rate constant on the potential energy surface (PES) of Gao, Meng and Song. A total of 110 partial waves are calculated by using the Chebyshev wave packet method with full Coriolis coupling (CC) and centrifugal sudden (CS) approximation in a collision energy range from 1.0 × 10–3 to 1.0 eV. The calculated probability decreases with the collision energy increasing except for J≤40. The calculation results indicate that the CS approximation will overestimate or underestimate the reaction probability . The ICS decreases with the collision energy increasing and shows an oscillatory structure due to the$\rm{SiH_2^+} $ well on the reaction path. The results show that the neglect of the Coriolis coupling leads to the overestimation of the cross section and the rate constant. Besides, the discrepancy between the integral cross sections from the CC and CS calculations decreases clearly with collision energy increasing. Comparison with the corresponding results of H+CH+ reaction indicates that isotope substitution reaction makes the cross section and the rate constant underestimated. The resulting integral reaction cross section displays less oscillatory structure, especially in the exact quantum calculation with the full Coriolis coupling effect taken into consideration. The kinetic isotope effect$(\kappa_{\rm H+SiH^+}(T)/\kappa_{\rm D+SiD^+}(T))$ is found to decrease with temperature increasing. It can be seen that the reduced mass of reactant can exert a certain effect on dynamic behavior.-
Keywords:
- Chebyshev wave packet method /
- reaction probability /
- integral cross section /
- rate constant
[1] Bender C F, Schaefer H F 1971 J. Mol. Spectrosc. 37 423
 Google Scholar
Google Scholar
[2] Stoecklin T, Halvick P 2005 Phys. Chem. Chem. Phys. 7 2446
 Google Scholar
Google Scholar
[3] Warmbier R, Schneider R 2011 Phys. Chem. Chem. Phys. 13 10285
 Google Scholar
Google Scholar
[4] Herráez-Aguilar D, Jambrina P G, Menéndez M, Aldegunde J, Warmbier R, Aoiz F J 2014 Phys. Chem. Chem. Phys. 16 24800
 Google Scholar
Google Scholar
[5] Li Y Q, Zhang P Y, Han K L 2015 J. Chem. Phys. 142 124302
 Google Scholar
Google Scholar
[6] Guo J, Zhang A J, Zhou Y, Liu J Y, Jia J F, Wu H S 2017 Chem. Phys. Lett. 689 121
 Google Scholar
Google Scholar
[7] Sundaram P, Manivannan V, Padmanaban R 2017 Phys. Chem. Chem. Phys. 19 20172
 Google Scholar
Google Scholar
[8] Scheier P, Marsen B, Lonfat M, Schneider W D 2000 Surf. Sci. 458 113
 Google Scholar
Google Scholar
[9] Maus M, Gantefor G, Eberhardt W 2000 Appl. Phys. A 70 535
 Google Scholar
Google Scholar
[10] Kasap S, Capper P 2017 Springer Handbook of Electronic and Photonic Materials (Heidelberg: Springer) pp573–576
[11] Zhang Y G, Dou G, Cui J, Yu Y 2018 J. Mol. Struct. 1165 318
 Google Scholar
Google Scholar
[12] Bauer C, Hirst D M, Hall D I, Sarre P J, Rosmus P J. 1994 Chem. Sot. Faraday Trans. 90 517
 Google Scholar
Google Scholar
[13] Vach H, Chaâbane N, Peslherbe G H 2002 Chem. Phys. Lett. 352 127
 Google Scholar
Google Scholar
[14] Chaâbane N, Vach H, Cabarrocas P R I 2004 J. Phys. Chem. A 108 1818
 Google Scholar
Google Scholar
[15] Gao F, Zhang L L, Zhao W L, Meng Q T, Song Y Z 2019 J. Chem. Phys. 150 224304
 Google Scholar
Google Scholar
[16] Langhoff S R, Davidson E R 1974 Int. J. Quantum Chem. 8 61
 Google Scholar
Google Scholar
[17] 高峰 2020 博士学位论文 (济南: 山东师范大学)
Gao F 2020 Ph. D. Dissertation (Jinan: Shandong Normal University) (in Chinese)
[18] Zhao W L, Tan R S, Cao X C, Gao F, Meng Q T 2021 Chin. Phys. B 30 123403
 Google Scholar
Google Scholar
[19] 王茗馨, 王美山, 杨传路, 刘佳, 马晓光, 王立志 2015 64 043402
 Google Scholar
Google Scholar
Wang M X, Wang M S, Yang C L, Liu J, Ma X G, Wang L Z 2015 Acta Phys. Sin. 64 043402
 Google Scholar
Google Scholar
[20] 夏文泽, 于永江, 杨传路 2012 61 223401
 Google Scholar
Google Scholar
Xia W Z, Yu Y J, Yang C L 2012 Acta Phys. Sin 61 223401
 Google Scholar
Google Scholar
[21] Guo L, Yang Y F, Fan X X, Ma F C, Li Y Q 2017 Commun. Theor. Phys. 67 549
 Google Scholar
Google Scholar
[22] Zhang J Z H 1999 Theory and Application of Quantum Molecular Dynamics (Singapore: World Scientific) pp149–150
[23] Lin S Y, Guo H 2003 J. Chem. Phys. 119 11602
 Google Scholar
Google Scholar
[24] Mandelshtam V A, Taylor H S 1995 J. Chem. Phys. 102 7390
 Google Scholar
Google Scholar
[25] Mandelshtam V A, Taylor H S 1995 J. Chem. Phys. 103 2903
 Google Scholar
Google Scholar
[26] Tal-Ezer H, Kosloff R 1984 J. Chem. Phys. 81 3967
 Google Scholar
Google Scholar
[27] Neuhauser D, Baer M, Judson R S, Kouri D J 1990 J. Chem. Phys. 93 312
 Google Scholar
Google Scholar
[28] Althorpe S C 2001 J. Chem. Phys. 114 1601
 Google Scholar
Google Scholar
[29] Zhai H C, Lin S Y 2015 Chem. Phys. 455 57
 Google Scholar
Google Scholar
[30] Bowman J M 1991 J. Phys. Chem. 95 4960
 Google Scholar
Google Scholar
[31] Gray S K, Goldfield, E M, Schatz G C, Balint-Kurti G G 1999 Phys. Chem. Chem. Phys. 1 1141
 Google Scholar
Google Scholar
[32] Clary D C 1984 Mol. Phys. 53 3
 Google Scholar
Google Scholar
[33] Chu T S, Han K L 2008 Phys. Chem. Chem. Phys. 10 2431
 Google Scholar
Google Scholar
[34] De Fazio D, Castillo J F 1999 Phys. Chem. Chem. Phys. 1 1165
 Google Scholar
Google Scholar
[35] Lu R F, Wang Y H, Deng K M, 2013 J. Comput. Chem. 34 1735
 Google Scholar
Google Scholar
-
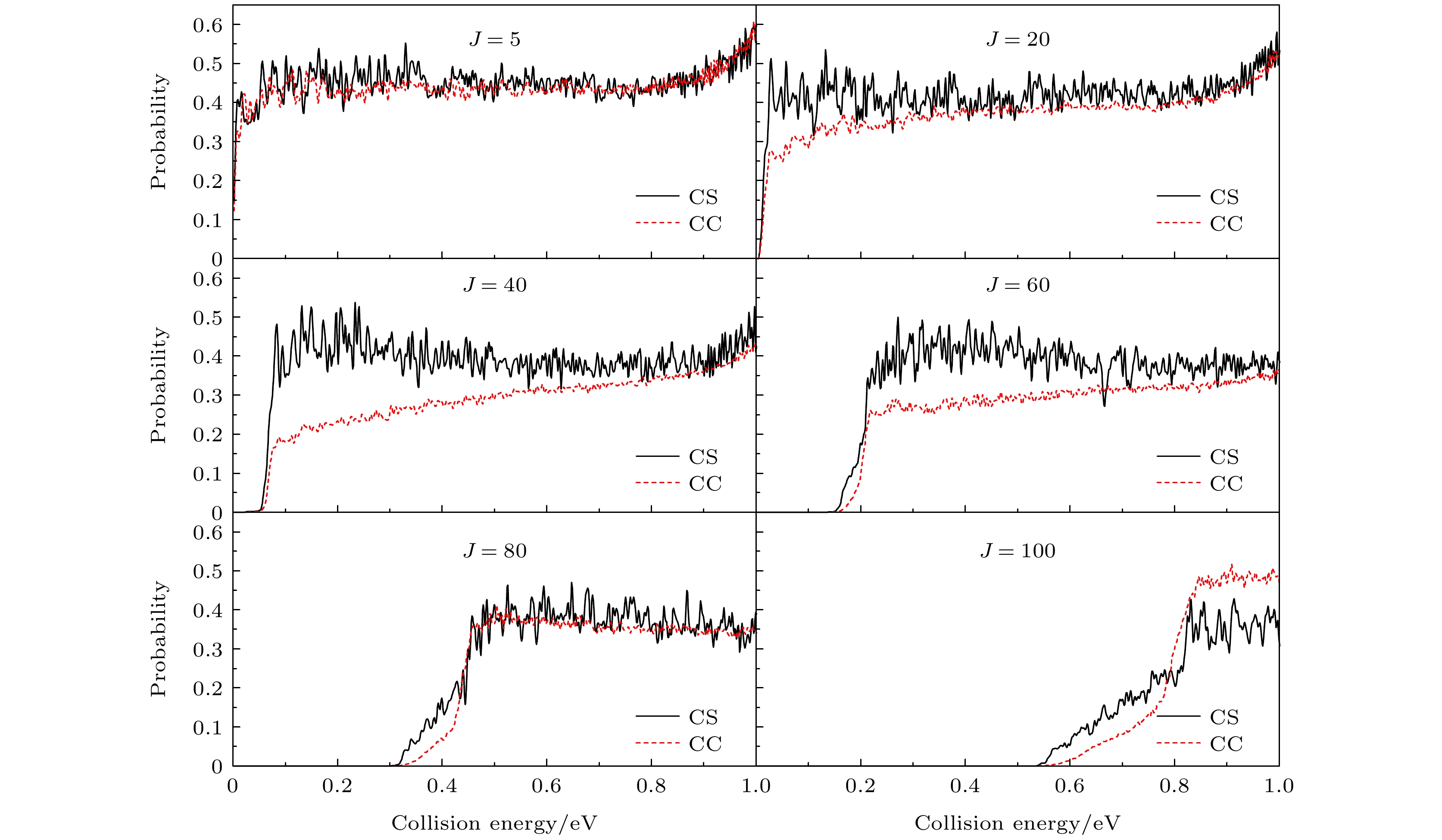
图 3
$ {\text{D}} + {\text{Si}}{{\text{D}}^ + } \to {{\text{D}}_2} + {\text{S}}{{\text{i}}^ + } $ 反应不同分波 (J = 5, 20, 40, 60, 80, 100)的反应概率随碰撞能量的变化, 黑色实线对应CS概率, 红色虚线对应CC概率Fig. 3. The reaction probabilities of CC and CS calculations for
$ {\text{D}} + {\text{Si}}{{\text{D}}^ + } \to {{\text{D}}_2} + {\text{S}}{{\text{i}}^ + } $ reaction at J = 5, 20, 40, 60, 80, and 100, The black soid line is for CS probability and the red dashed line is for CC probability.表 1 波包计算中的数值参量(除特殊说明, 均采用原子单位 a.u.)
Table 1. Model parameters of wave packet calculation (The atomic unit is used in the calculation unlessotherwise stated).
坐标取值范围和基组数 吸收势 初始波包 光谱控制 流计算的位置 传播步数 $ R \in (0.2, \, 22) \; ({N_R} = 383) $
$ r \in (0.5, \, 16) \; ({N_r} = 255) $
$ \gamma \in (0, \, {180^ \circ }) \; ({N_\gamma } = 200) $$ {R_d} = 18.0 \;\; {d_R} = 0.0005 $
$ {r_d} = 14.0 \;\; {d_r} = 0.001 $$ {R_0} = 16.0 $
$ {E_0} = 0.15{\text{ eV}} $
$ \delta = 0.3 $1.0 $ {r_f} = 13.8 $ 50000 -
[1] Bender C F, Schaefer H F 1971 J. Mol. Spectrosc. 37 423
 Google Scholar
Google Scholar
[2] Stoecklin T, Halvick P 2005 Phys. Chem. Chem. Phys. 7 2446
 Google Scholar
Google Scholar
[3] Warmbier R, Schneider R 2011 Phys. Chem. Chem. Phys. 13 10285
 Google Scholar
Google Scholar
[4] Herráez-Aguilar D, Jambrina P G, Menéndez M, Aldegunde J, Warmbier R, Aoiz F J 2014 Phys. Chem. Chem. Phys. 16 24800
 Google Scholar
Google Scholar
[5] Li Y Q, Zhang P Y, Han K L 2015 J. Chem. Phys. 142 124302
 Google Scholar
Google Scholar
[6] Guo J, Zhang A J, Zhou Y, Liu J Y, Jia J F, Wu H S 2017 Chem. Phys. Lett. 689 121
 Google Scholar
Google Scholar
[7] Sundaram P, Manivannan V, Padmanaban R 2017 Phys. Chem. Chem. Phys. 19 20172
 Google Scholar
Google Scholar
[8] Scheier P, Marsen B, Lonfat M, Schneider W D 2000 Surf. Sci. 458 113
 Google Scholar
Google Scholar
[9] Maus M, Gantefor G, Eberhardt W 2000 Appl. Phys. A 70 535
 Google Scholar
Google Scholar
[10] Kasap S, Capper P 2017 Springer Handbook of Electronic and Photonic Materials (Heidelberg: Springer) pp573–576
[11] Zhang Y G, Dou G, Cui J, Yu Y 2018 J. Mol. Struct. 1165 318
 Google Scholar
Google Scholar
[12] Bauer C, Hirst D M, Hall D I, Sarre P J, Rosmus P J. 1994 Chem. Sot. Faraday Trans. 90 517
 Google Scholar
Google Scholar
[13] Vach H, Chaâbane N, Peslherbe G H 2002 Chem. Phys. Lett. 352 127
 Google Scholar
Google Scholar
[14] Chaâbane N, Vach H, Cabarrocas P R I 2004 J. Phys. Chem. A 108 1818
 Google Scholar
Google Scholar
[15] Gao F, Zhang L L, Zhao W L, Meng Q T, Song Y Z 2019 J. Chem. Phys. 150 224304
 Google Scholar
Google Scholar
[16] Langhoff S R, Davidson E R 1974 Int. J. Quantum Chem. 8 61
 Google Scholar
Google Scholar
[17] 高峰 2020 博士学位论文 (济南: 山东师范大学)
Gao F 2020 Ph. D. Dissertation (Jinan: Shandong Normal University) (in Chinese)
[18] Zhao W L, Tan R S, Cao X C, Gao F, Meng Q T 2021 Chin. Phys. B 30 123403
 Google Scholar
Google Scholar
[19] 王茗馨, 王美山, 杨传路, 刘佳, 马晓光, 王立志 2015 64 043402
 Google Scholar
Google Scholar
Wang M X, Wang M S, Yang C L, Liu J, Ma X G, Wang L Z 2015 Acta Phys. Sin. 64 043402
 Google Scholar
Google Scholar
[20] 夏文泽, 于永江, 杨传路 2012 61 223401
 Google Scholar
Google Scholar
Xia W Z, Yu Y J, Yang C L 2012 Acta Phys. Sin 61 223401
 Google Scholar
Google Scholar
[21] Guo L, Yang Y F, Fan X X, Ma F C, Li Y Q 2017 Commun. Theor. Phys. 67 549
 Google Scholar
Google Scholar
[22] Zhang J Z H 1999 Theory and Application of Quantum Molecular Dynamics (Singapore: World Scientific) pp149–150
[23] Lin S Y, Guo H 2003 J. Chem. Phys. 119 11602
 Google Scholar
Google Scholar
[24] Mandelshtam V A, Taylor H S 1995 J. Chem. Phys. 102 7390
 Google Scholar
Google Scholar
[25] Mandelshtam V A, Taylor H S 1995 J. Chem. Phys. 103 2903
 Google Scholar
Google Scholar
[26] Tal-Ezer H, Kosloff R 1984 J. Chem. Phys. 81 3967
 Google Scholar
Google Scholar
[27] Neuhauser D, Baer M, Judson R S, Kouri D J 1990 J. Chem. Phys. 93 312
 Google Scholar
Google Scholar
[28] Althorpe S C 2001 J. Chem. Phys. 114 1601
 Google Scholar
Google Scholar
[29] Zhai H C, Lin S Y 2015 Chem. Phys. 455 57
 Google Scholar
Google Scholar
[30] Bowman J M 1991 J. Phys. Chem. 95 4960
 Google Scholar
Google Scholar
[31] Gray S K, Goldfield, E M, Schatz G C, Balint-Kurti G G 1999 Phys. Chem. Chem. Phys. 1 1141
 Google Scholar
Google Scholar
[32] Clary D C 1984 Mol. Phys. 53 3
 Google Scholar
Google Scholar
[33] Chu T S, Han K L 2008 Phys. Chem. Chem. Phys. 10 2431
 Google Scholar
Google Scholar
[34] De Fazio D, Castillo J F 1999 Phys. Chem. Chem. Phys. 1 1165
 Google Scholar
Google Scholar
[35] Lu R F, Wang Y H, Deng K M, 2013 J. Comput. Chem. 34 1735
 Google Scholar
Google Scholar
计量
- 文章访问数: 4676
- PDF下载量: 54
- 被引次数: 0

























 下载:
下载: