-
通过提拉法成功生长了GdScO3及Yb:GdScO3晶体, 分别对这两种晶体进行了空气气氛退火和氢气气氛退火, 并进行了X射线粉末衍射、激光拉曼光谱和透射光谱测试, 通过Rietveld精修给出了晶体的晶胞参数、原子坐标和温度因子等. 发现空气气氛退火使晶胞体积增大, 氢气气氛退火使晶胞体积减小, 说明氮气气氛中生长的晶体存在氧填隙缺陷, 空气气氛退火使晶体氧填隙缺陷增加, 氢气气氛退火减少了氧填隙缺陷. 退火气氛对GdScO3和Yb:GdScO3晶体的拉曼峰都不敏感, 掺Yb3+离子后使155 cm–1, 298 cm–1, 351 cm–1拉曼峰减弱或消失. 可见, 850 nm波段的GdScO3吸收损耗可能主要来自于氧填隙引起的缺陷能级吸收; Yb:GdScO3和GdScO3在1000—3000 nm波段的吸收损耗则由于空气或氢气气氛退火在导带或价带附近产生了陷阱能级所致. 这些结果为进一步优化和研究稀土掺杂GdScO3晶体的激光性能奠定了基础.GdScO3 and Yb:GdScO3 single crystals are grown by the chzochralski method in nitrogen atmosphere, and they are characterized by X-ray diffraction(XRD), Raman spectra and transmission spectra . Their lattice parameters, atomic coordinates and temperature factors are determined by Rietveld refinement. It is found that the cell volume of GdScO3 and Yb:GdScO3 annealed in air atmosphere increase, but after these sample are annealed in H2 atmosphere their cell volumes decrease. Based on these results, we demonstrate that the crystal grown in nitrogen atmosphere has interstitial oxygen atoms, and the number of interstitial oxygen atoms in the sample annealed in air atmosphere increases, but that annealed in H2 atmosphere decreases. The Raman peaks of 155 cm–1, 298 cm–1, 351 cm–1 of GdScO3 are weakened or even disappear when Yb3+ ions are doped into it. The Raman spectra of the Yb:GdScO3 unannealed and annealed in H2 and air atmosphere are nearly consistent with each other, which indicates that Raman spectrum is insensitive to the defects such as oxygen interstitial caused by annealing. It is suggested that the optical loss of GdScO3 in the visible wavelength originates mainly from the defect energy level absorption of oxygen interstitial, and transmissivity of Yb:GdScO3 increases when it is annealed in hydrogen atmosphere, which results from the fact that ytterbium ion can reduce some interstitial oxygen atoms. When GdScO3 and Yb:GdScO3 are annealed in air or hydrogen atmosphere, the optical absorption loss of GdScO3 and Yb:GdScO3 in a wavelength range of 1000–3000 nm increase due to the trap level produced near the conduction or valence band. The effect on structure and spectral properties of Yb:GdScO3 and GdScO3 are explored preliminarily, which is useful for further studying and optimizing laser performance of rare earth doped GdScO3 crystal.
-
Keywords:
- GdScO3 /
- X-ray diffraction /
- transmission spectrum /
- Raman spectrum
[1] Chaix-Pluchery O, Kreisel J 2011 Phase Transitions 84 542
 Google Scholar
Google Scholar
[2] Sheng J M, Kan X C, Ge H, Yuan P Q, Zhang L, Zhao N, Song Z M, Yao Y Y, Tang J N, Wang S M, Tian M L, Tong X, Wu L S 2020 Chin. Phys. B 29 66
 Google Scholar
Google Scholar
[3] Jia J H, Ke Y J, Zhang X X, Wang J F, Su L, Wu Y D, Xia Z C 2019 J. Alloys Compd. 803 992
 Google Scholar
Google Scholar
[4] Rong S S, Faheem M B, Li Y B 2021 J. Electron. Sci. Technol. 19 119
 Google Scholar
Google Scholar
[5] Aamir M, Bibi I, Ata S, Jilani K, Majid F, Kamal S, Alwadai N, Raza M A S, Bashir M, Iqbal S, Aadil M, Iqbal M 2021 Ceram. Int. 47 16696
 Google Scholar
Google Scholar
[6] Lin S H, Lin Z Q, Chen C W 2021 Ceram. Int 47 16828
 Google Scholar
Google Scholar
[7] Rumyantsev S, Stillman W, Shur M, Heeg T, Schlom D G, Koveshnikov S, Kambhampati R, Tokranov V, Oktyabrsky S 2012 Int. J. High Speed Electron. Syst. 20 105
 Google Scholar
Google Scholar
[8] Mizzi C A, Koirala P, Marks L D 2018 Phys. Rev. Mater. 2 025001
 Google Scholar
Google Scholar
[9] Schäfer A, Besmehn A, Luysberg M, Winden A, Stoica T, Schnee M, Zander W, Niu G, Schroeder T, Mantl S, Hardtdegen H, Mikulics M, Schubert J 2014 Semicond. Sci. Technol. 29 075005
 Google Scholar
Google Scholar
[10] Schäfer A, Rahmanizadeh K, Bihlmayer G, Luysberg M, Wendt F, Besmehn A, Fox A, Schnee M, Niu G, Schroeder T, Mantl S, Hardtdegen H, Mikulics M, Schubert J 2015 J. Alloys Compd. 651 514
 Google Scholar
Google Scholar
[11] Uecker R, Velickov B, Klimm D, Bertram R, Bernhagen M, Rabe M, Albrecht M, Fornari R, Schlom D G 2008 J. Cryst. Growth 310 2649
 Google Scholar
Google Scholar
[12] Mansley Z R, Mizzi C A, Koirala P, Wen J, Marks L D 2020 Phys. Rev. Mater. 4 045003
 Google Scholar
Google Scholar
[13] Paull R J, Mansley Z R, Ly T, Marks L D, Poeppelmeier K R 2018 Inorg. Chem. 57 4104
 Google Scholar
Google Scholar
[14] Seidel S, Schmid A, Miersch C, Schubert J, Heitmann J 2021 Appl. Phys. Lett. 118 052902
 Google Scholar
Google Scholar
[15] Briones J, Guinto M C, Pelicano C M 2021 Mater. Lett. 298 130040
 Google Scholar
Google Scholar
[16] Liu Y 2021 IOP Conference Series:Earth and Environmental Science 781 022069
 Google Scholar
Google Scholar
[17] Hidde J, Guguschev C, Ganschow S, Klimm D 2018 J. Alloys Compd. 738 415
 Google Scholar
Google Scholar
[18] Wu Y D, Chen H, Hua J Y, Qin Y L, Ma X H, Wei Y Y, Zi Z F 2019 Ceram. Int. 45 13094
 Google Scholar
Google Scholar
[19] Peng F, Liu W, Zhang Q, Luo J, Sun D, Sun G, Zhang D, Wang X 2018 J. Lumin. 201 176
 Google Scholar
Google Scholar
[20] Li Q, Dong J, Wang Q, Xue Y, Tang H, Xu X, Xu J 2020 Opt. Mater. 109 110298
 Google Scholar
Google Scholar
[21] Li Q, Dong J, Wang Q, Zhao H, Xue Y, Tang H, Xu X, Xu J 2021 J. Lumin. 230 117681
 Google Scholar
Google Scholar
[22] Hou W, Zhao H, Qin Z, Liu J, Wang D, Xue Y, Wang Q, Xie G, Xu X, Xu J 2020 Opt. Mater. Express 10 2730
 Google Scholar
Google Scholar
[23] Wang D, Hou W, Li N, Xue Y, Wang Q, Xu X, Li D, Zhao H, Xu J 2019 Opt. Mater. Express 9 4218
 Google Scholar
Google Scholar
[24] Peng F, Liu W, Luo J, Sun D, Chen Y, Zhang H, Ding S, Zhang Q 2018 Crystengcomm 20 6291
 Google Scholar
Google Scholar
[25] Yamaji A, Kochurikhin V, Fujimoto Y, Futami Y, Yanagida T, Yokota Y, Kurosawa S, Yoshikawa A 2012 Phys. Status SolidiC 9 2267
 Google Scholar
Google Scholar
[26] Gupta S K, Grover V, Shukla R, Srinivasu K, Natarajan V, Tyagi A K 2016 Chem. Eng. J. 283 114
 Google Scholar
Google Scholar
[27] Arsenev P A, Bienert K E, Sviridova R K 1972 Phys. Status Solidi A 9 K103
 Google Scholar
Google Scholar
[28] Rietveld H 1967 Acta Crystallogr. 22 151
 Google Scholar
Google Scholar
[29] Rietveld H 1969 J. Appl. Crystallogr 2 65
 Google Scholar
Google Scholar
[30] 张克从, 张乐潓 1997 晶体生长科学与技术(上) (北京: 科学出版社) 第472页
Zhang K C, Zhang L H 1997 Crystal Growth Science and Technology (Vol. 1) (Beijing: Science Press) p472 (in Chinese)
[31] Chopelas A 2011 Phys. Chem. Miner. 38 709
 Google Scholar
Google Scholar
[32] Grover V, Shukla R, Jain D, Deshpande S K, Arya A, Pillai C G S, Tyagi A K 2012 Chem. Mater. 24 2186
 Google Scholar
Google Scholar
[33] Chaix-Pluchery O, Kreisel J 2009 J. Phys. Condens. Matter 21 175901
 Google Scholar
Google Scholar
[34] Weber M C, Guennou M, Zhao H J, Íñiguez J, Vilarinho R, Almeida A, Moreira J A, Kreisel J 2016 Phys. Rev. B 94 214103
 Google Scholar
Google Scholar
[35] Iliev M N, Abrashev M V, Laverdière J, Jandl S, Gospodinov M M, Wang Y Q, Sun Y Y 2006 Phys. Rev. B 73 064302
 Google Scholar
Google Scholar
[36] Alsowayigh M M, Timco G A, Borilovic I, Alanazi A, Vitorica-Yrezabal I J, Whitehead G F S, McNaughter P D, Tuna F, O'Brien P, Winpenny R E P, Lewis D J, Collison D 2020 Inorg. Chem. 59 15796
 Google Scholar
Google Scholar
[37] Singh M K, Jang H M, Gupta H C, Katiyar R S 2008 J. Raman Spectrosc. 39 842
 Google Scholar
Google Scholar
[38] Ruffo A, Mozzati M C, Albini B, Galinetto P, Bini M 2020 J. Mater. Sci.: Mater. Electron. 31 18263
 Google Scholar
Google Scholar
[39] Nikl M, Nitsch K, Hybler J, Chval J, Reiche P 1996 Phys. Status Solidi B 196 7
 Google Scholar
Google Scholar
[40] 李涛, 赵广军, 何晓明, 徐 军, 潘守夔 2002 人工晶体学报 31 456
Li T, Zhao G J, He X M, Xu J, Pan S K 2002 J. Artif. Cryst. 31 456
-
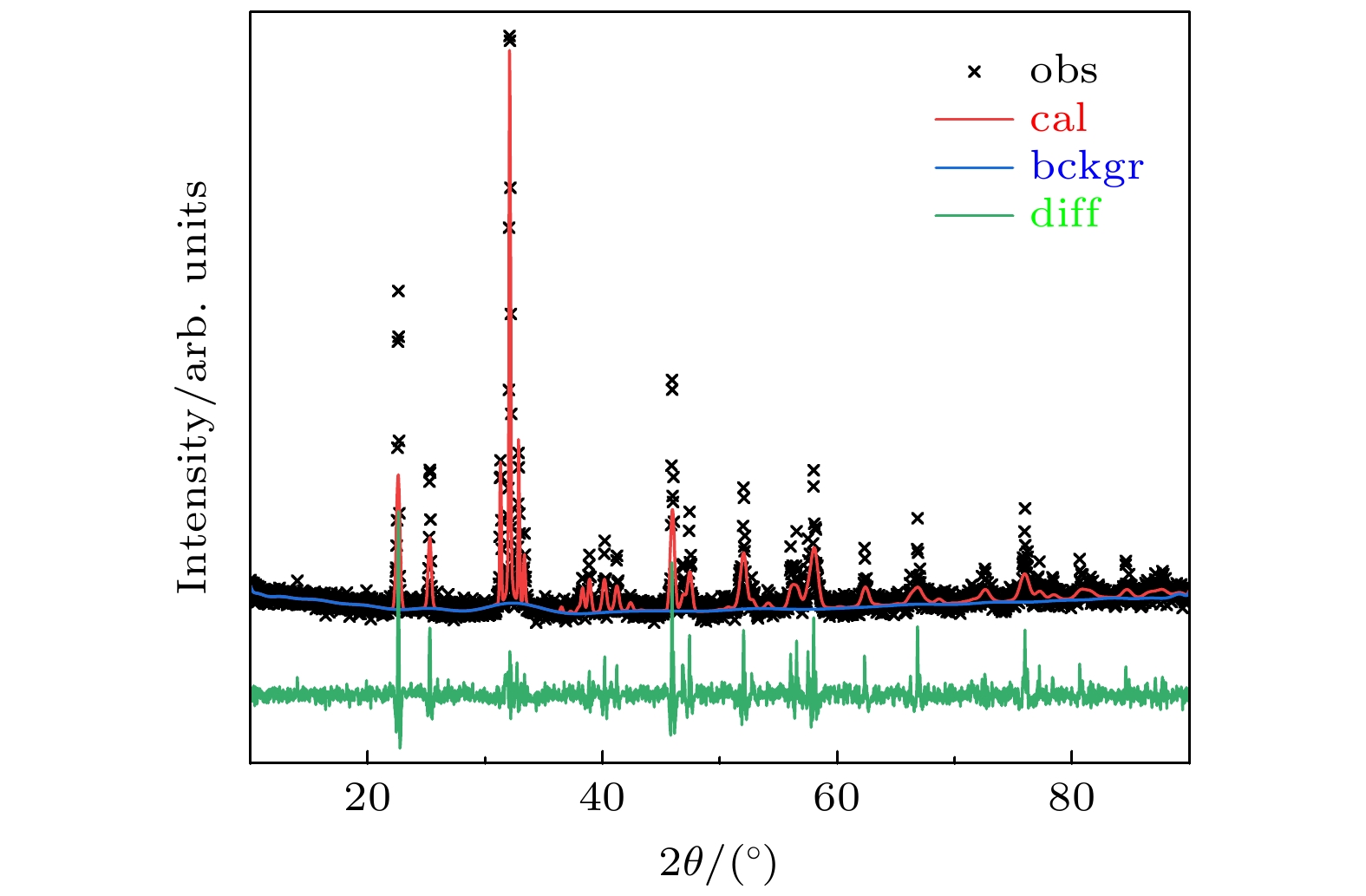
图 1 GdScO3晶体XRD数据Rietveld精修结果(cal, obs, bckgr和diff表示计算值、实验值、背底以及实验值和计算值之间的误差)
Fig. 1. Rietveld refinement results of the GdScO3 crystal obtained from the XRD data. (cal, obs, bckgr, and diff mean calculated data, observed data, background, and the difference between observed data and calculated data).
图 2 不同气氛退火GdScO3晶体XRD精修结果与GdScO3标准卡片(ICSD#65513) (a) Yb:GdScO3未退火; (b) Yb:GdScO3空气气氛退火; (c) Yb:GdScO3 H2气氛退火; (d) GdScO3空气气氛退火; (e) GdScO3 H2气氛退火; (f) GdScO3(ICSD#65513)
Fig. 2. Rietveld refinement results of the GdScO3 crystal obtained from the XRD data annealed in different atmospheres and (ICSD#65513): (a) Yb:GdScO3 unannealed; (b) Yb:GdScO3 annealed in air atmosphere; (c) Yb:GdScO3 annealed in H2; (d) GdScO3 annealed in air atmosphere; (e) GdScO3 annealed in H2; (f) GdScO3(ICSD#65513).
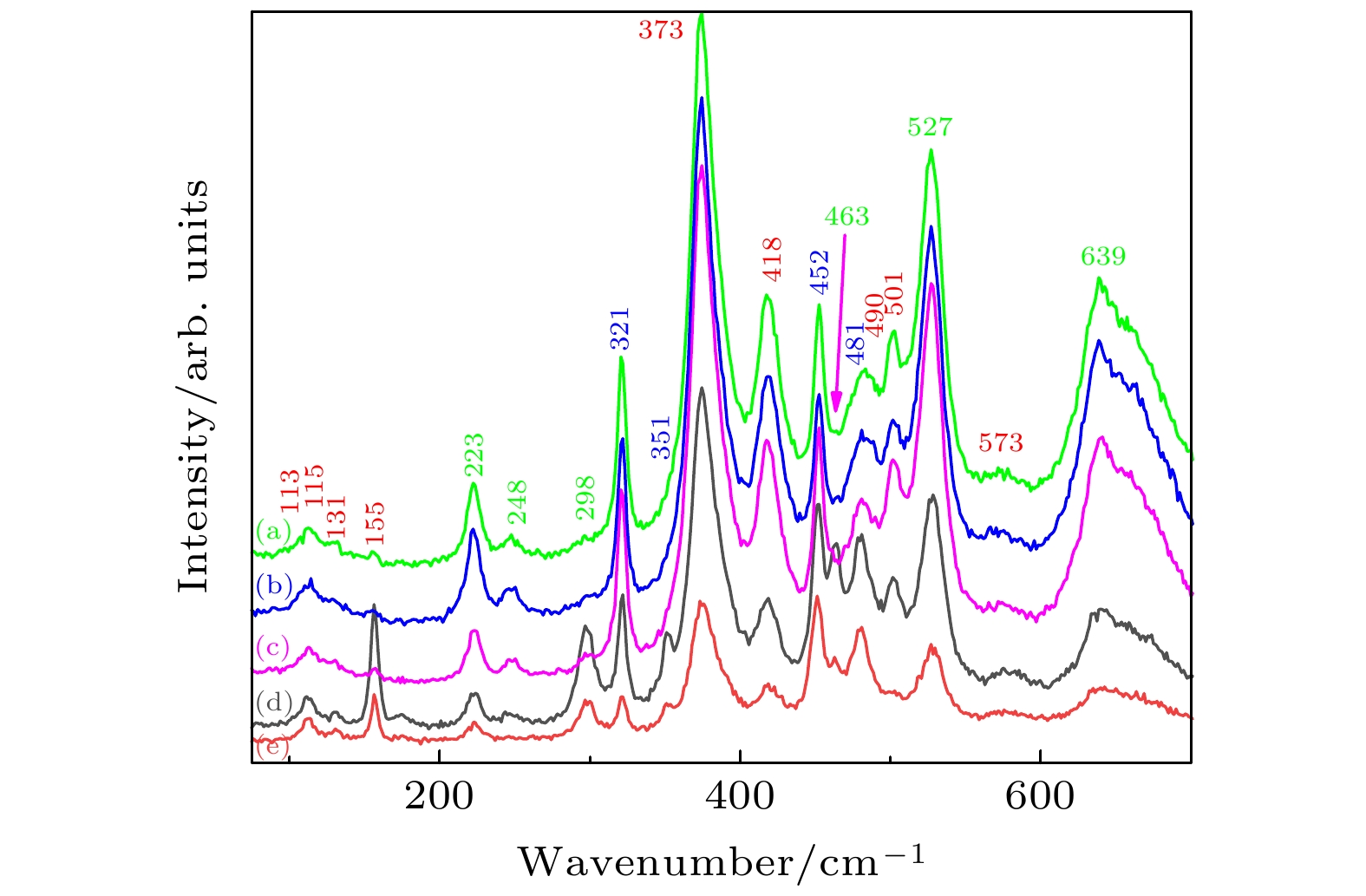
图 3 不同退火气氛下Yb:GdScO3和GdScO3晶体的拉曼光谱 (a) Yb:GdScO3 未退火; (b) Yb:GdScO3 空气气氛退火; (c) Yb:GdScO3 H2气氛退火; (d) GdScO3 空气气氛退火; (e) GdScO3 H2气氛退火
Fig. 3. Raman spectra of Yb:GdScO3 and GdScO3 crystals annealed in different atmospheres: (a) Yb:GdScO3 unannealed; ( b) Yb:GdScO3 annealed in air atmosphere; (c) Yb:GdScO3 annealed in H2; (d) GdScO3 annealed in air atmosphere; (e) GdScO3 annealed in H2.
图 4 不同退火气氛条件下GdScO3晶体在250—3000 nm范围内的透射光谱 (a) GdScO3 H2气氛退火(样品厚度d = 1.60 mm); (b) GdScO3 未退火(样品厚度d = 1.63 mm); (c) GdScO3 空气气氛退火(样品厚度d=1.62 mm)
Fig. 4. Transmittance spectra of the GdScO3 crystal before and after annealed in the range of 250–3000 nm: (a) GdScO3 annealed in H2; (b) GdScO3 unannealed; (c) GdScO3 annealedin air atmosphere.
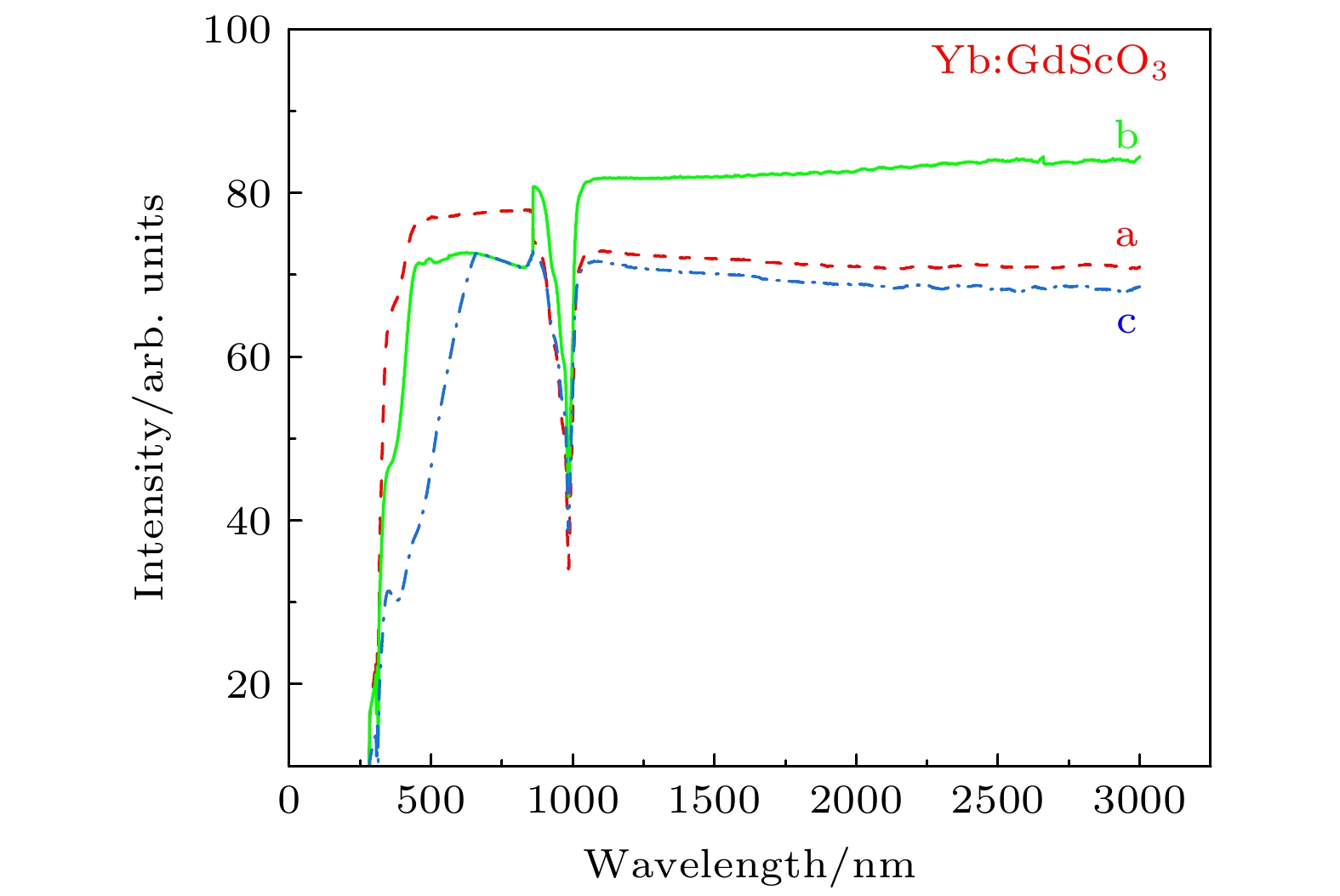
图 5 不同气氛条件下Yb:GdScO3晶体在250—3000 nm范围内的透射光谱 (a) Yb:GdScO3 H2气氛退火(样品厚度d = 1.65 mm); (b) Yb:GdScO3 未退火(样品厚度d = 1.60 mm); (c) Yb:GdScO3 空气气氛退火(样品厚度d = 1.66 mm)
Fig. 5. Transmittance spectra of the Yb:GdScO3 crystal before and after annealed in the range of 250—3000 nm: (a) Yb:GdScO3 annealed in H2; (b) Yb:GdScO3 unannealed; (c) Yb:GdScO3 annealed in air atmosphere.
表 1 GdScO3晶体XRD数据精修结构参数
Table 1. Refined structural parameters of GdScO3 crystal obtained from XRD data.
Atom Multiplicity
Wyckoff letterx y z Occupancy Uiso R factor Rp Rwp GdScO3 annealed in H2 O1 4c 0.451078 0.250000 0.112375 1.0144 0.01800 Sc1 4b 0.000000 0.000000 0.500000 0.9994 0.02418 4.12% 5.19% O2 8d 0.193300 0.557177 0.182774 0.9985 0.04452 Gd1 4c 0.440567 0.750000 0.482340 0.9268 0.01880 GdScO3 annealed in air atmosphere O1 4c 0.468803 0.250000 0.108393 0.0298 0.01489 Sc1 4b 0.000000 0.000000 0.500000 0.9984 0.01157 4.56% 5.83% O2 8d 0.205347 0.561459 0.188795 1.0616 0.01910 Gd1 4c 0.440566 0.750000 0.486551 1.0022 0.01328 Yb:GdScO3 annealed in H2 O1 4c 0.459576 0.250000 0.103701 1.1295 0.00793 Sc1 4b 0.000000 0.000000 0.500000 1.0062 0.01648 4.45% 5.66% O2 8d 0.199195 0.559033 0.183212 1.0482 0.01898 Gd1 4c 0.439748 0.750000 0.485806 0.9781 0.01645 Yb1 4c 0.451014 0.750000 0.404342 0.0189 0.07962 Yb:GdScO3 annealed in air atmosphere O1 4c 0.462904 0.250000 0.092804 1.0952 0.01311 Sc1 4b 0.000000 0.000000 0.500000 1.0241 0.02387 4.67% 5.99% O2 8d 0.184743 0.552496 0.189058 1.0101 0.03297 Gd1 4c 0.438027 0.750000 0.483159 0.9807 0.02051 Yb1 4c 0.443478 0.750000 0.413392 0.0192 0.04427 Yb:GdScO3 unannealed O1 4c 0.465020 0.250000 0.338290 1.3635 0.01824 Sc1 4b 0.000000 0.000000 0.500000 0.9707 0.01780 5.20% 7.13% O2 8d 0.177886 0.573337 0.202298 1.0466 0.03207 Gd1 4c 0.437167 0.750000 0.482011 0.9540 0.01456 Yb 1 4c 0.459029 0.750000 0.370978 0.0181 0.09000 表 2 不同气氛退火GdScO3和Yb: GdScO3的晶胞参数、晶胞体积和计算密度
Table 2. Refined lattice parameters, unit cell volumes and calculated densities of GdScO3 and Yb:GdScO3 annealed in different atmospheres.
GdScO3 a/Å b/Å c/Å V/Å3 ρ/(g·cm–3) GdScO3 annealed in H2 5.750224 7.936361 5.485410 250.331367 6.6380 GdScO3 annealed in air 5.750860 7.936271 5.485696 250.369268 6.6399 Yb:GdScO3 annealed in H2 5.747629 7.931895 5.481167 249.884155 6.6584 Yb:GdScO3 annealed in air 5.754268 7.942210 5.488072 250.843313 6.6328 Yb:GdScO3 unannealed 5.748397 7.936244 5.482695 250.124281 6.6519 -
[1] Chaix-Pluchery O, Kreisel J 2011 Phase Transitions 84 542
 Google Scholar
Google Scholar
[2] Sheng J M, Kan X C, Ge H, Yuan P Q, Zhang L, Zhao N, Song Z M, Yao Y Y, Tang J N, Wang S M, Tian M L, Tong X, Wu L S 2020 Chin. Phys. B 29 66
 Google Scholar
Google Scholar
[3] Jia J H, Ke Y J, Zhang X X, Wang J F, Su L, Wu Y D, Xia Z C 2019 J. Alloys Compd. 803 992
 Google Scholar
Google Scholar
[4] Rong S S, Faheem M B, Li Y B 2021 J. Electron. Sci. Technol. 19 119
 Google Scholar
Google Scholar
[5] Aamir M, Bibi I, Ata S, Jilani K, Majid F, Kamal S, Alwadai N, Raza M A S, Bashir M, Iqbal S, Aadil M, Iqbal M 2021 Ceram. Int. 47 16696
 Google Scholar
Google Scholar
[6] Lin S H, Lin Z Q, Chen C W 2021 Ceram. Int 47 16828
 Google Scholar
Google Scholar
[7] Rumyantsev S, Stillman W, Shur M, Heeg T, Schlom D G, Koveshnikov S, Kambhampati R, Tokranov V, Oktyabrsky S 2012 Int. J. High Speed Electron. Syst. 20 105
 Google Scholar
Google Scholar
[8] Mizzi C A, Koirala P, Marks L D 2018 Phys. Rev. Mater. 2 025001
 Google Scholar
Google Scholar
[9] Schäfer A, Besmehn A, Luysberg M, Winden A, Stoica T, Schnee M, Zander W, Niu G, Schroeder T, Mantl S, Hardtdegen H, Mikulics M, Schubert J 2014 Semicond. Sci. Technol. 29 075005
 Google Scholar
Google Scholar
[10] Schäfer A, Rahmanizadeh K, Bihlmayer G, Luysberg M, Wendt F, Besmehn A, Fox A, Schnee M, Niu G, Schroeder T, Mantl S, Hardtdegen H, Mikulics M, Schubert J 2015 J. Alloys Compd. 651 514
 Google Scholar
Google Scholar
[11] Uecker R, Velickov B, Klimm D, Bertram R, Bernhagen M, Rabe M, Albrecht M, Fornari R, Schlom D G 2008 J. Cryst. Growth 310 2649
 Google Scholar
Google Scholar
[12] Mansley Z R, Mizzi C A, Koirala P, Wen J, Marks L D 2020 Phys. Rev. Mater. 4 045003
 Google Scholar
Google Scholar
[13] Paull R J, Mansley Z R, Ly T, Marks L D, Poeppelmeier K R 2018 Inorg. Chem. 57 4104
 Google Scholar
Google Scholar
[14] Seidel S, Schmid A, Miersch C, Schubert J, Heitmann J 2021 Appl. Phys. Lett. 118 052902
 Google Scholar
Google Scholar
[15] Briones J, Guinto M C, Pelicano C M 2021 Mater. Lett. 298 130040
 Google Scholar
Google Scholar
[16] Liu Y 2021 IOP Conference Series:Earth and Environmental Science 781 022069
 Google Scholar
Google Scholar
[17] Hidde J, Guguschev C, Ganschow S, Klimm D 2018 J. Alloys Compd. 738 415
 Google Scholar
Google Scholar
[18] Wu Y D, Chen H, Hua J Y, Qin Y L, Ma X H, Wei Y Y, Zi Z F 2019 Ceram. Int. 45 13094
 Google Scholar
Google Scholar
[19] Peng F, Liu W, Zhang Q, Luo J, Sun D, Sun G, Zhang D, Wang X 2018 J. Lumin. 201 176
 Google Scholar
Google Scholar
[20] Li Q, Dong J, Wang Q, Xue Y, Tang H, Xu X, Xu J 2020 Opt. Mater. 109 110298
 Google Scholar
Google Scholar
[21] Li Q, Dong J, Wang Q, Zhao H, Xue Y, Tang H, Xu X, Xu J 2021 J. Lumin. 230 117681
 Google Scholar
Google Scholar
[22] Hou W, Zhao H, Qin Z, Liu J, Wang D, Xue Y, Wang Q, Xie G, Xu X, Xu J 2020 Opt. Mater. Express 10 2730
 Google Scholar
Google Scholar
[23] Wang D, Hou W, Li N, Xue Y, Wang Q, Xu X, Li D, Zhao H, Xu J 2019 Opt. Mater. Express 9 4218
 Google Scholar
Google Scholar
[24] Peng F, Liu W, Luo J, Sun D, Chen Y, Zhang H, Ding S, Zhang Q 2018 Crystengcomm 20 6291
 Google Scholar
Google Scholar
[25] Yamaji A, Kochurikhin V, Fujimoto Y, Futami Y, Yanagida T, Yokota Y, Kurosawa S, Yoshikawa A 2012 Phys. Status SolidiC 9 2267
 Google Scholar
Google Scholar
[26] Gupta S K, Grover V, Shukla R, Srinivasu K, Natarajan V, Tyagi A K 2016 Chem. Eng. J. 283 114
 Google Scholar
Google Scholar
[27] Arsenev P A, Bienert K E, Sviridova R K 1972 Phys. Status Solidi A 9 K103
 Google Scholar
Google Scholar
[28] Rietveld H 1967 Acta Crystallogr. 22 151
 Google Scholar
Google Scholar
[29] Rietveld H 1969 J. Appl. Crystallogr 2 65
 Google Scholar
Google Scholar
[30] 张克从, 张乐潓 1997 晶体生长科学与技术(上) (北京: 科学出版社) 第472页
Zhang K C, Zhang L H 1997 Crystal Growth Science and Technology (Vol. 1) (Beijing: Science Press) p472 (in Chinese)
[31] Chopelas A 2011 Phys. Chem. Miner. 38 709
 Google Scholar
Google Scholar
[32] Grover V, Shukla R, Jain D, Deshpande S K, Arya A, Pillai C G S, Tyagi A K 2012 Chem. Mater. 24 2186
 Google Scholar
Google Scholar
[33] Chaix-Pluchery O, Kreisel J 2009 J. Phys. Condens. Matter 21 175901
 Google Scholar
Google Scholar
[34] Weber M C, Guennou M, Zhao H J, Íñiguez J, Vilarinho R, Almeida A, Moreira J A, Kreisel J 2016 Phys. Rev. B 94 214103
 Google Scholar
Google Scholar
[35] Iliev M N, Abrashev M V, Laverdière J, Jandl S, Gospodinov M M, Wang Y Q, Sun Y Y 2006 Phys. Rev. B 73 064302
 Google Scholar
Google Scholar
[36] Alsowayigh M M, Timco G A, Borilovic I, Alanazi A, Vitorica-Yrezabal I J, Whitehead G F S, McNaughter P D, Tuna F, O'Brien P, Winpenny R E P, Lewis D J, Collison D 2020 Inorg. Chem. 59 15796
 Google Scholar
Google Scholar
[37] Singh M K, Jang H M, Gupta H C, Katiyar R S 2008 J. Raman Spectrosc. 39 842
 Google Scholar
Google Scholar
[38] Ruffo A, Mozzati M C, Albini B, Galinetto P, Bini M 2020 J. Mater. Sci.: Mater. Electron. 31 18263
 Google Scholar
Google Scholar
[39] Nikl M, Nitsch K, Hybler J, Chval J, Reiche P 1996 Phys. Status Solidi B 196 7
 Google Scholar
Google Scholar
[40] 李涛, 赵广军, 何晓明, 徐 军, 潘守夔 2002 人工晶体学报 31 456
Li T, Zhao G J, He X M, Xu J, Pan S K 2002 J. Artif. Cryst. 31 456
计量
- 文章访问数: 6263
- PDF下载量: 97
- 被引次数: 0













 下载:
下载: