-
由于量子限域效应和态密度的限制, 石墨烯、硅烯等二维材料的量子电容在费米能级附近趋近于零. 基于密度泛函理论的第一性原理研究发现, 掺杂和吸附使石墨烯等二维电极材料的电子结构得以有效的调制, 它促进狄拉克点附近局域电子态的形成和/或费米能级的移动, 从而使量子电容得到了提高. 比较Ti (Au, Ag, Cu, Al)和3-B (N, P, S)掺杂单空位石墨烯(硅烯, 锗烯)的量子电容, 发现3-N掺杂单空位石墨烯和Ti原子吸附单空位硅烯、锗烯的量子电容明显得到了提升, 量子电容分别为118.42 μF/cm2, 79.84 μF/cm2和76.54 μF/cm2. 另外还研究了3-N掺杂三种烯类的浓度效应, 随掺杂浓度的增加, 量子电容呈增加趋势. 通过研究各掺杂体系的热力学稳定性问题, 发现Ti是最稳定的吸附原子, 因为Ti和C原子之间可以形成强键. 在B, N, P, S掺杂单空位硅烯和锗烯中, S是最稳定的掺杂原子, 而对于石墨烯, N掺杂的形成能最低, 量子电容最高. 上述二维电极材料的理论模拟计算为超级电容器和场效应晶体管中的实际应用做出了探索性的工作.Double electric layer capacitor is a kind of supercapacitor with high power density, but has relatively low energy density. Improving the quantum capacitances of materials will be a new way to increase their total interface capacitances. We design a two-dimensional electrode material with a high specific capacity and stable crystal structure. Due to the quantum confinement effect and the density of states, the quantum capacitances of two-dimensional materials such as graphene and silicene approach to zero when they are near the Fermi level. On the basis of the first principles of density functional theory, doping and adsorption can effectively modulate the electronic structure of two-dimensional electrode material such as graphene. It promotes the formation of the local state of the electrode material near the Dirac point and/or the movement of the Fermi level, thereby improving the quantum capacitance. Compared with the quantum capacitance of Ti (Au, Ag, Cu, Al), and 3-B (N, P, S) doped single-vacancy graphene (silicene, germanene), the quantum capacitance of 3-N doped single-vacancy graphene and of Ti atom adsorbed single-vacancy silicene/germanene are both significantly improved, and their quantum capacitances are as high as 118.42 μF/cm2, 79.84 μF/cm2, and 76.54 μF/cm2. The concentration effects of 3N-doped three kinds of alkenes are studied, and the results show that the quantum capacitance is enhanced with the doping concentration increasing. It is also found by studying the thermodynamic stability of the doped systems that Ti is the most stable adsorbed atom because of the strong bond between Ti atom and C atom. The S is the most stable doping atom in B, N, P, S doped single-vacancy silicene and germanene. For graphene, N doping has the lowest formation energy and the best quantum capacitance. This study intends to clarify the controversy regarding the energy storage enhancement of two-dimensional double-layer supercapacitor materials, and to improve the quantum capacitance. The research results provide the guidance for understanding the quantum effects caused by optimizing the structure of two-dimensional electrode material. The above theoretical calculation of the mentioned two-dimensional electrode material provides some research ideas for improving the low energy density of electric double-layer supercapacitors.
-
Keywords:
- quantum capacitance /
- two-dimensional electrode material /
- doping /
- first principles
[1] Yang Z, Zhang J, Kintner-Meyer MC, Lu X, Choi D, Lemmon JP, Liu J 2011 Chem. Rev. 111 3577
 Google Scholar
Google Scholar
[2] Simon P, Gogotsi Y 2008 Nat. Mater. 7 845
 Google Scholar
Google Scholar
[3] Premathilake D, Outlaw R A, Parler S G, Butler S M, Miller J R 2017 Carbon 111 231
 Google Scholar
Google Scholar
[4] Paek E, Pak A J, Kweon K E, Hwang G S 2013 J. Phys. Chem. C 11 75610
[5] Zhang L L, Zhao X, Ji H, Stoller M D, Lai L, Murali S, Mcdonnell S, Cleveger B, Wallace R M, Ruoff R S 2012 Energy Environ. Sci. 5 9618
 Google Scholar
Google Scholar
[6] Stoller M D, Magnuson C W, Zhu Y W, Murali S, Suk J W, Piner R, Ruoff R S 2011 Energy Environ. Sci. 4 4685
 Google Scholar
Google Scholar
[7] Jeong H M, Lee J W S, Hin W H, Choi Y J, Shin H J, Kang J K, Choi J W 2011 Nano Lett. 11 2472
 Google Scholar
Google Scholar
[8] Singh V, Joung D, Zhai L, Das S, Khondaker S I, Seal S 2011 Prog. Mater. Sci. 56 1178
 Google Scholar
Google Scholar
[9] You B, Wang L, Yao L, Yang J 2013 Chem. Commun. 49 5016
 Google Scholar
Google Scholar
[10] Pak A J, Paek E, Hwang G S 2013 Phys. Chem. Chem. Phys. 15 19741
 Google Scholar
Google Scholar
[11] Fleurence A, Friedlein R, Ozaki T, Kawai H, Wang Y, Yamada-Takamura Y 2012 Phys. Rev. Lett. 108 245501
 Google Scholar
Google Scholar
[12] Chiappe D, Scalise E, Cinquanta E, Grazianetti C, Van den Broek B, Fanciulli, Houssa M, Molle A 2014 Adv. Mater. 26 2096
 Google Scholar
Google Scholar
[13] Yang G M, Zhang H Z, Fan X F, Zheng W T 2015 J. Phys. Chem. C 119 6464
 Google Scholar
Google Scholar
[14] Yang G M, Xu Q, Fan X F, Zheng W T 2018 J. Phys. Chem. C 122 1903
 Google Scholar
Google Scholar
[15] Kresse G, Furthmüller J 1996 J. Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[16] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[17] Perdew J P, Chevary J A, Vosko S H, Jackson K A, Pederson M R, Singh D J, Fiolhais C 1992 Phys. Rev. B 4 66671
[18] Sivek J, Sahin H, Partoens B, Peeters F M 2013 Phys. Rev. B 87 085444
 Google Scholar
Google Scholar
[19] De Padova P, Vogt P, Resta A, Avila J, Razado-Colambo I, Quaresima C, Ottaviani C, Bruhn T, Hirahara T, Shirai T, Hasegawa S, Asensio M C, Le Lay G 2013 Appl. Phys. Lett. 102 163106
 Google Scholar
Google Scholar
[20] Zhan C, Zhang Y, Cummings P T, Jiang D R 2016 Phys. Chem. Chem. Phys. 18 4668
 Google Scholar
Google Scholar
-
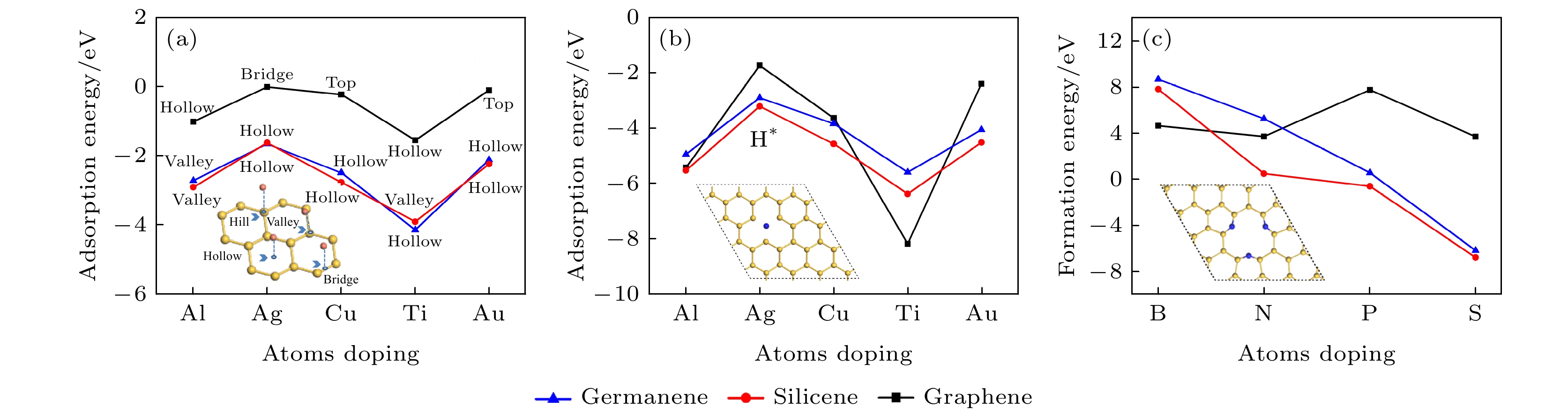
图 1 (a), (b) 金属原子分别在本征和单空位锗烯(硅烯, 石墨烯)上的最稳定吸附位置的吸附能, 掺杂浓度为3.1%; (c) 单空位锗烯(硅烯, 石墨烯)掺杂3-B (N, P, S)原子的形成能, 掺杂浓度9.3%
Fig. 1. (a), (b) The adsorption energies of adsorbing metal atoms in the strongest position on pristine and single-vacancy germanene (silicene, graphene) with thedoping concentration of 3.1%; (c) the formation energy of single-vacancy germanene (silicene, graphene) doped with triple-B (N, P, S) with the doping concentration of 9.3%.
图 2 (a), (b)分别为本征石墨烯、硅烯、锗烯的质量比量子电容, 面积比量子电容(锗烯: 1 μF/cm2 = 11.48 F/g, 硅烯: 1 μF/cm2 = 27.37 F/g, 石墨烯: 1 μF/cm2 = 26.3 F/g)
Fig. 2. (a), (b) calculated specific quantum capacitance (CQ), areal CQ vs. potential drop of pristine graphene, siliceneand germanene. (For germanene 1 μF/cm2 = 11.48 F/g, silicene 1 μF/cm2 = 27.37 F/g, and graphene 1 μF/cm2 = 26.3 F/g).
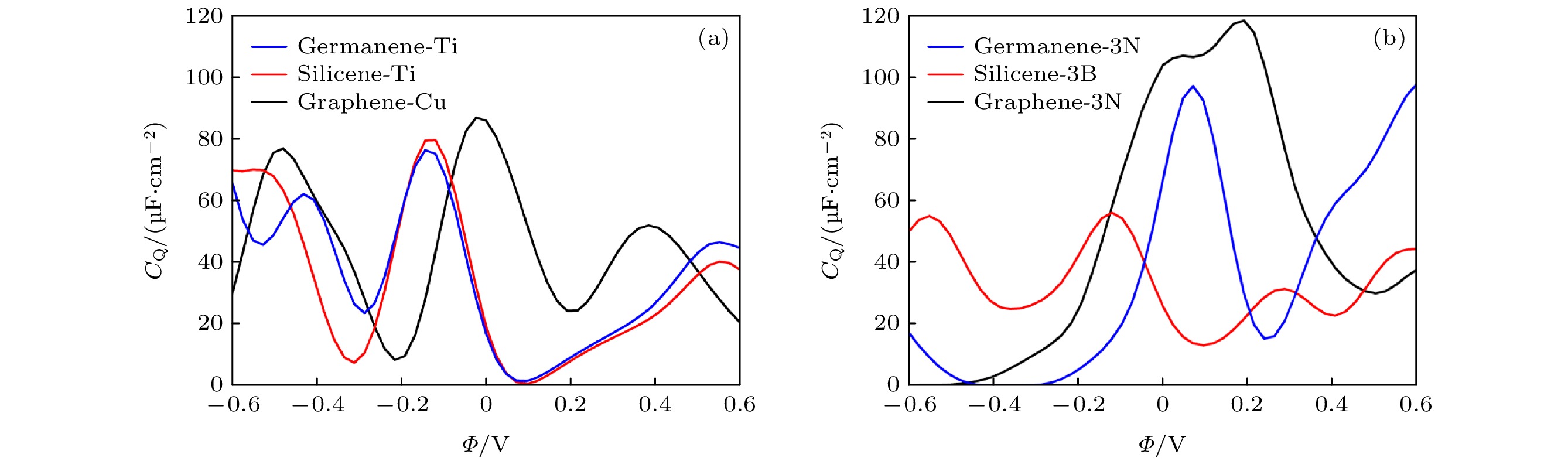
图 3 (a)−(c) 单空位石墨烯(硅烯, 锗烯)吸附Ti (Cu, Au, Ag, Al)和(d)−(f) 3-B (P, N, S)掺杂单空位石墨烯(硅烯, 锗烯)量子电容最大值的变化趋势图
Fig. 3. (a)−(c) Change trend chart of the maximum value of CQ for Ti (Cu, Au, Ag and Al) adsorbed single-vacancy grapheme (silicene, germanene), and (d)−(f) 3-B (P, N, S)-doped single-vacancy grapheme (silicene, germanene).
图 4 (a) 单空位石墨烯(硅烯, 锗烯)吸附Ti, Au, Ag, Cu, Al体系中, 最高的量子电容与电势的关系图, 掺杂浓度为3.1%; (b) 单空位石墨烯(硅烯, 锗烯)掺杂三个B, N, P, S原子体系中, 最高的量子电容-电势关系图, 掺杂浓度为9.3%
Fig. 4. (a) Under the condition of doping concentration of 3.1%, the calculated quantum capacitance of single-vacancy grapheme (silicene, germanene) adsorbed withTi, Au, Ag, Cu, Al with the best properties, as a function of local electrode potential (Φ); (b) CQ of single-vacancy germanene (silicene, graphene) doping with triple-B (N, P, S)with the best properties, under the condition of doping concentration of 9.3%.
图 5 (a) Ti掺杂单空位硅烯浓度的态密度(DOS)和局域态密度(LDOS)图, 掺杂浓度为3.1%; (b) 3-N原子掺杂单空位石墨烯的态密度(DOS)和局域态密度(LDOS)图, 掺杂浓度为9.4%
Fig. 5. Density of states (DOS) and local density of states (LDOS) of adsorbed Ti atoms on single-vacancy silicene with Ti concentration3.1%, and (b) triple N-doping single-vacancy grapheme with N concentration 9.4%.
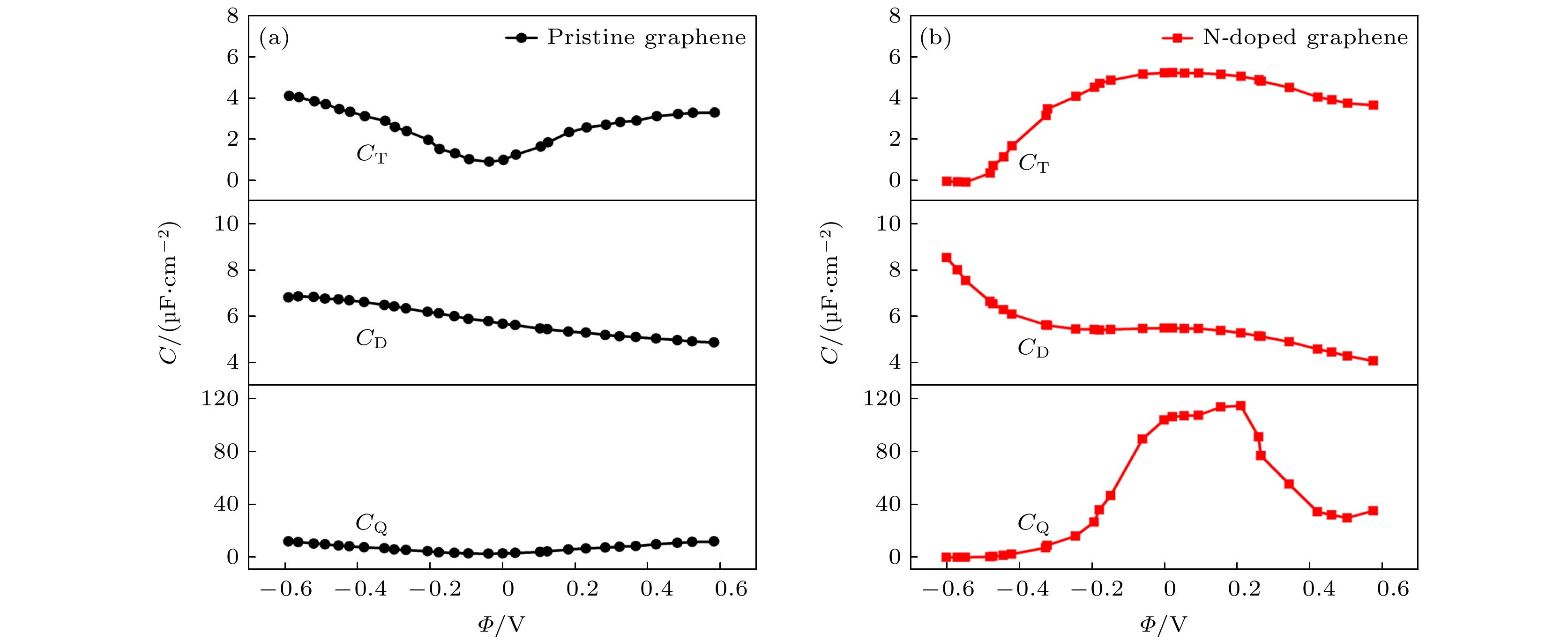
图 7 本征石墨烯和石墨烯掺杂3-N原子的总界面电容, 双电层电容以及量子电容. 双电层电容是根据经典分子动力学模拟获得, 模拟条件为1 M NaCl水性电解液[20]
Fig. 7. The capacitance of triple-N doped with graphene and pristine grapheme, including quantum capacitance (CQ), electric double-layer capacitance (CD) and total interfacial capacitance(CT).The CD is obtained by classical MD simulation under the condition of 1 M NaCl aqueous electrolyte[20].
表 1 在本征和单空位石墨烯、硅烯、锗烯上分别以不同位置吸附Al, Ag, Cu, Ti和Au金属原子的吸附能
Table 1. The adsorption energy of Al, Ag, Cu, Ti, Au adsorbed on pristine and single-vacancy graphene (silicene, germanene) with different configurations.
System
ΔEad (eV)Hill Valley Bridge Hollow H* Graphene Al –0.888 — –0.902 –1.012 –5.399 Ag 0.035 — –0.001 0.011 –1.695 Cu –0.220 — –0.217 –0.057 –3.599 Ti –1.075 — –0.827 –1.544 –8.159 Au –0.096 — –0.082 –0.073 –2.359 Silicene Al –2.794 –2.907 — –2.573 –5.502 Ag –0.991 –1.313 –1.244 –1.616 –3.182 Cu –1.549 –2.368 — –2.764 –4.538 Ti –3.694 –3.907 — –3.893 –6.348 Au –1.843 — –1.933 –2.238 –4.486 Germanene Al –1.815 –2.716 — –2.520 –4.908 Ag –0.984 –1.322 — –1.650 –2.869 Cu –1.377 –2.091 — –2.489 –3.812 Ti –3.569 –3.968 — –4.144 –5.565 Au –1.636 –1.700 –1.818 –2.113 –4.020 表 2 在单空位石墨烯、硅烯、锗烯上掺杂3-B(N, P, S)原子的形成能
Table 2. The formation energy of triple-B (N, P, S) doped single-vacancy graphene (silicene, germanene).
System ΔEf (eV) B N P S Graphene 4.663 3.735 7.773 3.708 Silicene 7.825 0.503 –0.590 –6.759 Germanene 8.684 5.256 0.575 –6.149 -
[1] Yang Z, Zhang J, Kintner-Meyer MC, Lu X, Choi D, Lemmon JP, Liu J 2011 Chem. Rev. 111 3577
 Google Scholar
Google Scholar
[2] Simon P, Gogotsi Y 2008 Nat. Mater. 7 845
 Google Scholar
Google Scholar
[3] Premathilake D, Outlaw R A, Parler S G, Butler S M, Miller J R 2017 Carbon 111 231
 Google Scholar
Google Scholar
[4] Paek E, Pak A J, Kweon K E, Hwang G S 2013 J. Phys. Chem. C 11 75610
[5] Zhang L L, Zhao X, Ji H, Stoller M D, Lai L, Murali S, Mcdonnell S, Cleveger B, Wallace R M, Ruoff R S 2012 Energy Environ. Sci. 5 9618
 Google Scholar
Google Scholar
[6] Stoller M D, Magnuson C W, Zhu Y W, Murali S, Suk J W, Piner R, Ruoff R S 2011 Energy Environ. Sci. 4 4685
 Google Scholar
Google Scholar
[7] Jeong H M, Lee J W S, Hin W H, Choi Y J, Shin H J, Kang J K, Choi J W 2011 Nano Lett. 11 2472
 Google Scholar
Google Scholar
[8] Singh V, Joung D, Zhai L, Das S, Khondaker S I, Seal S 2011 Prog. Mater. Sci. 56 1178
 Google Scholar
Google Scholar
[9] You B, Wang L, Yao L, Yang J 2013 Chem. Commun. 49 5016
 Google Scholar
Google Scholar
[10] Pak A J, Paek E, Hwang G S 2013 Phys. Chem. Chem. Phys. 15 19741
 Google Scholar
Google Scholar
[11] Fleurence A, Friedlein R, Ozaki T, Kawai H, Wang Y, Yamada-Takamura Y 2012 Phys. Rev. Lett. 108 245501
 Google Scholar
Google Scholar
[12] Chiappe D, Scalise E, Cinquanta E, Grazianetti C, Van den Broek B, Fanciulli, Houssa M, Molle A 2014 Adv. Mater. 26 2096
 Google Scholar
Google Scholar
[13] Yang G M, Zhang H Z, Fan X F, Zheng W T 2015 J. Phys. Chem. C 119 6464
 Google Scholar
Google Scholar
[14] Yang G M, Xu Q, Fan X F, Zheng W T 2018 J. Phys. Chem. C 122 1903
 Google Scholar
Google Scholar
[15] Kresse G, Furthmüller J 1996 J. Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[16] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[17] Perdew J P, Chevary J A, Vosko S H, Jackson K A, Pederson M R, Singh D J, Fiolhais C 1992 Phys. Rev. B 4 66671
[18] Sivek J, Sahin H, Partoens B, Peeters F M 2013 Phys. Rev. B 87 085444
 Google Scholar
Google Scholar
[19] De Padova P, Vogt P, Resta A, Avila J, Razado-Colambo I, Quaresima C, Ottaviani C, Bruhn T, Hirahara T, Shirai T, Hasegawa S, Asensio M C, Le Lay G 2013 Appl. Phys. Lett. 102 163106
 Google Scholar
Google Scholar
[20] Zhan C, Zhang Y, Cummings P T, Jiang D R 2016 Phys. Chem. Chem. Phys. 18 4668
 Google Scholar
Google Scholar
计量
- 文章访问数: 8196
- PDF下载量: 237
- 被引次数: 0













 下载:
下载: