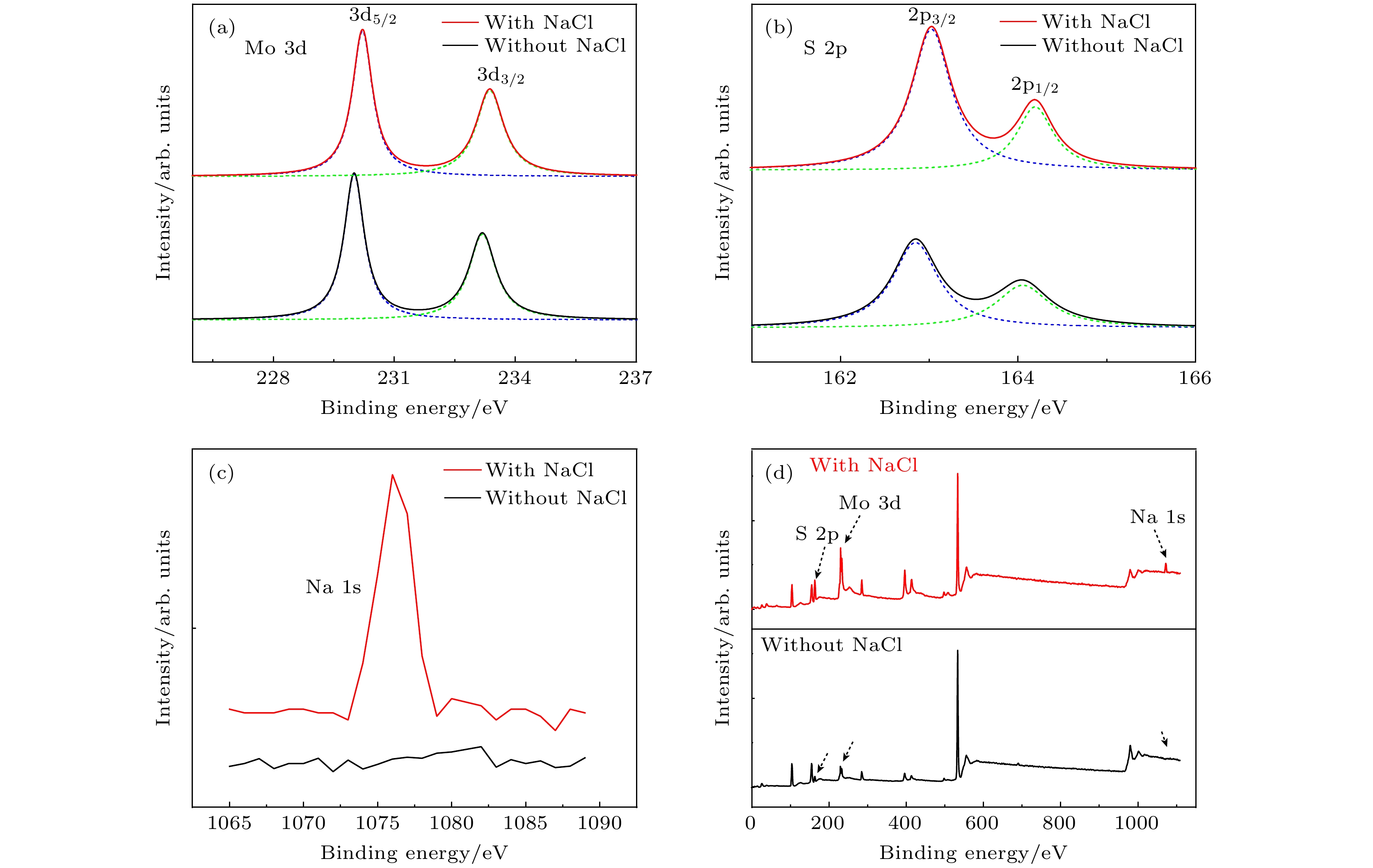
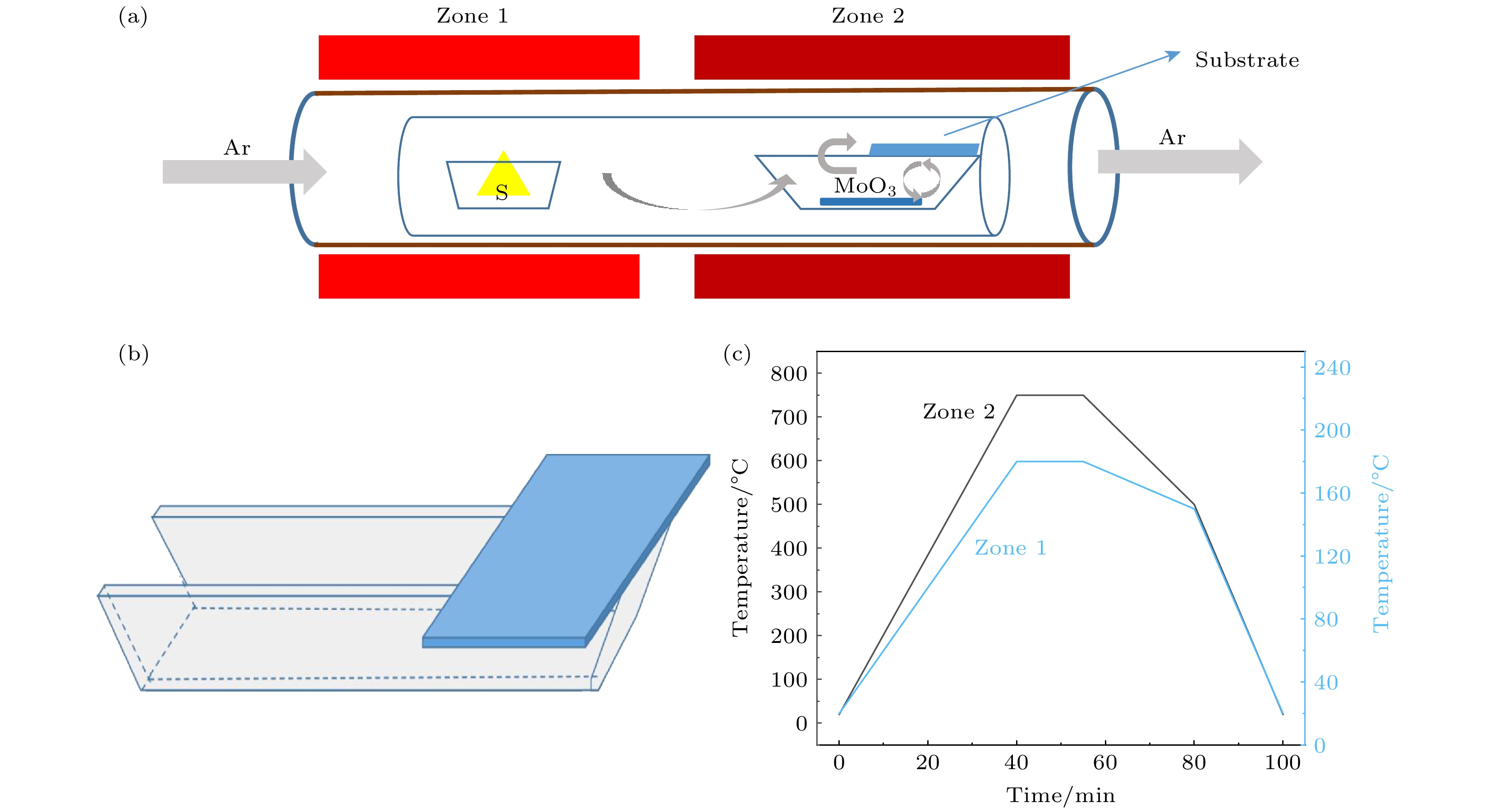
-
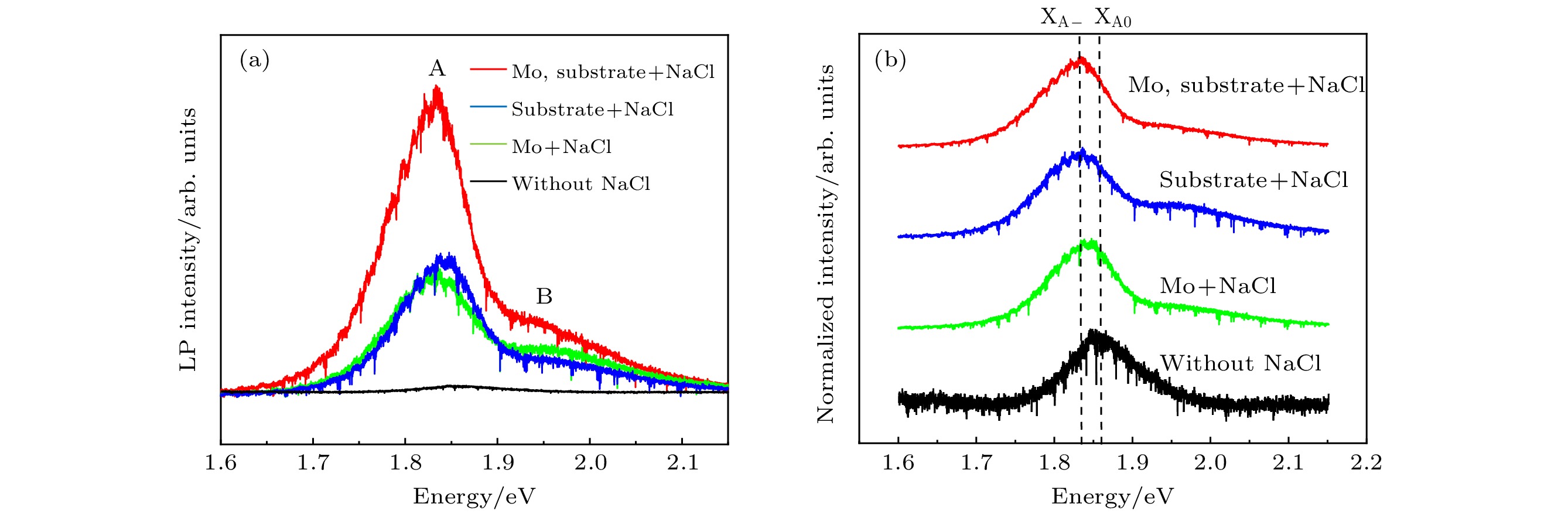
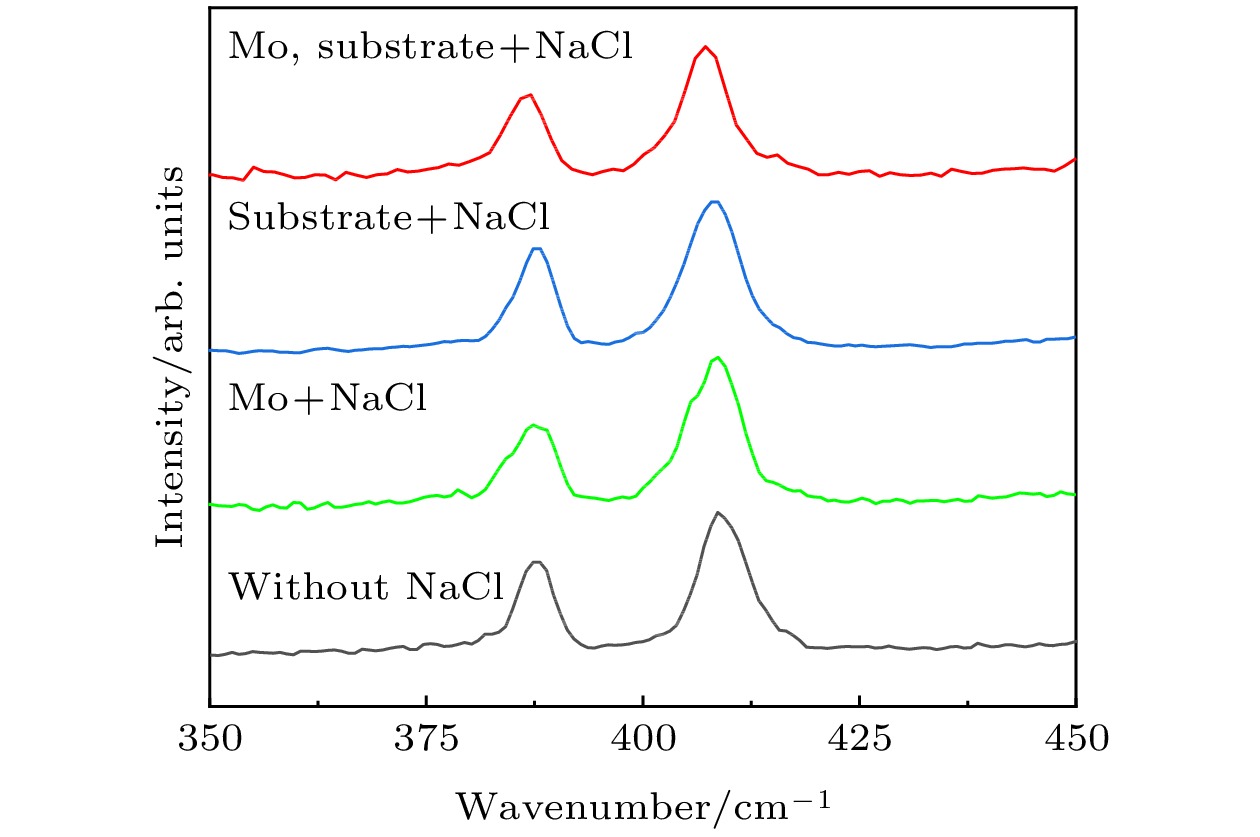
以单层二硫化钼(MoS2)为代表的过渡金属硫族化合物半导体材料具有良好的光学、电学性质, 近十年来引起了人们广泛的研究兴趣. 合成高质量单层MoS2薄膜是科学研究及工业应用的基础. 最近科研人员提出了盐辅助化学气相沉积生长单层薄膜的方法, 大大提高了单层MoS2薄膜的生长速度及晶体质量. 本文基于此方法, 提出利用氯化钠(NaCl)的双辅助方法, 成功制备了高质量的单层MoS2薄膜. 光致发光(PL)谱显示其发光强度比无NaCl辅助生长的样品有了明显的提高. 本文提出的NaCl双辅助生长方法为二维材料的大规模生长提供了思路.In recent years, transition metal dichalcogenides materials represented by monolayer molybdenum disulfide (MoS2) have aroused great interest due to their excellent optical and electrical properties. The synthesis method of high-quality monolayer MoS2 film is a key problem for scientific research and industrial application. Recently, researchers have proposed a salt-assisted chemical vapor deposition method for growing the monolayer films, which greatly promotes the growth rate and quality of monolayer film. By using this method, we design a growth source of semi-enclosed quartz boat, and successfully obtain high-quality monolayer MoS2 films by using the double auxiliary action of sodium chloride (NaCl). Scanning electron microscopy shows the excellent film formation, and the photoluminescence spectra show that the luminescence intensity is significantly higher than that of the sample grown without NaCl. The NaCl double-assisted growth method proposed in this study can reduce the growth temperature of MoS2, shorten the growth time, and improve the optical properties of the films. Besides, the operation is simple and the cost is low, which provides an idea for growing the large-scale two-dimensional materials.
-
Keywords:
- MoS2 /
- chemical vapor deposition /
- NaCl dual-assist strategy /
- monolayer film growth
[1] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, DUbonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[2] Xie Y, Wang Z, Zhan Y, Zhang P, Wu R, Jiang T, Wu S, Wang H, Zhao Y, Nan T, Ma X 2017 Nanotechnology 28 084001
 Google Scholar
Google Scholar
[3] Chen J, Zhao X, Tan S J, Xu H, Wu B, Liu B, Fu D, Fu W, Geng D, Liu Y, Liu W, Tang W, Li L, Zhou W, Sum T C, Loh K P 2017 J. Am. Chem. Soc. 139 1073
 Google Scholar
Google Scholar
[4] Wu T, Zhang X, Yuan Q, Xue J, Lu G, Liu Z, Wang H, Wang H, Ding F, Yu Q, Xie X, Jiang M 2016 Nat. Mater. 15 43
 Google Scholar
Google Scholar
[5] 徐依全, 王聪 2020 69 184216
 Google Scholar
Google Scholar
Xu Y Q, Wang C 2020 Acta Phys. Sin. 69 184216
 Google Scholar
Google Scholar
[6] Mak K F, Lee C, Hone J, Shan J, Heinz T F 2010 Phys. Rev. Lett. 105 136805
 Google Scholar
Google Scholar
[7] Yu Z, Ong Z Y, Li S, Xu J B, Zhang G, Zhang Y W, Shi Y, Wang X 2017 Adv. Funct. Mater. 27 1604093
 Google Scholar
Google Scholar
[8] Han T, Liu H, Wang S, Chen S, Li W, Yang X, Cai M, Yang K 2019 Nanomaterials 9 740
 Google Scholar
Google Scholar
[9] Lembke D, Kis A 2012 ACS Nano 6 10070
 Google Scholar
Google Scholar
[10] Zhang W, Huang J K, Chen C H, Chang Y H, Cheng Y J, Li L J 2013 Adv. Mater. 25 3456
 Google Scholar
Google Scholar
[11] Yin X B, Ye Z L, Chenet D A, Ye Y, Brien K O, Hone J C, Zhang X 2014 Science 344 488
 Google Scholar
Google Scholar
[12] 樊子冉, 孔洋洋, 李宇豪, 李志, 贾婷婷 2019 人工晶体学报 48 1190
 Google Scholar
Google Scholar
Fan Z R, Kong Y Y, Li Y H, Li Z, Jia T T 2019 J. Synth. Cryst. 48 1190
 Google Scholar
Google Scholar
[13] Fu D, Zhao X, Zhang Y Y, Li L, Xu H, Jang A R, Yoon S I, Song P, Poh S M, Ren T, Ding Z, Fu W, Shin T J, Shin H S, Pantelides S T, Zhou W, Loh K P 2017 J. Am. Chem. Soc. 139 9392
 Google Scholar
Google Scholar
[14] Zeng Z, Yin Z, Huang X, Li H, He Q, Lu G, Boey F, Zhang H 2011 Angew. Chem. Int. Ed. 50 11093
 Google Scholar
Google Scholar
[15] Wang S, Rong Y, Fan Y, Pacios M, Bhaskaran H, He K, Warner J H 2014 Chem. Mater. 26 6371
 Google Scholar
Google Scholar
[16] 魏晓旭, 程英, 霍达, 张宇涵, 王军转, 胡勇, 施毅 2014 63 217802
 Google Scholar
Google Scholar
Wei X X, Cheng Y, Huo D, Zhang Y H, Wang J Z, Hu Y, Shi Y 2014 Acta Phys. Sin. 63 217802
 Google Scholar
Google Scholar
[17] Huang Y, Pan Y H, Yang R, Bao L H, Meng L, Luo H L, Cai Y Q, Liu G D, Zhao W J, Zhou Z, Wu L M, Zhu Z L, Huang M, Liu L W, Liu L, Cheng P, Wu K H, Tian S B, Gu C Z, Shi Y G, Guo Y F, Cheng Z G, Hu J P, Zhao L, Yang G H, Sutter E, Sutter P, Wang Y L, Ji W, Zhou X J, Gao H J 2020 Nat. Commun. 11 2453
 Google Scholar
Google Scholar
[18] Liu X, Fechler N, Antonietti M 2013 Chem. Soc. Rev. 42 8237
 Google Scholar
Google Scholar
[19] Huang L, Hu Z, Jin H, Wu J, Liu K, Xu Z, Wan J, Zhou H, Duan J, Hu B, Zhou J 2020 Adv. Funct. Mater. 30 1908486
 Google Scholar
Google Scholar
[20] Chen K, Chen Z, Wan X, Zheng Z, Xie F, Chen W, Gui X, Chen H, Xie W, Xu J 2017 Adv. Mater. 29 1700704
 Google Scholar
Google Scholar
[21] Xie Y, Ma X, Wang Z, Nan T, Wu R, Zhang P, Wang H, Wang Y, Zhan Y, Hao Y 2018 MRS Adv. 3 365
 Google Scholar
Google Scholar
[22] Yang P, Zou X, Zhang Z, Hong M, Shi J, Chen S, Shu J, Zhao L, Jiang S, Zhou X, Huan Y, Xie C, Gao P, Chen Q, Zhang Q, Liu Z, Zhang Y 2018 Nat. Commun. 9 979
 Google Scholar
Google Scholar
[23] Lin Y C, Yeh C H, Lin H C, Siao M D, Liu Z, Nakajima H, Okazaki T, Chou M Y, Suenaga K, Chiu P W 2018 ACS Nano 12 12080
 Google Scholar
Google Scholar
[24] Pandey S K, Alsalman H, Azadani J G, Izquierdo N, Low T, Campbell S A 2018 Nanoscale 10 21374
 Google Scholar
Google Scholar
[25] Huan Y, Shi J, Zou X, Gong Y, Xie C, Yang Z, Zhang Z, Gao Y, Shi Y, Li M, Yang P, Jiang S, Hong M, Gu L, Zhang Q, Yan X, Zhang Y 2019 J. Am. Chem. Soc. 141 18694
 Google Scholar
Google Scholar
[26] Li P, Cui J, Zhou J, Guo D, Zhao Z, Yi J, Fan J, Ji Z, Jing X, Qu F, Yang C, Lu L, Lin J, Liu Z, Liu G 2019 Adv. Mater. 31 e1904641
 Google Scholar
Google Scholar
[27] Lan F, Yang R, Xu Y, Qian S, Zhang S, Cheng H, Zhang Y 2018 Nanomaterials 8 100
 Google Scholar
Google Scholar
[28] Zhou J, Lin J, Huang X, Zhou Y, Chen Y, Xia J, Wang H, Xie Y, Yu H, Lei J, Wu D, Liu F, Fu Q, Zeng Q, Hsu C H, Yang C, Lu L, Yu T, Shen Z, Lin H, Yakobson B I, Liu Q, Suenaga K, Liu G, Liu Z 2018 Nature 556 355
 Google Scholar
Google Scholar
[29] Chen L, Zang L, Chen L, Wu J, Jiang C, Song J 2021 CrystEngComm 23 5337
 Google Scholar
Google Scholar
[30] Li S, Wang S, Tang D M, Zhao W, Xu H, Chu L, Bando Y, Golberg D, Eda G 2015 Appl. Mater. Today 1 60
 Google Scholar
Google Scholar
[31] Wang P, Lei J, Qu J, Cao S, Jiang H, He M, Shi H, Sun X, Gao B, Liu W 2019 Chem. Mater. 31 873
 Google Scholar
Google Scholar
[32] Xie C, Yang P, Huan Y, Cui F, Zhang Y 2020 Dalton Trans. 49 10319
 Google Scholar
Google Scholar
[33] Wang W, Shu H, Wang J, Cheng Y, Liang P, Chen X 2020 ACS Appl. Mater. Interfaces 12 9563
 Google Scholar
Google Scholar
[34] Song J G, Ryu G H, Lee S J, Sim S, Lee C W, Choi T, Jung H, Kim Y, Lee Z, Myoung J M, Dussarrat C, Lansalot-Matras C, Park J, Choi H, Kim H 2015 Nat. Commun. 6 7817
 Google Scholar
Google Scholar
[35] Splendiani A, Sun L, Zhang Y, Li T, Kim J, Chim C Y, Galli G, Wang F 2010 Nano Lett. 10 1271
 Google Scholar
Google Scholar
[36] Mak K F, He K, Lee C, Lee G H, Hone J, Heinz T F, Shan J 2013 Nat. Mater. 12 207
 Google Scholar
Google Scholar
-
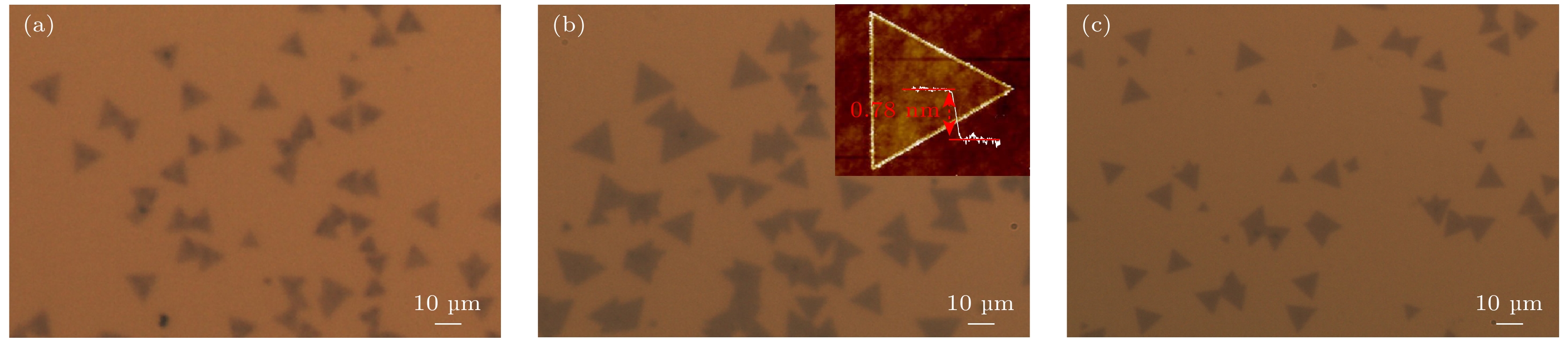
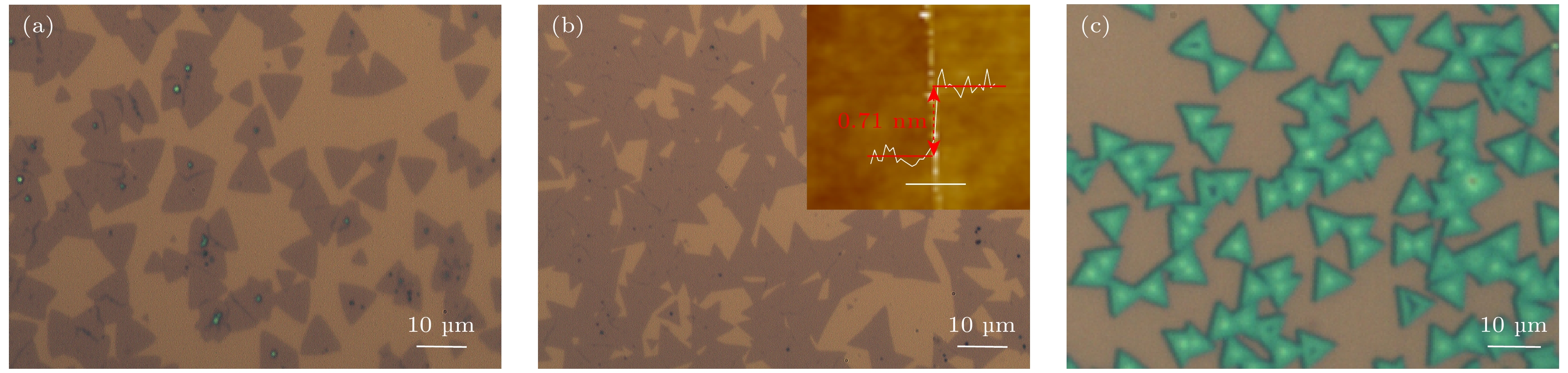
图 5 (a)—(c) 不同浓度的NaCl搭配下的光学显微镜图像 (a) 10 mg + 0.1 mmol/L; (b) 10 mg + 0.3 mmol/L, 内插图为MoS2边界的AFM图; (c) 10 mg + 0.5 mmol/L. (d) 10 mg + 0.3 mmol/L条件下生长的MoS2在Si衬底的照片以及左中右三块区域的大范围SEM图像
Fig. 5. (a)–(c) Optical microscope images with different concentrations of NaCl: (a) 10 mg + 0.1 mmol/L; (b) 10 mg + 0.3 mmol/L, the inside image is an AFM diagram of MoS2 boundary; (c) 10 mg + 0.5 mmol/L. (d) Photographs of MoS2 grown under the condition of 10 mg + 0.3 mmol/L and large range SEM images corresponding to the three regions of left, middle and right
-
[1] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, DUbonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[2] Xie Y, Wang Z, Zhan Y, Zhang P, Wu R, Jiang T, Wu S, Wang H, Zhao Y, Nan T, Ma X 2017 Nanotechnology 28 084001
 Google Scholar
Google Scholar
[3] Chen J, Zhao X, Tan S J, Xu H, Wu B, Liu B, Fu D, Fu W, Geng D, Liu Y, Liu W, Tang W, Li L, Zhou W, Sum T C, Loh K P 2017 J. Am. Chem. Soc. 139 1073
 Google Scholar
Google Scholar
[4] Wu T, Zhang X, Yuan Q, Xue J, Lu G, Liu Z, Wang H, Wang H, Ding F, Yu Q, Xie X, Jiang M 2016 Nat. Mater. 15 43
 Google Scholar
Google Scholar
[5] 徐依全, 王聪 2020 69 184216
 Google Scholar
Google Scholar
Xu Y Q, Wang C 2020 Acta Phys. Sin. 69 184216
 Google Scholar
Google Scholar
[6] Mak K F, Lee C, Hone J, Shan J, Heinz T F 2010 Phys. Rev. Lett. 105 136805
 Google Scholar
Google Scholar
[7] Yu Z, Ong Z Y, Li S, Xu J B, Zhang G, Zhang Y W, Shi Y, Wang X 2017 Adv. Funct. Mater. 27 1604093
 Google Scholar
Google Scholar
[8] Han T, Liu H, Wang S, Chen S, Li W, Yang X, Cai M, Yang K 2019 Nanomaterials 9 740
 Google Scholar
Google Scholar
[9] Lembke D, Kis A 2012 ACS Nano 6 10070
 Google Scholar
Google Scholar
[10] Zhang W, Huang J K, Chen C H, Chang Y H, Cheng Y J, Li L J 2013 Adv. Mater. 25 3456
 Google Scholar
Google Scholar
[11] Yin X B, Ye Z L, Chenet D A, Ye Y, Brien K O, Hone J C, Zhang X 2014 Science 344 488
 Google Scholar
Google Scholar
[12] 樊子冉, 孔洋洋, 李宇豪, 李志, 贾婷婷 2019 人工晶体学报 48 1190
 Google Scholar
Google Scholar
Fan Z R, Kong Y Y, Li Y H, Li Z, Jia T T 2019 J. Synth. Cryst. 48 1190
 Google Scholar
Google Scholar
[13] Fu D, Zhao X, Zhang Y Y, Li L, Xu H, Jang A R, Yoon S I, Song P, Poh S M, Ren T, Ding Z, Fu W, Shin T J, Shin H S, Pantelides S T, Zhou W, Loh K P 2017 J. Am. Chem. Soc. 139 9392
 Google Scholar
Google Scholar
[14] Zeng Z, Yin Z, Huang X, Li H, He Q, Lu G, Boey F, Zhang H 2011 Angew. Chem. Int. Ed. 50 11093
 Google Scholar
Google Scholar
[15] Wang S, Rong Y, Fan Y, Pacios M, Bhaskaran H, He K, Warner J H 2014 Chem. Mater. 26 6371
 Google Scholar
Google Scholar
[16] 魏晓旭, 程英, 霍达, 张宇涵, 王军转, 胡勇, 施毅 2014 63 217802
 Google Scholar
Google Scholar
Wei X X, Cheng Y, Huo D, Zhang Y H, Wang J Z, Hu Y, Shi Y 2014 Acta Phys. Sin. 63 217802
 Google Scholar
Google Scholar
[17] Huang Y, Pan Y H, Yang R, Bao L H, Meng L, Luo H L, Cai Y Q, Liu G D, Zhao W J, Zhou Z, Wu L M, Zhu Z L, Huang M, Liu L W, Liu L, Cheng P, Wu K H, Tian S B, Gu C Z, Shi Y G, Guo Y F, Cheng Z G, Hu J P, Zhao L, Yang G H, Sutter E, Sutter P, Wang Y L, Ji W, Zhou X J, Gao H J 2020 Nat. Commun. 11 2453
 Google Scholar
Google Scholar
[18] Liu X, Fechler N, Antonietti M 2013 Chem. Soc. Rev. 42 8237
 Google Scholar
Google Scholar
[19] Huang L, Hu Z, Jin H, Wu J, Liu K, Xu Z, Wan J, Zhou H, Duan J, Hu B, Zhou J 2020 Adv. Funct. Mater. 30 1908486
 Google Scholar
Google Scholar
[20] Chen K, Chen Z, Wan X, Zheng Z, Xie F, Chen W, Gui X, Chen H, Xie W, Xu J 2017 Adv. Mater. 29 1700704
 Google Scholar
Google Scholar
[21] Xie Y, Ma X, Wang Z, Nan T, Wu R, Zhang P, Wang H, Wang Y, Zhan Y, Hao Y 2018 MRS Adv. 3 365
 Google Scholar
Google Scholar
[22] Yang P, Zou X, Zhang Z, Hong M, Shi J, Chen S, Shu J, Zhao L, Jiang S, Zhou X, Huan Y, Xie C, Gao P, Chen Q, Zhang Q, Liu Z, Zhang Y 2018 Nat. Commun. 9 979
 Google Scholar
Google Scholar
[23] Lin Y C, Yeh C H, Lin H C, Siao M D, Liu Z, Nakajima H, Okazaki T, Chou M Y, Suenaga K, Chiu P W 2018 ACS Nano 12 12080
 Google Scholar
Google Scholar
[24] Pandey S K, Alsalman H, Azadani J G, Izquierdo N, Low T, Campbell S A 2018 Nanoscale 10 21374
 Google Scholar
Google Scholar
[25] Huan Y, Shi J, Zou X, Gong Y, Xie C, Yang Z, Zhang Z, Gao Y, Shi Y, Li M, Yang P, Jiang S, Hong M, Gu L, Zhang Q, Yan X, Zhang Y 2019 J. Am. Chem. Soc. 141 18694
 Google Scholar
Google Scholar
[26] Li P, Cui J, Zhou J, Guo D, Zhao Z, Yi J, Fan J, Ji Z, Jing X, Qu F, Yang C, Lu L, Lin J, Liu Z, Liu G 2019 Adv. Mater. 31 e1904641
 Google Scholar
Google Scholar
[27] Lan F, Yang R, Xu Y, Qian S, Zhang S, Cheng H, Zhang Y 2018 Nanomaterials 8 100
 Google Scholar
Google Scholar
[28] Zhou J, Lin J, Huang X, Zhou Y, Chen Y, Xia J, Wang H, Xie Y, Yu H, Lei J, Wu D, Liu F, Fu Q, Zeng Q, Hsu C H, Yang C, Lu L, Yu T, Shen Z, Lin H, Yakobson B I, Liu Q, Suenaga K, Liu G, Liu Z 2018 Nature 556 355
 Google Scholar
Google Scholar
[29] Chen L, Zang L, Chen L, Wu J, Jiang C, Song J 2021 CrystEngComm 23 5337
 Google Scholar
Google Scholar
[30] Li S, Wang S, Tang D M, Zhao W, Xu H, Chu L, Bando Y, Golberg D, Eda G 2015 Appl. Mater. Today 1 60
 Google Scholar
Google Scholar
[31] Wang P, Lei J, Qu J, Cao S, Jiang H, He M, Shi H, Sun X, Gao B, Liu W 2019 Chem. Mater. 31 873
 Google Scholar
Google Scholar
[32] Xie C, Yang P, Huan Y, Cui F, Zhang Y 2020 Dalton Trans. 49 10319
 Google Scholar
Google Scholar
[33] Wang W, Shu H, Wang J, Cheng Y, Liang P, Chen X 2020 ACS Appl. Mater. Interfaces 12 9563
 Google Scholar
Google Scholar
[34] Song J G, Ryu G H, Lee S J, Sim S, Lee C W, Choi T, Jung H, Kim Y, Lee Z, Myoung J M, Dussarrat C, Lansalot-Matras C, Park J, Choi H, Kim H 2015 Nat. Commun. 6 7817
 Google Scholar
Google Scholar
[35] Splendiani A, Sun L, Zhang Y, Li T, Kim J, Chim C Y, Galli G, Wang F 2010 Nano Lett. 10 1271
 Google Scholar
Google Scholar
[36] Mak K F, He K, Lee C, Lee G H, Hone J, Heinz T F, Shan J 2013 Nat. Mater. 12 207
 Google Scholar
Google Scholar
计量
- 文章访问数: 9455
- PDF下载量: 312
- 被引次数: 0













 下载:
下载: