-
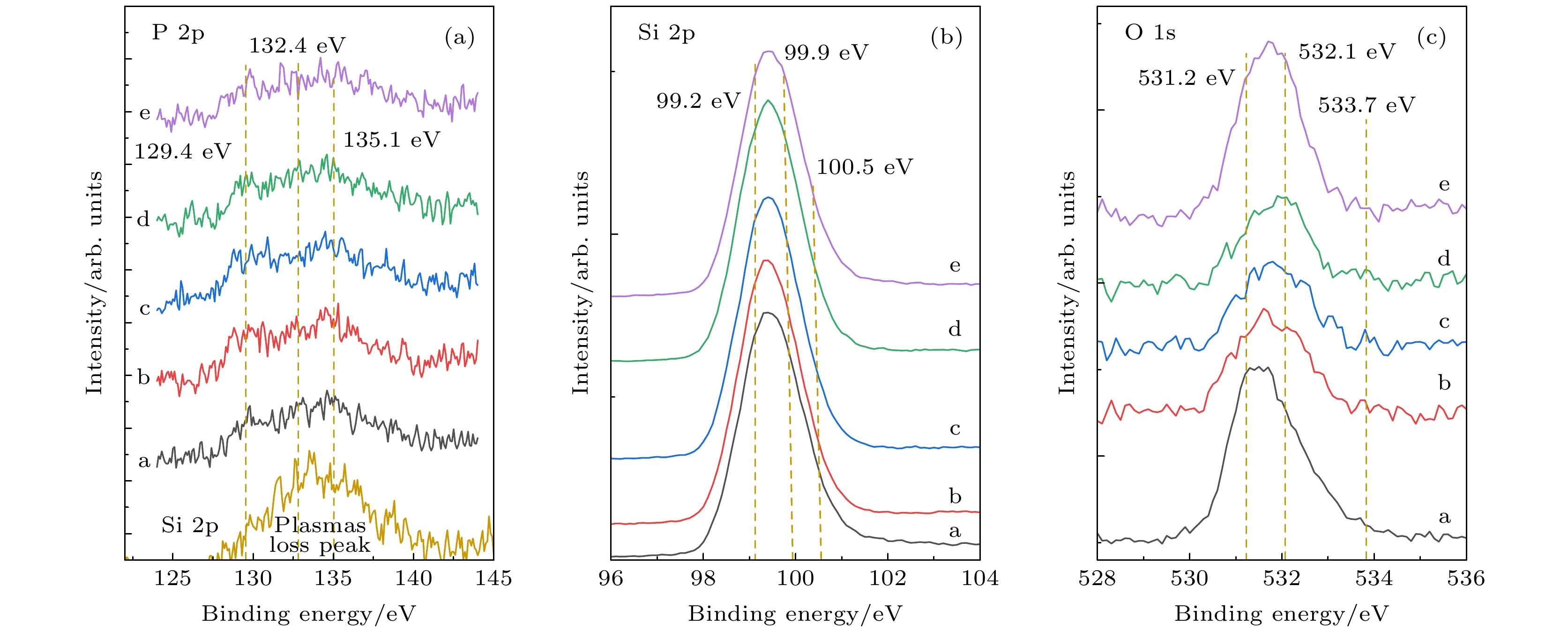
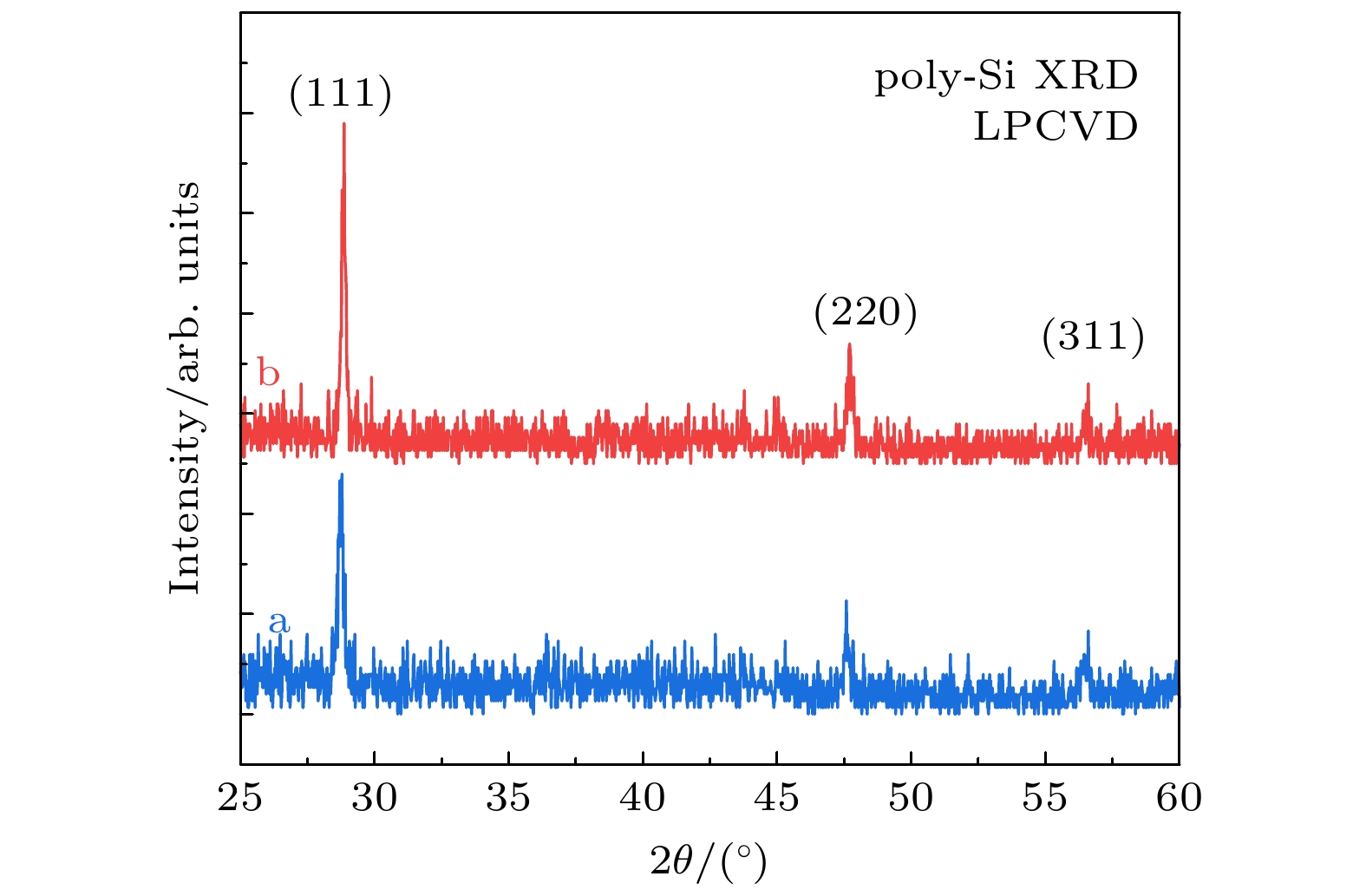
在 n-型隧穿氧化物钝化接触(n-TOPCon)光伏器件中, 高浓度磷掺杂的多晶硅薄膜 (n+ poly-Si) 是电子选择性钝化的关键材料. 它的光学和电子学性能取决于化学组态与多晶结构的物相, 并依赖于晶态转化过程中的高温退火与结构弛豫. 采用低压化学气相沉积技术在 SiOx/n-Si 衬底上生长制备poly-Si (n+) 薄膜, 利用带有深度刻蚀特征的 X-射线光电子能谱、高分辨率透射电子显微术和 X-光衍射分析方法, 研究了该薄膜的微结构. 发现薄膜中氧(O)元素的 O 1s 态的两个拟合峰(O2 和 O3)的结合能分别为 532.1 eV 和 533.7 eV, 对应 O—Si 和 O—P 的成键态; 而磷(P)元素的P 2p态的两个拟合峰(P2 和 P3)的结合能分别为 132.4 eV和 135.1 eV, 对应着O—P*的同根成键态. 电子显微与光衍射分析表明, 该多晶硅薄膜具有 (111)晶向择优生长的特点, 晶面间距为0.313 nm, 平均晶粒尺寸在43.6—55.0 nm. 而(111)晶面簇在920 ℃高温退火过程中, 产生力学形变与晶界, 在局域范围内呈现大晶粒的单晶态. 结合热力学函数, 如生成焓、反应熵、热容、形成能与吉布斯自由能, 以及能量最低原理分析可知, 多晶硅薄膜内存在O—Si和 O—P键形成的条件, 产生了氧化硅和氧化磷的成键态.In tunneling oxide passivation contact (n-TOPCon) photovoltaic devices, poly-Si (n+) films with high-concentration phosphorus doping are the key materials for electron selective passivation. Its optical and electronic properties strongly depend on the chemical configuration and physical phase, and also on high temperature annealing and structural relaxation in the recrystallization process. The poly-Si (n+) films grown on SiOx/n-Si substrates by low pressure chemical vapor deposition technology are investigated, while the microstructure of the film is studied by using X-ray photoelectron spectroscopy with depth etching, high-resolution transmission electron microscopy and X-ray diffraction analysis. It is found that the binding energy values of the two fitted peaks (O2 and O3) of O 1s state of the thin film are situated at 532.1 and 533.7 eV, corresponding to the bonding of O—Si and O—P, respectively. The binding energy values of the two fitted peaks (P2 and P3) of P 2p state are located at 132.4 and 135.1 eV, corresponding to O—P* bonding with the same origin. Electronic microscopy and light diffraction analyses show that the polycrystalline silicon film has the characteristic of (111) preferential orientation, and the space of crystal plane is 0.313 nm, for which the average grain size is in a range of about 43.6–55.0 nm. However, the mechanical deformation and grain boundaries are generated in the annealing process at 920 ℃ along (111) crystal cluster, resulting in the localized monocrystalline state within large grains. The comprehensive analyses of thermodynamic function parameters of formation enthalpy, reaction entropy, heat capacity, formation energy and Gibbs free energy and energy minimum principle analysis indicate that there exist conditions for forming Si—O and P—O bonds in the polysilicon film, and thus the bonding state of silicon and phosphorus oxides are formed.
-
Keywords:
- poly-Si (n+) /
- phosphorous oxide /
- silicon oxide /
- photoelectron spectroscopy /
- depth profile analysis
[1] Feldmann F, Bivour M, Reichel C, Steinkemper H, Hermle M, Glunz S W 2014 Sol. Energy Mater. Sol. Cells 131 46
 Google Scholar
Google Scholar
[2] Moldovan A, Feldmann F, Zimmer M, Rentsch J, Benick J, Hermle M 2015 Sol. Energy Mater. Sol. Cells 142 123
 Google Scholar
Google Scholar
[3] Shen W, Zhao Y, Liu F 2022 Front. Energy 16 40
 Google Scholar
Google Scholar
[4] Richter A, Müller R, Benick J, Feldmann F, Steinhauser B, Reichel C, Fell A, Bivour M, Hermle M, Glunz S W 2021 Nat. Energy 6 429
 Google Scholar
Google Scholar
[5] Long W, Yin S, Peng F G, Yang M, Fang L, Ru X N, Qu M H, Lin H F, Xu X X 2021 Sol. Energy Mater. Sol. Cells 231 111291
 Google Scholar
Google Scholar
[6] Yan D, Cuevas A, Michel J I, Zhang C, Wan Y, Zhang X, Bullock J 2021 Joule 5 811
 Google Scholar
Google Scholar
[7] Chen D, Chen Y, Wang Z, Gong J, Liu C, Zou Y, He Y, Wang Y, Yuan L, Lin W, Xia R, Yin L, Zhang X, Xu G, Yang Y, Shen H, Feng Z, Altermatt P P, Verlinden P J 2020 Sol. Energy Mater. Sol. Cells 206 110258
 Google Scholar
Google Scholar
[8] Yoshikawa K, Kawasaki H, Yoshida W, Irie T, Konishi K, Nakano K, Uto T, Adachi D, Kanematsu M, Uzu H 2017 Nat. Energy 2 17032
 Google Scholar
Google Scholar
[9] Chandra M N, Biswas S, Acharya S, Panda T, Sadhukhan S, Sharma J R, Nandi A, Bose S, Kole A, Das G, Maity S, Chaudhuri P, Saha H 2020 Mater. Sci. Semicond. Process. 119 105163
 Google Scholar
Google Scholar
[10] Padhamnath P, Khanna A, Nandakumar N, Nampalli N, Shanmugam V, Aberle A G, Duttagupta S 2020 Sol. Energy Mater. Sol. Cells 207 110358
 Google Scholar
Google Scholar
[11] Gao T, Yang Q, Guo X Q, Huang Y Q, Zhang Z, Wang Z X, Liao M D, Shou C H, Zeng Y H, Yan B J, Hou G F, Zhang X D, Zhao Y, Ye J C 2019 Sol. Energy Mater. Sol. Cells 200 109926
 Google Scholar
Google Scholar
[12] Kim D R, Lee C H, Weisse J M, Cho I S, Zheng X 2012 Nano Lett. 12 6485
 Google Scholar
Google Scholar
[13] Polzin J I, Hammann B, Niewelt T, Kwapil W, Hermle M, Feldmann F 2021 Sol. Energy Mater. Sol. Cells 230 111267
 Google Scholar
Google Scholar
[14] Padhamnath P, Khanna A, Balaji N, Shanmugam V, Nandakumar N, Wang D, Sun Q, Huang M, Huang S, Fan B, Ding B, Aberle A G, Duttagupta S 2020 Sol. Energy Mater. Sol. Cells 218 110751
 Google Scholar
Google Scholar
[15] Susa M, Kawagishi K, Tanaka N, Nagata K 1997 J. Electrochem. Soc. 144 2552
 Google Scholar
Google Scholar
[16] Hide I, Matsuyama T, Suzuki M, Yamashita H, Suzuki T, Moritani T, Maeda Y 1990 J. Cryst. Growth 99 1339
 Google Scholar
Google Scholar
[17] Fırat M, Payo M R, Duerinckx F, Luchies J-M, Lenes M, Poortmans J 2019 AIP Conf. Proc. 2147 040004
 Google Scholar
Google Scholar
[18] Kern W 1970 RCA Rev. 31 51
 Google Scholar
Google Scholar
[19] Lozac'h M, Nunomura S, Matsubara K 2020 Sol. Energy Mater. Sol. Cells 207 110357
 Google Scholar
Google Scholar
[20] Han L, Chen Z 2013 ECS J. Solid State Sci. Technol. 2 N228
 Google Scholar
Google Scholar
[21] Ying W B, Mizokawa Y, Kamiura Y, Kawamoto K, Yang W Y 2001 Appl. Surf. Sci. 181 1
 Google Scholar
Google Scholar
[22] Sherwood P M A 2002 Surf. Sci. Spectra 9 62
 Google Scholar
Google Scholar
[23] Moulder J F, Chastain J, King R C 1992 Handbook of X-ray Photoelectron Spectroscopy: a Reference Book of Standard Spectra for Identification and Interpretation of XPS Data (Waltham: Perkin-Elmer Corporation) pp230–232
[24] Chen K, Bothwell A, Guthrey H, Hartenstein M B, Polzin J I, Feldmann F, Nemeth W, Theingi S, Page M, Young D L, Stradins P, Agarwal S 2022 Sol. Energy Mater. Sol. Cells 236 111510
 Google Scholar
Google Scholar
[25] Monshi A, Foroughi M R, Monshi M R 2012 World J. Nano Sci. Eng. 2 154
 Google Scholar
Google Scholar
[26] Kale A S, Nemeth W, Guthrey H, Nanayakkara S U, LaSalvia V, Theingi S, Findley D, Page M, Al-Jassim M, Young D L, Stradins P, Agarwal S 2019 ACS Appl. Mater. Interfaces 11 42021
 Google Scholar
Google Scholar
[27] De Jong M, Chen W, Angsten T, Jain A, Notestine R, Gamst A, Sluiter M, Krishna Ande C, van der Zwaag S, Plata J J, Toher C, Curtarolo S, Ceder G, Persson K A, Asta M 2015 Sci. Data 2 150009
 Google Scholar
Google Scholar
[28] De Jong M, Chen W, Geerlings H, Asta M, Persson K A 2015 Sci. Data 2 150053
 Google Scholar
Google Scholar
[29] Jain A, Hautier G, Ong S P, Moore C J, Fischer C C, Persson K A, Ceder G 2011 Phys. Rev. B 84 045115
 Google Scholar
Google Scholar
[30] Perry D L, Phillips S L 1998 Handbook of inorganic compounds (Boca Raton: CRC Press)
[31] Jung I H, Hudon P 2012 J. Am. Ceram. Soc. 95 3665
 Google Scholar
Google Scholar
[32] Rahman M, Hudon P, Jung I H 2013 Metall. Mater. Trans. B 44 837
 Google Scholar
Google Scholar
[33] Boigelot R, Graz Y, Bourgel C, Defoort F, Poirier J 2015 Ceram. Int. 41 2353
 Google Scholar
Google Scholar
-
图 2 P 2p, Si 2p, O 1s态的XPS能谱图(描述了能谱在薄膜不同深度处的强度变化, 对应刻蚀时间为a: 0 s, b: 500 s, c: 1500 s, d: 2000 s, e: 2600 s) (a) P 2p态的XPS能谱和Si 2p态的等离激元损失峰; (b) Si 2p 态的XPS能谱; (c) O 1s 态的XPS能谱. 为了方便显示各元素结合能的变化, 对(a)—(c)图谱曲线的强度均做出了调整
Fig. 2. The XPS spectra of P 2p, Si 2p and O 1s, which describes the changes of density with different depths at etching time of a 0 s, b 500 s, c 1500 s, d 2000 s, and e 2600 s. (a) XPS spectra of P 2p states and Si 2p for plasmon loss peak; (b) XPS spectra of Si 2p states; (c) XPS spectra of O 1s states. In order to display the change of binding energy of each elements intuitively, the intensity of spectrum curves in panel (a)–(c) are adjusted.
表 1 298 K(室温)时, SiP, SiO, SiO2 和P2O5的热力学函数参数(生成焓、热容、反应熵与形成能)
Table 1. Thermodynamic function parameters (formation enthalpy, heat capacity, reaction entropy and formation energy) of SiP, SiO, SiO2 and P2O5 at 298 K (RT).
$ {H}_{0}^{298} $/
eV${C}_{\mathrm{P} }$/
(J·mol–1·K–1)${ {S} }_{0}^{298}/$
(J·mol–1·K–1)形成能/
eVSiP –0.64 –5.65 33.35 –0.28 SiO –8.19 –2.05 211.18 –4.11 SiO2 –9.44 –10.08 43.63 –9.81 P2O5 –31.20 –15.61 17.07 –15.60 表 2 1193 K (920 ℃)时, SiP, SiO, SiO2 和 P2O5的热力学函数参数(生成焓、热容、反应熵与形成能)
Table 2. Thermodynamic function parameters (formation enthalpy, heat capacity, reaction entropy and Gibbs free energy) of SiP, SiO, SiO2 and P2O5 at 1193 K.
$ {H}_{0}^{1193} $/
eV$ {C}_{\mathrm{P}} $/
(J·mol–1·K–1)$ {S}_{0}^{1193} $/
(J·mol–1·K–1)$ {G}_{0}^{1193} $
/
eVSiP –53.05 –5.65 25.51 –53.37 SiO –27.21 –2.05 208.34 –29.79 SiO2 –102.95 –10.08 29.65 –103.31 P2O5 –176.01 –15.61 –4.58 –175.95 -
[1] Feldmann F, Bivour M, Reichel C, Steinkemper H, Hermle M, Glunz S W 2014 Sol. Energy Mater. Sol. Cells 131 46
 Google Scholar
Google Scholar
[2] Moldovan A, Feldmann F, Zimmer M, Rentsch J, Benick J, Hermle M 2015 Sol. Energy Mater. Sol. Cells 142 123
 Google Scholar
Google Scholar
[3] Shen W, Zhao Y, Liu F 2022 Front. Energy 16 40
 Google Scholar
Google Scholar
[4] Richter A, Müller R, Benick J, Feldmann F, Steinhauser B, Reichel C, Fell A, Bivour M, Hermle M, Glunz S W 2021 Nat. Energy 6 429
 Google Scholar
Google Scholar
[5] Long W, Yin S, Peng F G, Yang M, Fang L, Ru X N, Qu M H, Lin H F, Xu X X 2021 Sol. Energy Mater. Sol. Cells 231 111291
 Google Scholar
Google Scholar
[6] Yan D, Cuevas A, Michel J I, Zhang C, Wan Y, Zhang X, Bullock J 2021 Joule 5 811
 Google Scholar
Google Scholar
[7] Chen D, Chen Y, Wang Z, Gong J, Liu C, Zou Y, He Y, Wang Y, Yuan L, Lin W, Xia R, Yin L, Zhang X, Xu G, Yang Y, Shen H, Feng Z, Altermatt P P, Verlinden P J 2020 Sol. Energy Mater. Sol. Cells 206 110258
 Google Scholar
Google Scholar
[8] Yoshikawa K, Kawasaki H, Yoshida W, Irie T, Konishi K, Nakano K, Uto T, Adachi D, Kanematsu M, Uzu H 2017 Nat. Energy 2 17032
 Google Scholar
Google Scholar
[9] Chandra M N, Biswas S, Acharya S, Panda T, Sadhukhan S, Sharma J R, Nandi A, Bose S, Kole A, Das G, Maity S, Chaudhuri P, Saha H 2020 Mater. Sci. Semicond. Process. 119 105163
 Google Scholar
Google Scholar
[10] Padhamnath P, Khanna A, Nandakumar N, Nampalli N, Shanmugam V, Aberle A G, Duttagupta S 2020 Sol. Energy Mater. Sol. Cells 207 110358
 Google Scholar
Google Scholar
[11] Gao T, Yang Q, Guo X Q, Huang Y Q, Zhang Z, Wang Z X, Liao M D, Shou C H, Zeng Y H, Yan B J, Hou G F, Zhang X D, Zhao Y, Ye J C 2019 Sol. Energy Mater. Sol. Cells 200 109926
 Google Scholar
Google Scholar
[12] Kim D R, Lee C H, Weisse J M, Cho I S, Zheng X 2012 Nano Lett. 12 6485
 Google Scholar
Google Scholar
[13] Polzin J I, Hammann B, Niewelt T, Kwapil W, Hermle M, Feldmann F 2021 Sol. Energy Mater. Sol. Cells 230 111267
 Google Scholar
Google Scholar
[14] Padhamnath P, Khanna A, Balaji N, Shanmugam V, Nandakumar N, Wang D, Sun Q, Huang M, Huang S, Fan B, Ding B, Aberle A G, Duttagupta S 2020 Sol. Energy Mater. Sol. Cells 218 110751
 Google Scholar
Google Scholar
[15] Susa M, Kawagishi K, Tanaka N, Nagata K 1997 J. Electrochem. Soc. 144 2552
 Google Scholar
Google Scholar
[16] Hide I, Matsuyama T, Suzuki M, Yamashita H, Suzuki T, Moritani T, Maeda Y 1990 J. Cryst. Growth 99 1339
 Google Scholar
Google Scholar
[17] Fırat M, Payo M R, Duerinckx F, Luchies J-M, Lenes M, Poortmans J 2019 AIP Conf. Proc. 2147 040004
 Google Scholar
Google Scholar
[18] Kern W 1970 RCA Rev. 31 51
 Google Scholar
Google Scholar
[19] Lozac'h M, Nunomura S, Matsubara K 2020 Sol. Energy Mater. Sol. Cells 207 110357
 Google Scholar
Google Scholar
[20] Han L, Chen Z 2013 ECS J. Solid State Sci. Technol. 2 N228
 Google Scholar
Google Scholar
[21] Ying W B, Mizokawa Y, Kamiura Y, Kawamoto K, Yang W Y 2001 Appl. Surf. Sci. 181 1
 Google Scholar
Google Scholar
[22] Sherwood P M A 2002 Surf. Sci. Spectra 9 62
 Google Scholar
Google Scholar
[23] Moulder J F, Chastain J, King R C 1992 Handbook of X-ray Photoelectron Spectroscopy: a Reference Book of Standard Spectra for Identification and Interpretation of XPS Data (Waltham: Perkin-Elmer Corporation) pp230–232
[24] Chen K, Bothwell A, Guthrey H, Hartenstein M B, Polzin J I, Feldmann F, Nemeth W, Theingi S, Page M, Young D L, Stradins P, Agarwal S 2022 Sol. Energy Mater. Sol. Cells 236 111510
 Google Scholar
Google Scholar
[25] Monshi A, Foroughi M R, Monshi M R 2012 World J. Nano Sci. Eng. 2 154
 Google Scholar
Google Scholar
[26] Kale A S, Nemeth W, Guthrey H, Nanayakkara S U, LaSalvia V, Theingi S, Findley D, Page M, Al-Jassim M, Young D L, Stradins P, Agarwal S 2019 ACS Appl. Mater. Interfaces 11 42021
 Google Scholar
Google Scholar
[27] De Jong M, Chen W, Angsten T, Jain A, Notestine R, Gamst A, Sluiter M, Krishna Ande C, van der Zwaag S, Plata J J, Toher C, Curtarolo S, Ceder G, Persson K A, Asta M 2015 Sci. Data 2 150009
 Google Scholar
Google Scholar
[28] De Jong M, Chen W, Geerlings H, Asta M, Persson K A 2015 Sci. Data 2 150053
 Google Scholar
Google Scholar
[29] Jain A, Hautier G, Ong S P, Moore C J, Fischer C C, Persson K A, Ceder G 2011 Phys. Rev. B 84 045115
 Google Scholar
Google Scholar
[30] Perry D L, Phillips S L 1998 Handbook of inorganic compounds (Boca Raton: CRC Press)
[31] Jung I H, Hudon P 2012 J. Am. Ceram. Soc. 95 3665
 Google Scholar
Google Scholar
[32] Rahman M, Hudon P, Jung I H 2013 Metall. Mater. Trans. B 44 837
 Google Scholar
Google Scholar
[33] Boigelot R, Graz Y, Bourgel C, Defoort F, Poirier J 2015 Ceram. Int. 41 2353
 Google Scholar
Google Scholar
计量
- 文章访问数: 7690
- PDF下载量: 173
- 被引次数: 0














 下载:
下载: