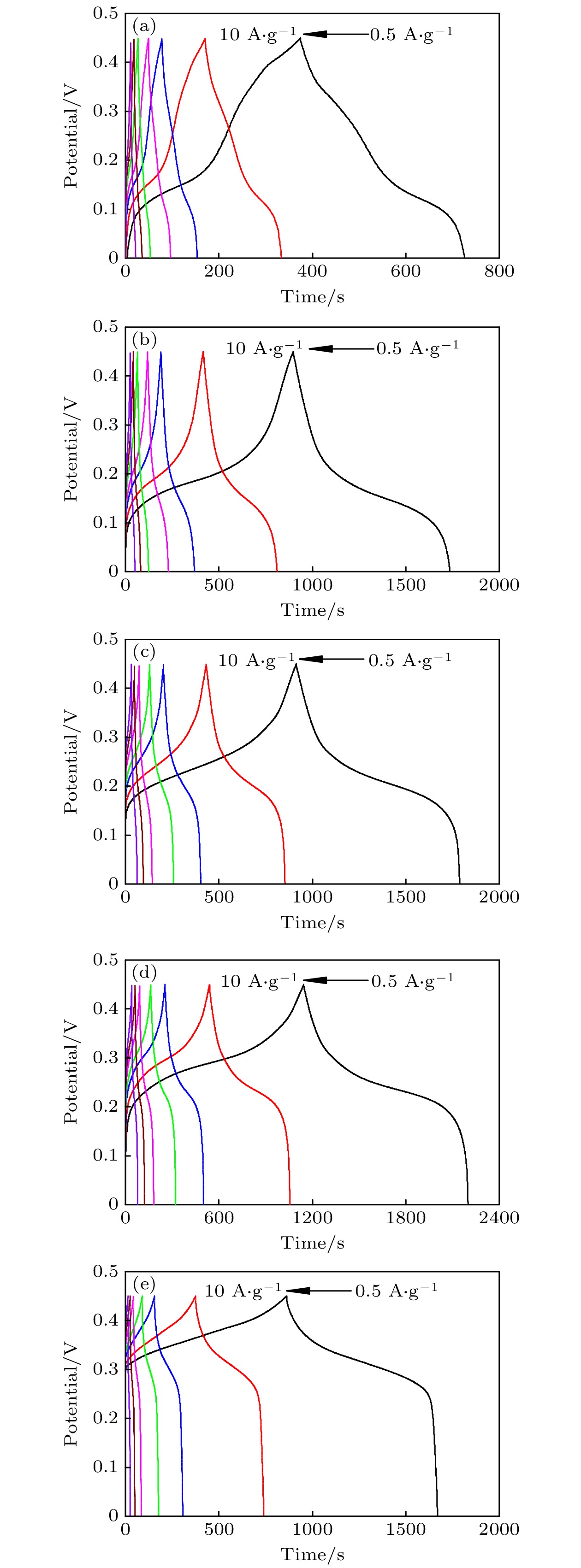
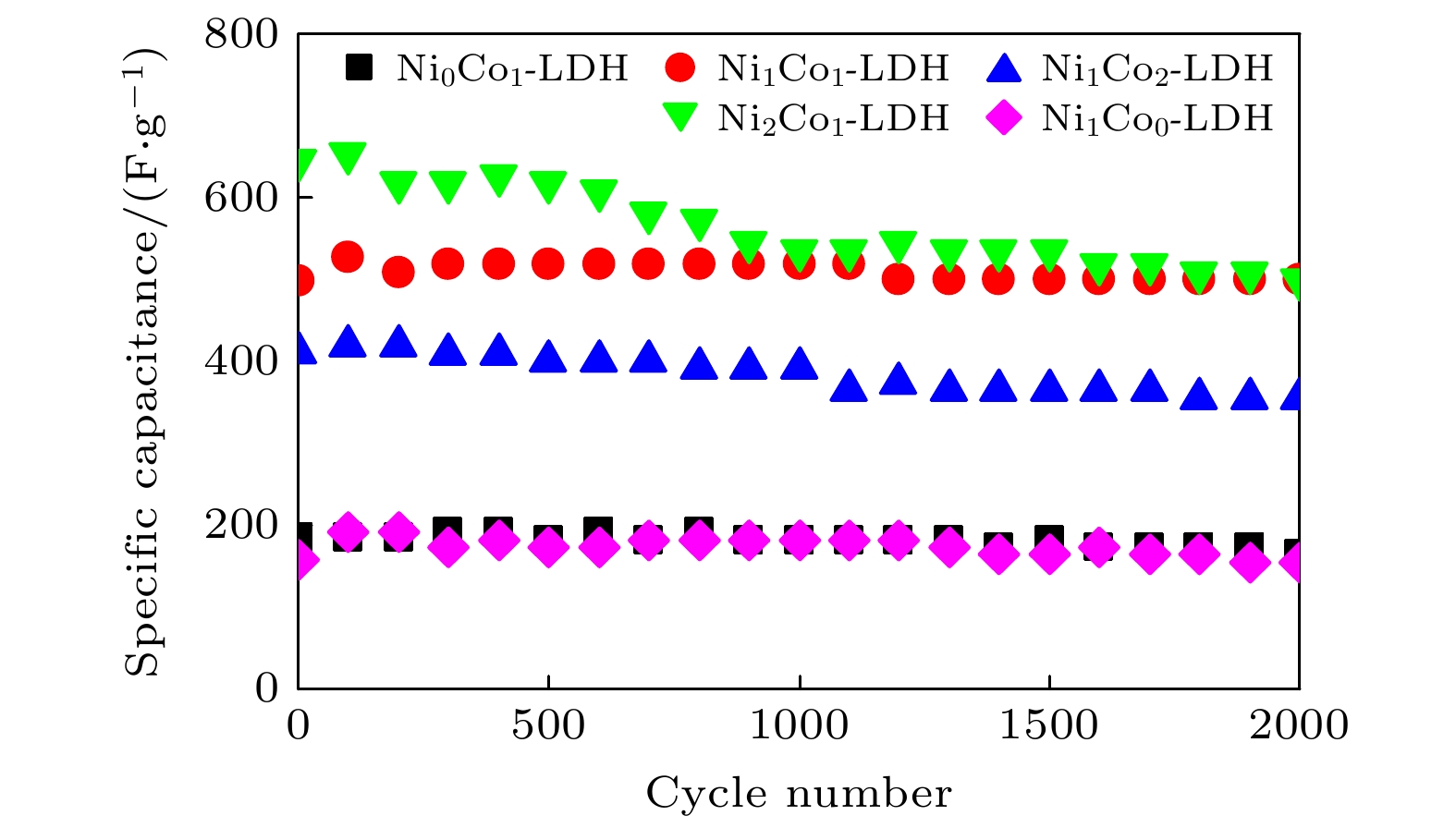
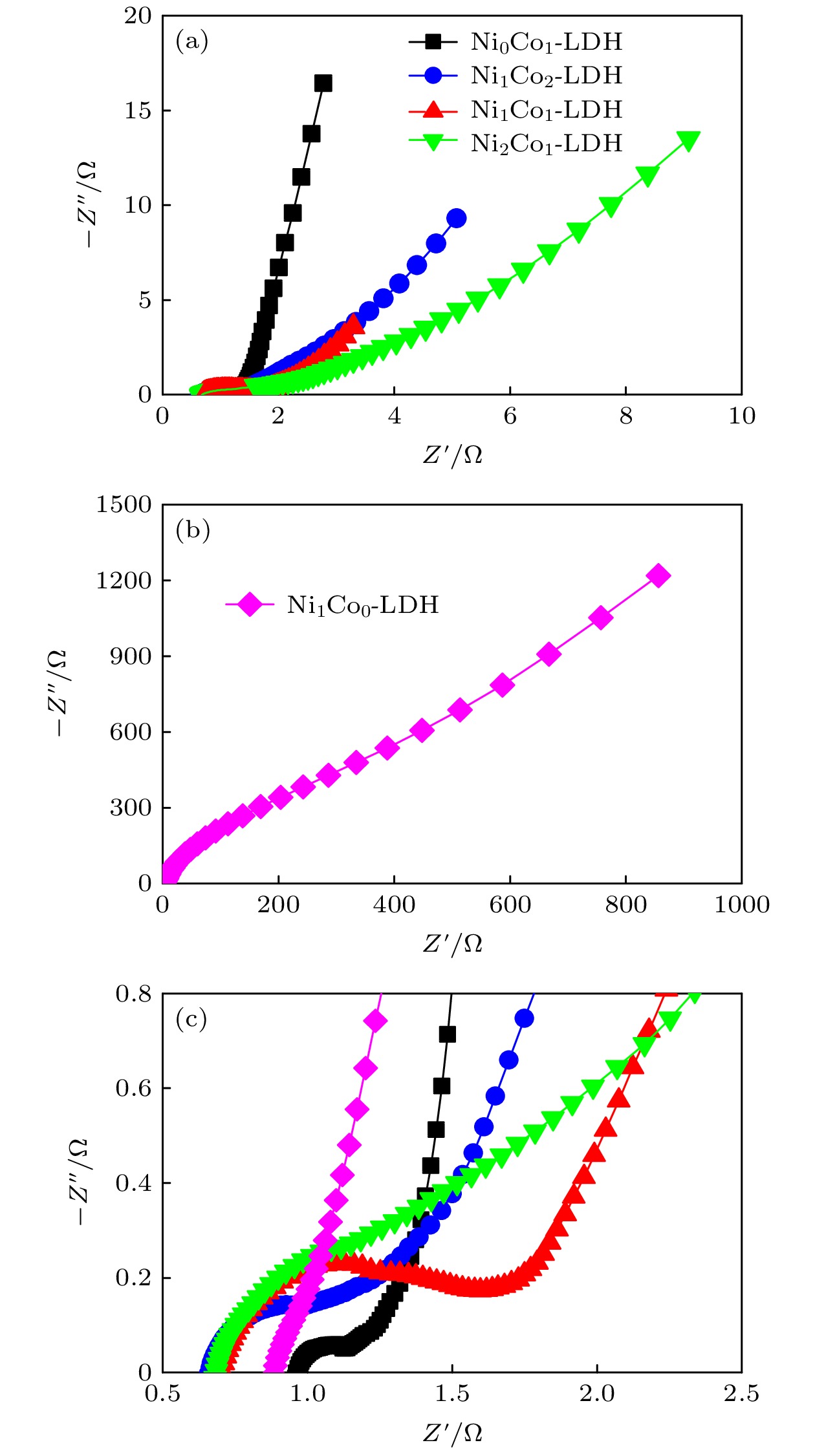
-
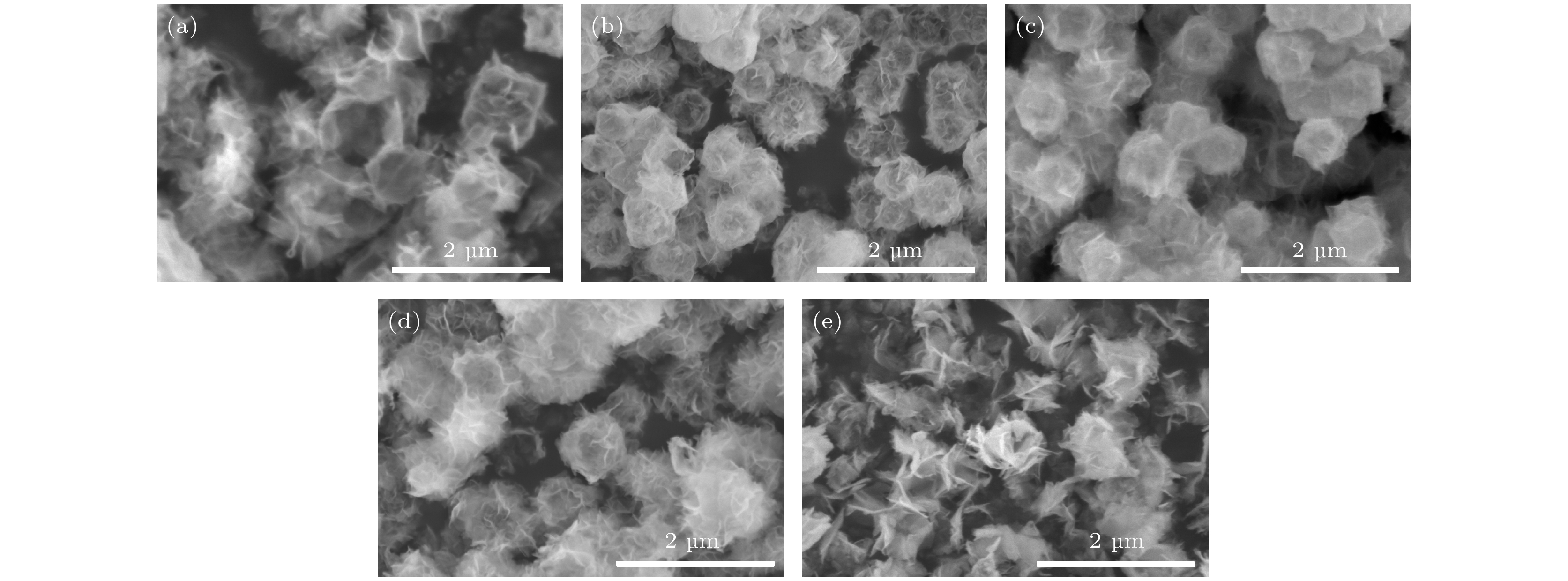
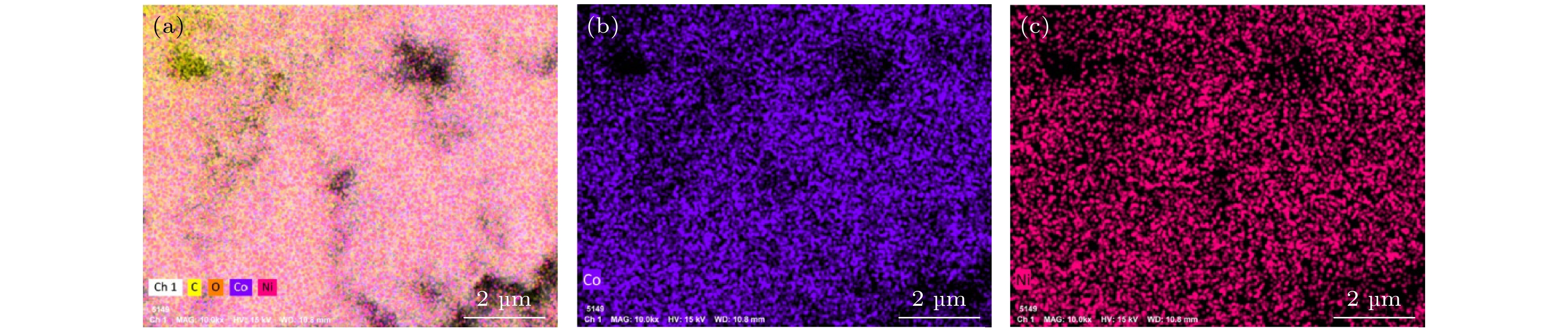
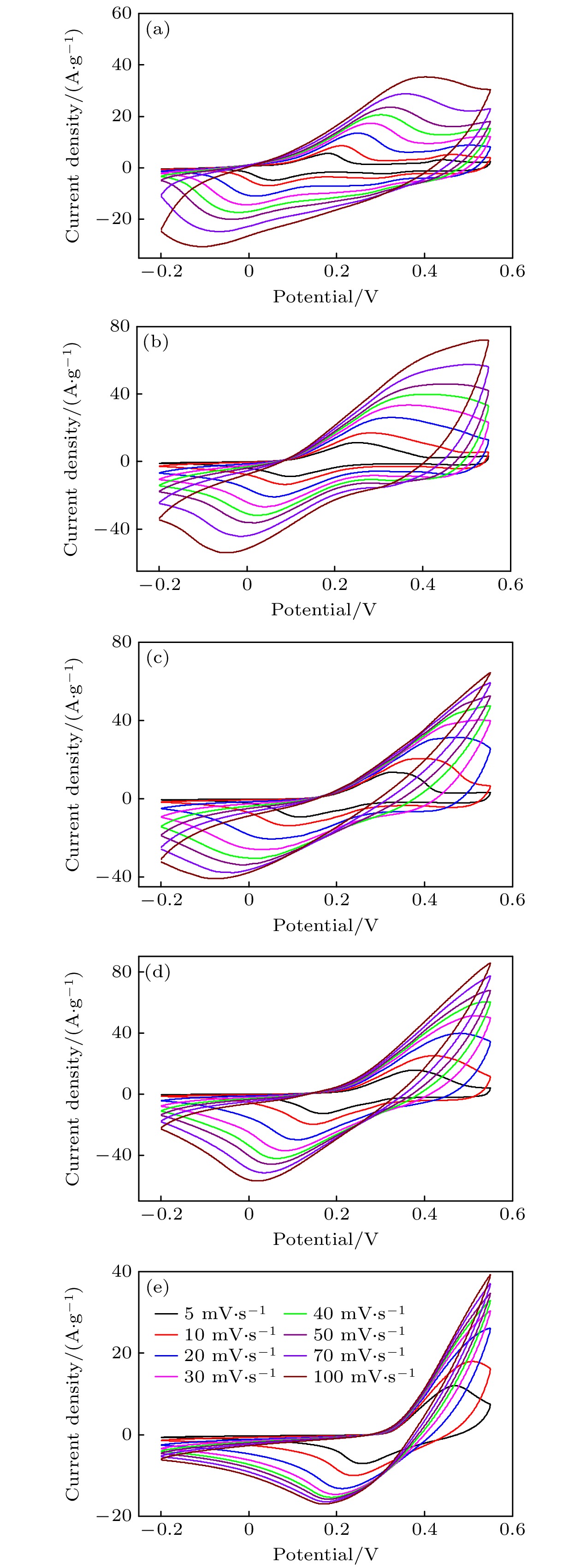
超级电容器以功率密度高、寿命长、环境友好等优点在各种能量存储设备中受到广泛关注. 所以, 提高电极材料的储能性能对超级电容器的开发与应用具有重要的意义. 具有特定纳米结构的功能材料作为超级电容器电极材料时具有优异的电化学性能, 原因在于其能提供丰富的电化学活性位点、高的比表面积和增加电解质与材料的接触面积. 因此, 本文以ZIF-67纳米晶为模板, 利用硝酸盐刻蚀的方法制备中空笼状镍钴层状氢氧化物(NiCo-LDH), 并研究其作为超级电容器电极材料的储能性能. 借助X射线衍射、扫描电镜、透射电镜、低温氮气吸附/脱附和电化学测试等手段分析所得NiCo-LDH的结构、形貌和电化学性能. 结果表明: NiCo-LDH由纳米片组装形成中空笼状结构, 拥有丰富的介孔和大孔孔道以及较高的比表面积, 从而有助于增加电活性位点, 促使电解液与电极材料的充分接触, 进而显著提高材料的储能性能. 当刻蚀用镍、钴盐质量比为1∶1时, 样品Ni 1Co 1-LDH的比电容可达801 F·g –1(电流密度为0.5 A·g –1), 且在大电流密度下(10 A·g –1)仍能保持582 F·g –1的比电容; 在电流密度15 A·g –1的条件下经过2000次循环后, 其比电容值保持为初始值的100.2%, 表现出优异的储能性能和潜在的应用价值.Supercapacitors have attracted extensive attention in various storage devices due to their high power density, long life and friendly environment. Hence, improving the energy storage performances of electrode materials are of great significance for supercapacitors. Functional materials with specific nanostructures, as energy storage materials, can display excellent electrochemical performances, for they will provide rich electrochemically active sites, high specific surface area and enhance electrolyte contact area. Consequently, hollow cage-like nickel cobalt layered hydroxides (NiCo-LDH) are prepared via nitrate etching of ZIF-67 nanocrystals, and investigated as electrode materials of supercapacitor. The morphology, structure and electrochemical properties of the obtained materials are investigated by X-ray diffraction, scanning electron microscope, transmission electron microscope, N 2 adsorption/desorption and a series of electrochemical tests (including cyclic voltammetry, galvanostatic charge and discharge and AC impedance). The results show that the NiCo-LDH samples assembled by nanosheets present a porous structure with hollow cages and high specific area surfaces, which conduces to increasing the electroactive sites, enhancing the contact between the electrolyte and the electrode material, and thus significantly improving the electrochemical performance of the materials. With the mass ratio of nickel to cobalt salt being 1∶1, the specific capacitance of Ni 1Co 1-LDH is 801 F·g –1 at a current density of 0.5 A·g –1, and a specific capacitance of 582 F·g –1 can still be maintained at a high current density of 10 A·g –1. Moreover, the specific capacitance retention of Ni 1Co 1-LDH is 100.2% after 2000 cycles at a current density of 15 A·g –1, displaying good electrochemical performance and great potential in supercapacitor applications.
-
Keywords:
- ZIF-67 /
- nickel cobalt double hydroxides /
- porous structure /
- hollow cage
[1] González A, Goikolea E, Barrena J A, Mysyk R 2016 Renewable Sustainable Energy Rev. 58 1189
 Google Scholar
Google Scholar
[2] Zha D S, Sun H H, Fu Y S, Ouyang X P, Wang X 2017 Electrochim. Acta 236 18
 Google Scholar
Google Scholar
[3] Zhang L J, Hui K N, Hui S K, Lee H 2016 J. Power Sources 318 76
 Google Scholar
Google Scholar
[4] Wang H T, Jin C, Liu Y N, Kang X H, Bian S W, Zhu Quan 2018 Electrochim. Acta 283 1789
 Google Scholar
Google Scholar
[5] Cai Z X, Wang Z L, Kim J, Yamauchi Y 2019 Adv. Mater. 31 1804903
 Google Scholar
Google Scholar
[6] Li L, Liu X, Liu C, Wang H Z, Zhang J, Liang P, Wang H B, Wang H 2018 Electrochim. Acta 259 303
 Google Scholar
Google Scholar
[7] 张诚, 邓明森, 蔡绍洪 2017 66 128201
 Google Scholar
Google Scholar
Zang C, Deng M S, Cai S H 2017 Acta Phys. Sin. 66 128201
 Google Scholar
Google Scholar
[8] Xiao P W, Meng Q H, Zhao L, Li J J, Wei Z X, Han B H 2017 Mater. Des. 129 164
 Google Scholar
Google Scholar
[9] Liu D, Du P C, Wei W L, Wang H X, Liu P 2018 J. Colloid. Interface Sci. 513 295
 Google Scholar
Google Scholar
[10] 冯艳艳, 李彦杰, 杨文, 牛潇迪 2020 化工进展 39 2734
Feng Y Y, Li Y J, Yang W, Niu X D 2020 Chem. Ind. Eng. Prog. 39 2734
[11] Ryu I, Yang M H, Kwon H, Park H K, Do Y R, Lee S B, Yim S 2014 Langmuir 30 1704
 Google Scholar
Google Scholar
[12] Shi P P, Li L, Hua L, Qian Q Q, Wang P F, Zhou J Y, Sun G Z, Huang W 2017 ACS Nano 11 444
 Google Scholar
Google Scholar
[13] Shen K W, Ran F, Zhang X X, Liu C, Wang N J, Niu X Q, Liu Y, Zhang D J, Kong L B, Kang L, Chen S W 2015 Synth. Met. 209 369
 Google Scholar
Google Scholar
[14] Nanwani A, Deshmukh K A, Sivaraman P, Peshwe D R, Sharma I, Dhoble S J, Swart H C, Deshmukh A D, Gupta B K 2019 Npj 2 D Mater. Appl. 3 1
 Google Scholar
Google Scholar
[15] Xuan X Y, Qian M, Han L, Wan L J, Li Y Q, Lu T, Pan L K, Niu Y P, Gong S Q 2019 Electrochim. Acta 321 134710
 Google Scholar
Google Scholar
[16] 冯艳艳, 黄宏斌, 张心桔, 易亚军, 杨文 2017 66 248202
 Google Scholar
Google Scholar
Feng Y Y, Huang H b, Zhang X J, Yi Y J, Yang W 2017 Acta Phys. Sin. 66 248202
 Google Scholar
Google Scholar
[17] Huang Q, Liu K Y, He F, Zhang S R, Xie Q L, Chen C 2017 Trans. Nonferrous Met. Soc. 27 1804
 Google Scholar
Google Scholar
[18] Huang W G, Zhang A T, Li X R, Tian J M, Yue L J, Cui L, Zheng R K, Wei D, Liu J Q 2019 J. Power Sources 440 227123
 Google Scholar
Google Scholar
[19] Xu J, Ma C J, Cao J Y, Chen Z D 2017 Dalton Trans. 46 3276
 Google Scholar
Google Scholar
[20] Xiao Z Y, Bao Y X, Li Z J, Huai X D, Wang M H, Liu P, Wang L 2019 ACS Appl. Energy Mater. 2 1086
 Google Scholar
Google Scholar
[21] Yang Z, Wang X M, Zhang H, Yan S H, Zhang C, Liu S X 2019 ChemElectroChem 6 4456
 Google Scholar
Google Scholar
[22] Cheng C, Wei C Z, He Y Y, Liu L Y, Hu J Y, Du W M 2021 J. Energy Storage 33 102105
 Google Scholar
Google Scholar
[23] Li X Y, Yu L, Wang G L, Wan G P, Peng X G, Wang K, Wang G Z 2017 Electrochim. Acta 255 15
 Google Scholar
Google Scholar
[24] Xiao Z Y, Mei Y J, Yuan S, Mei H, Xu B, Bao Y X, Fan L L, Kang W P, Dai F N, Wang R, Wang L, Hu S Q, Sun D F, Zhou H C 2019 ACS Nano 13 7024
 Google Scholar
Google Scholar
[25] Xu Y Q, Hou S J, Yang G, Wang X J, Lu T, Pan L K 2018 Electrochim. Acta 285 192
 Google Scholar
Google Scholar
[26] Yu L, Hu H, Wu H B, Lou X W 2017 Adv. Mater. 29 1604563
 Google Scholar
Google Scholar
[27] Hu H, Guan B Y, Xia B Y, Lou X W 2015 J. Am. Chem. Soc. 137 5590
 Google Scholar
Google Scholar
[28] Liu D, Wan J W, Pang G S, Tang Z Y 2019 Adv. Mater. 31 1803291
 Google Scholar
Google Scholar
[29] Rashti A, Lu X, Dobson A, Hassani E, Feyzbar-Khalkhali-Nejad F, He K, Oh T S 2021 ACS Appl. Energy Mater. 4 1537
 Google Scholar
Google Scholar
[30] Liu K, Yu M L, Wang H Y, Wang J, Liu W P, Hoffmann M R 2019 Environ. Sci. Technol. 53 6474
 Google Scholar
Google Scholar
[31] Zhu Y Y, Zhou Y N, Zhang X, Sun Z G, Jiao C Q 2021 Adv. Opt. Mater. 9 2001889
 Google Scholar
Google Scholar
[32] Li R, Che R, Liu Q, Su S Z, Li Z S, Zhang H S, Liu J Y, Liu L H, Wang J 2017 J. Hazard. Mater. 338 167
 Google Scholar
Google Scholar
[33] Song X K, Jiang Y, Cheng F, Earnshaw J, Na J, Li X P, Yamauchi Y 2021 Small 17 2004142
 Google Scholar
Google Scholar
[34] Hou S Y, Lian Y, Bai Y Q, Zhou Q P, Ban C L, Wang Z F, Zhao J, Zhang H H 2020 Electrochim. Acta 341 136053
 Google Scholar
Google Scholar
[35] Wu H, Zhang Y N, Yuan W Y, Zhao Y X, Luo S H, Yuan X W, Zheng L X, Cheng L F 2018 J. Mater. Chem. A 6 16617
 Google Scholar
Google Scholar
[36] Wang D, Tian L Y, Li D W, Xu Y, Wei Q F 2020 J. Electroanal. Chem. 873 114377
 Google Scholar
Google Scholar
[37] Liu Y X, Wang Y Z, Shi C J, Chen Y J, Li D, He Z F, Wang C, Guo L, Ma J M 2020 Carbon 165 129
 Google Scholar
Google Scholar
[38] Tahir M. U, Arshad H, Xie W Y, Wang X L, Nawaz M, Yang C, Su X T 2020 Appl. Surf. Sci. 529 147073
 Google Scholar
Google Scholar
[39] Chu H L, Zhu Y, Fang T T, Hua J Q, Qiu S J, Liu H D, Qin L Y, Wei Q H, Zou Y J, Xiang C L, Xu F, Sun L X 2020 Sustainable Energy Fuel 4 337
 Google Scholar
Google Scholar
[40] Zang Y, Luo H, Zhang H, Xue H G 2021 ACS Appl. Energy Mater. 4 1189
 Google Scholar
Google Scholar
[41] Jiang Z, Li Z P, Qin Z H, Sun H Y, Jiao X L, Chen D R 2013 Nanoscale 5 11770
 Google Scholar
Google Scholar
[42] Liu L L, Fang L, Wu F, Hu J, Zhang S F, Luo H J, Hu B S, Zhou M 2020 J. Alloys Compd. 824 153929
 Google Scholar
Google Scholar
[43] Yang F, Chu J, Cheng Y P, Gong J F, Wang X Q, Xiong S X 2021 Chem. Res. Chin. U. 37 772
 Google Scholar
Google Scholar
[44] Wan H Z, Li L, Xu Y, Tan Q Y, Liu X, Zhang J, Wang H B, Wang H 2018 Nanotechnology 29 194003
 Google Scholar
Google Scholar
[45] Li Y L, Li Q, Zhao S H, Chen C, Zhou J J, Tao K, Han L 2018 ChemistrySelect 3 13596
 Google Scholar
Google Scholar
[46] Lv Z J, Zhong Q, Bu Y F 2018 Adv. Mater. Interfaces 5 1800438
 Google Scholar
Google Scholar
[47] DinariI M, Allami H, Momeni M M 2020 Energy Fuel. 35 1831
[48] Wu S H, Zhang J Z, Sun C, Chen J S 2020 J. Inorg. Organomet. Polym. 30 3179
 Google Scholar
Google Scholar
-
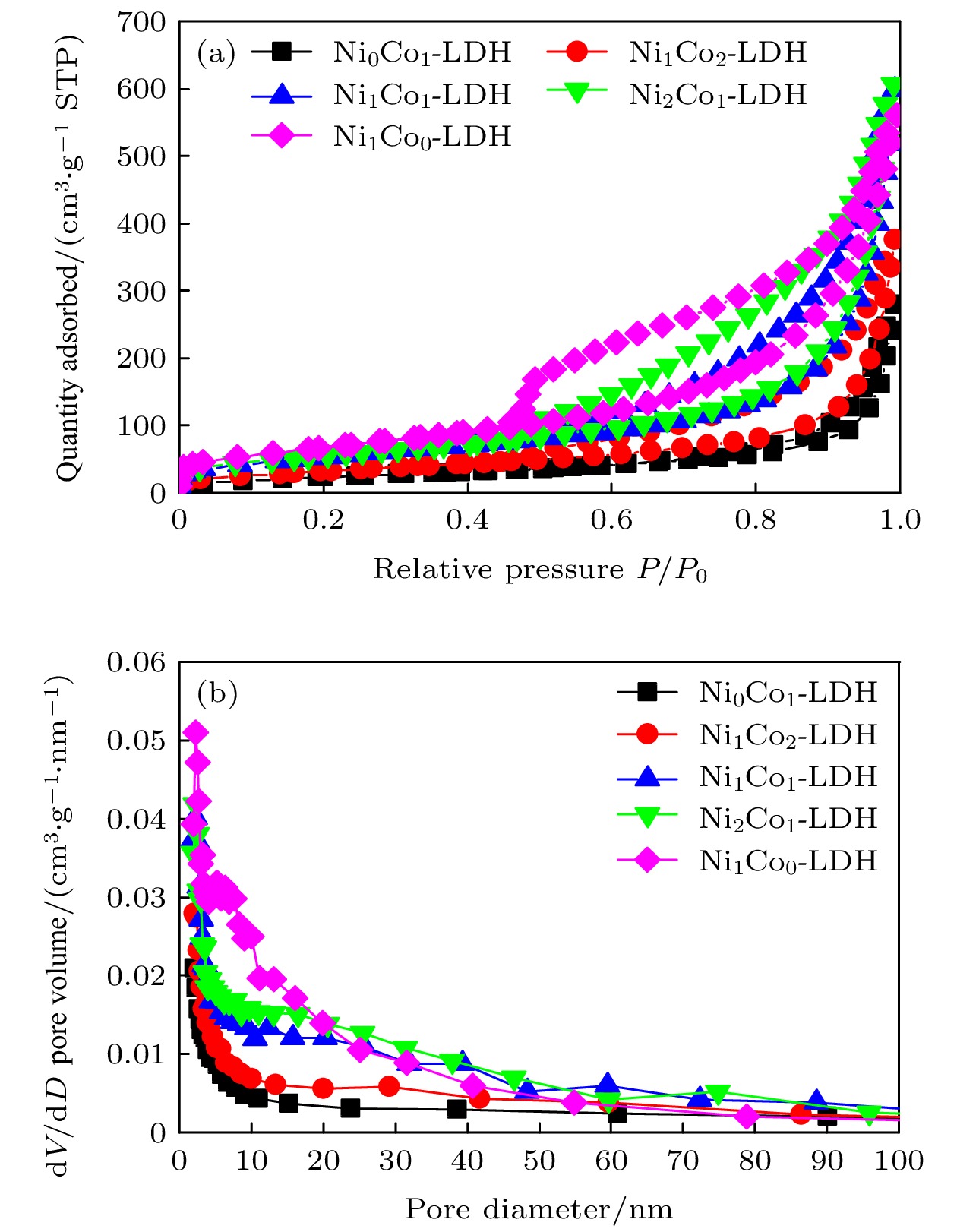
表 1 样品的孔结构参数
Table 1. Pore structure parameters of the samples.
Sample Ni/Co molar ratio BET specific surface area/m 2·g –1 Pore volume (meso)/(cm 3·g –1) Average pore width/nm Average pore width (meso)/nm Ni 0Co 1-LDH 0 81.4 0.443 18.2 17.5 Ni 1Co 2-LDH 0.18 112.7 0.600 17.9 16.5 Ni 1Co 1-LDH 0.39 182.6 0.954 17.9 16.3 Ni 2Co 1-LDH 0.45 194.3 0.973 17.8 15.4 Ni 1Co 0-LDH 2.37 233.3 0.913 13.2 11.5 表 2 NiCo-LDH基电极材料的比电容值比较
Table 2. Comparison of specific capacitances of various NiCo-LDH based electrodes materials.
Sample Electrolyte Specific capacitance/(F·g –1) Reference Ni 2Co 1-LDH 2 M KOH 963 (0.5 A·g –1) 本文 MnO 2-2/NiCo-LDH/CC 1 M NaSO 4 312 (0.2 A·g –1) [ 42] NiCo@BC 6 M KOH 606.4 (0.5 A·g –1) [ 43] Ni-Co LDH/NiNw 6 M KOH 466.6 (0.125 A·g –1) [ 44] NiCo LDH@Ni-CAT 1 M KOH 882 (1 A·g –1) [ 45] NCLDH@CNTs 6 M KOH 916.8 (1 A·g –1) [ 46] 10%Ce-NiCo-LDH/CNT 1 M KOH 187.2 (1 A·g –1) [ 47] MnO 2/NiCo-LDH 6 M KOH 555.6 (1 A·g –1) [ 48] -
[1] González A, Goikolea E, Barrena J A, Mysyk R 2016 Renewable Sustainable Energy Rev. 58 1189
 Google Scholar
Google Scholar
[2] Zha D S, Sun H H, Fu Y S, Ouyang X P, Wang X 2017 Electrochim. Acta 236 18
 Google Scholar
Google Scholar
[3] Zhang L J, Hui K N, Hui S K, Lee H 2016 J. Power Sources 318 76
 Google Scholar
Google Scholar
[4] Wang H T, Jin C, Liu Y N, Kang X H, Bian S W, Zhu Quan 2018 Electrochim. Acta 283 1789
 Google Scholar
Google Scholar
[5] Cai Z X, Wang Z L, Kim J, Yamauchi Y 2019 Adv. Mater. 31 1804903
 Google Scholar
Google Scholar
[6] Li L, Liu X, Liu C, Wang H Z, Zhang J, Liang P, Wang H B, Wang H 2018 Electrochim. Acta 259 303
 Google Scholar
Google Scholar
[7] 张诚, 邓明森, 蔡绍洪 2017 66 128201
 Google Scholar
Google Scholar
Zang C, Deng M S, Cai S H 2017 Acta Phys. Sin. 66 128201
 Google Scholar
Google Scholar
[8] Xiao P W, Meng Q H, Zhao L, Li J J, Wei Z X, Han B H 2017 Mater. Des. 129 164
 Google Scholar
Google Scholar
[9] Liu D, Du P C, Wei W L, Wang H X, Liu P 2018 J. Colloid. Interface Sci. 513 295
 Google Scholar
Google Scholar
[10] 冯艳艳, 李彦杰, 杨文, 牛潇迪 2020 化工进展 39 2734
Feng Y Y, Li Y J, Yang W, Niu X D 2020 Chem. Ind. Eng. Prog. 39 2734
[11] Ryu I, Yang M H, Kwon H, Park H K, Do Y R, Lee S B, Yim S 2014 Langmuir 30 1704
 Google Scholar
Google Scholar
[12] Shi P P, Li L, Hua L, Qian Q Q, Wang P F, Zhou J Y, Sun G Z, Huang W 2017 ACS Nano 11 444
 Google Scholar
Google Scholar
[13] Shen K W, Ran F, Zhang X X, Liu C, Wang N J, Niu X Q, Liu Y, Zhang D J, Kong L B, Kang L, Chen S W 2015 Synth. Met. 209 369
 Google Scholar
Google Scholar
[14] Nanwani A, Deshmukh K A, Sivaraman P, Peshwe D R, Sharma I, Dhoble S J, Swart H C, Deshmukh A D, Gupta B K 2019 Npj 2 D Mater. Appl. 3 1
 Google Scholar
Google Scholar
[15] Xuan X Y, Qian M, Han L, Wan L J, Li Y Q, Lu T, Pan L K, Niu Y P, Gong S Q 2019 Electrochim. Acta 321 134710
 Google Scholar
Google Scholar
[16] 冯艳艳, 黄宏斌, 张心桔, 易亚军, 杨文 2017 66 248202
 Google Scholar
Google Scholar
Feng Y Y, Huang H b, Zhang X J, Yi Y J, Yang W 2017 Acta Phys. Sin. 66 248202
 Google Scholar
Google Scholar
[17] Huang Q, Liu K Y, He F, Zhang S R, Xie Q L, Chen C 2017 Trans. Nonferrous Met. Soc. 27 1804
 Google Scholar
Google Scholar
[18] Huang W G, Zhang A T, Li X R, Tian J M, Yue L J, Cui L, Zheng R K, Wei D, Liu J Q 2019 J. Power Sources 440 227123
 Google Scholar
Google Scholar
[19] Xu J, Ma C J, Cao J Y, Chen Z D 2017 Dalton Trans. 46 3276
 Google Scholar
Google Scholar
[20] Xiao Z Y, Bao Y X, Li Z J, Huai X D, Wang M H, Liu P, Wang L 2019 ACS Appl. Energy Mater. 2 1086
 Google Scholar
Google Scholar
[21] Yang Z, Wang X M, Zhang H, Yan S H, Zhang C, Liu S X 2019 ChemElectroChem 6 4456
 Google Scholar
Google Scholar
[22] Cheng C, Wei C Z, He Y Y, Liu L Y, Hu J Y, Du W M 2021 J. Energy Storage 33 102105
 Google Scholar
Google Scholar
[23] Li X Y, Yu L, Wang G L, Wan G P, Peng X G, Wang K, Wang G Z 2017 Electrochim. Acta 255 15
 Google Scholar
Google Scholar
[24] Xiao Z Y, Mei Y J, Yuan S, Mei H, Xu B, Bao Y X, Fan L L, Kang W P, Dai F N, Wang R, Wang L, Hu S Q, Sun D F, Zhou H C 2019 ACS Nano 13 7024
 Google Scholar
Google Scholar
[25] Xu Y Q, Hou S J, Yang G, Wang X J, Lu T, Pan L K 2018 Electrochim. Acta 285 192
 Google Scholar
Google Scholar
[26] Yu L, Hu H, Wu H B, Lou X W 2017 Adv. Mater. 29 1604563
 Google Scholar
Google Scholar
[27] Hu H, Guan B Y, Xia B Y, Lou X W 2015 J. Am. Chem. Soc. 137 5590
 Google Scholar
Google Scholar
[28] Liu D, Wan J W, Pang G S, Tang Z Y 2019 Adv. Mater. 31 1803291
 Google Scholar
Google Scholar
[29] Rashti A, Lu X, Dobson A, Hassani E, Feyzbar-Khalkhali-Nejad F, He K, Oh T S 2021 ACS Appl. Energy Mater. 4 1537
 Google Scholar
Google Scholar
[30] Liu K, Yu M L, Wang H Y, Wang J, Liu W P, Hoffmann M R 2019 Environ. Sci. Technol. 53 6474
 Google Scholar
Google Scholar
[31] Zhu Y Y, Zhou Y N, Zhang X, Sun Z G, Jiao C Q 2021 Adv. Opt. Mater. 9 2001889
 Google Scholar
Google Scholar
[32] Li R, Che R, Liu Q, Su S Z, Li Z S, Zhang H S, Liu J Y, Liu L H, Wang J 2017 J. Hazard. Mater. 338 167
 Google Scholar
Google Scholar
[33] Song X K, Jiang Y, Cheng F, Earnshaw J, Na J, Li X P, Yamauchi Y 2021 Small 17 2004142
 Google Scholar
Google Scholar
[34] Hou S Y, Lian Y, Bai Y Q, Zhou Q P, Ban C L, Wang Z F, Zhao J, Zhang H H 2020 Electrochim. Acta 341 136053
 Google Scholar
Google Scholar
[35] Wu H, Zhang Y N, Yuan W Y, Zhao Y X, Luo S H, Yuan X W, Zheng L X, Cheng L F 2018 J. Mater. Chem. A 6 16617
 Google Scholar
Google Scholar
[36] Wang D, Tian L Y, Li D W, Xu Y, Wei Q F 2020 J. Electroanal. Chem. 873 114377
 Google Scholar
Google Scholar
[37] Liu Y X, Wang Y Z, Shi C J, Chen Y J, Li D, He Z F, Wang C, Guo L, Ma J M 2020 Carbon 165 129
 Google Scholar
Google Scholar
[38] Tahir M. U, Arshad H, Xie W Y, Wang X L, Nawaz M, Yang C, Su X T 2020 Appl. Surf. Sci. 529 147073
 Google Scholar
Google Scholar
[39] Chu H L, Zhu Y, Fang T T, Hua J Q, Qiu S J, Liu H D, Qin L Y, Wei Q H, Zou Y J, Xiang C L, Xu F, Sun L X 2020 Sustainable Energy Fuel 4 337
 Google Scholar
Google Scholar
[40] Zang Y, Luo H, Zhang H, Xue H G 2021 ACS Appl. Energy Mater. 4 1189
 Google Scholar
Google Scholar
[41] Jiang Z, Li Z P, Qin Z H, Sun H Y, Jiao X L, Chen D R 2013 Nanoscale 5 11770
 Google Scholar
Google Scholar
[42] Liu L L, Fang L, Wu F, Hu J, Zhang S F, Luo H J, Hu B S, Zhou M 2020 J. Alloys Compd. 824 153929
 Google Scholar
Google Scholar
[43] Yang F, Chu J, Cheng Y P, Gong J F, Wang X Q, Xiong S X 2021 Chem. Res. Chin. U. 37 772
 Google Scholar
Google Scholar
[44] Wan H Z, Li L, Xu Y, Tan Q Y, Liu X, Zhang J, Wang H B, Wang H 2018 Nanotechnology 29 194003
 Google Scholar
Google Scholar
[45] Li Y L, Li Q, Zhao S H, Chen C, Zhou J J, Tao K, Han L 2018 ChemistrySelect 3 13596
 Google Scholar
Google Scholar
[46] Lv Z J, Zhong Q, Bu Y F 2018 Adv. Mater. Interfaces 5 1800438
 Google Scholar
Google Scholar
[47] DinariI M, Allami H, Momeni M M 2020 Energy Fuel. 35 1831
[48] Wu S H, Zhang J Z, Sun C, Chen J S 2020 J. Inorg. Organomet. Polym. 30 3179
 Google Scholar
Google Scholar
计量
- 文章访问数: 9002
- PDF下载量: 168
- 被引次数: 0














 下载:
下载: