-
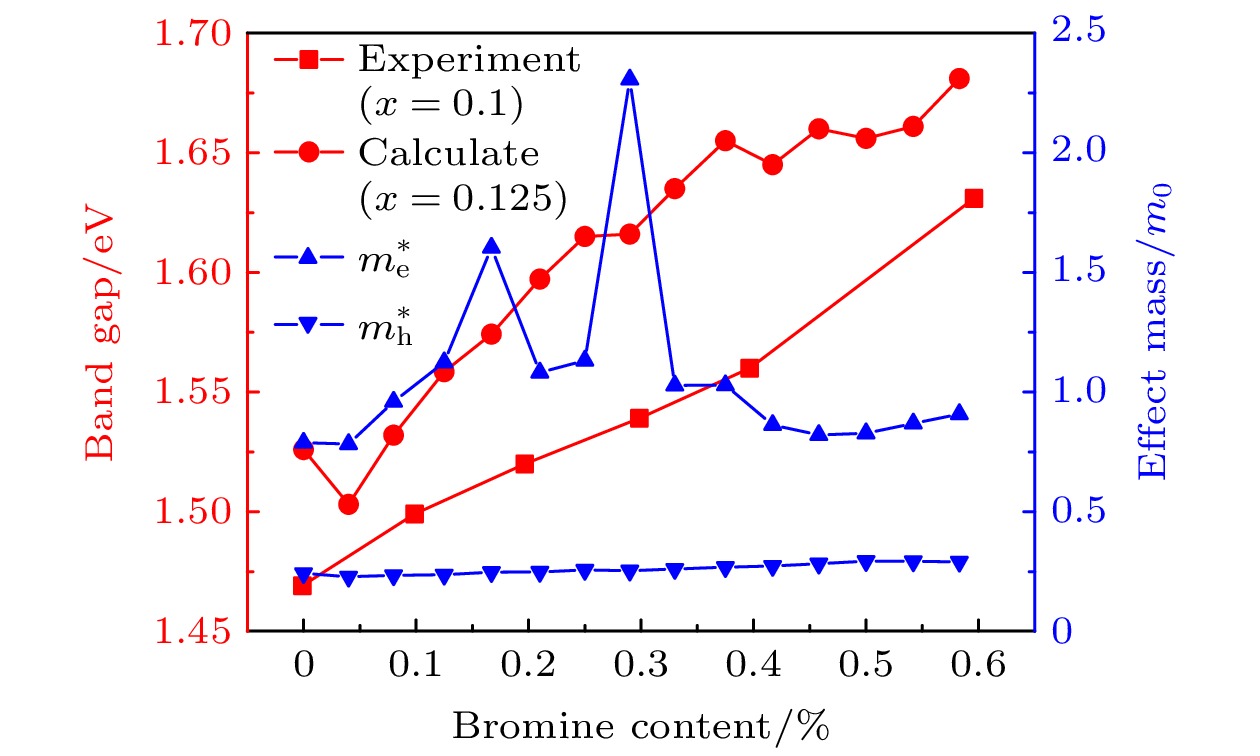
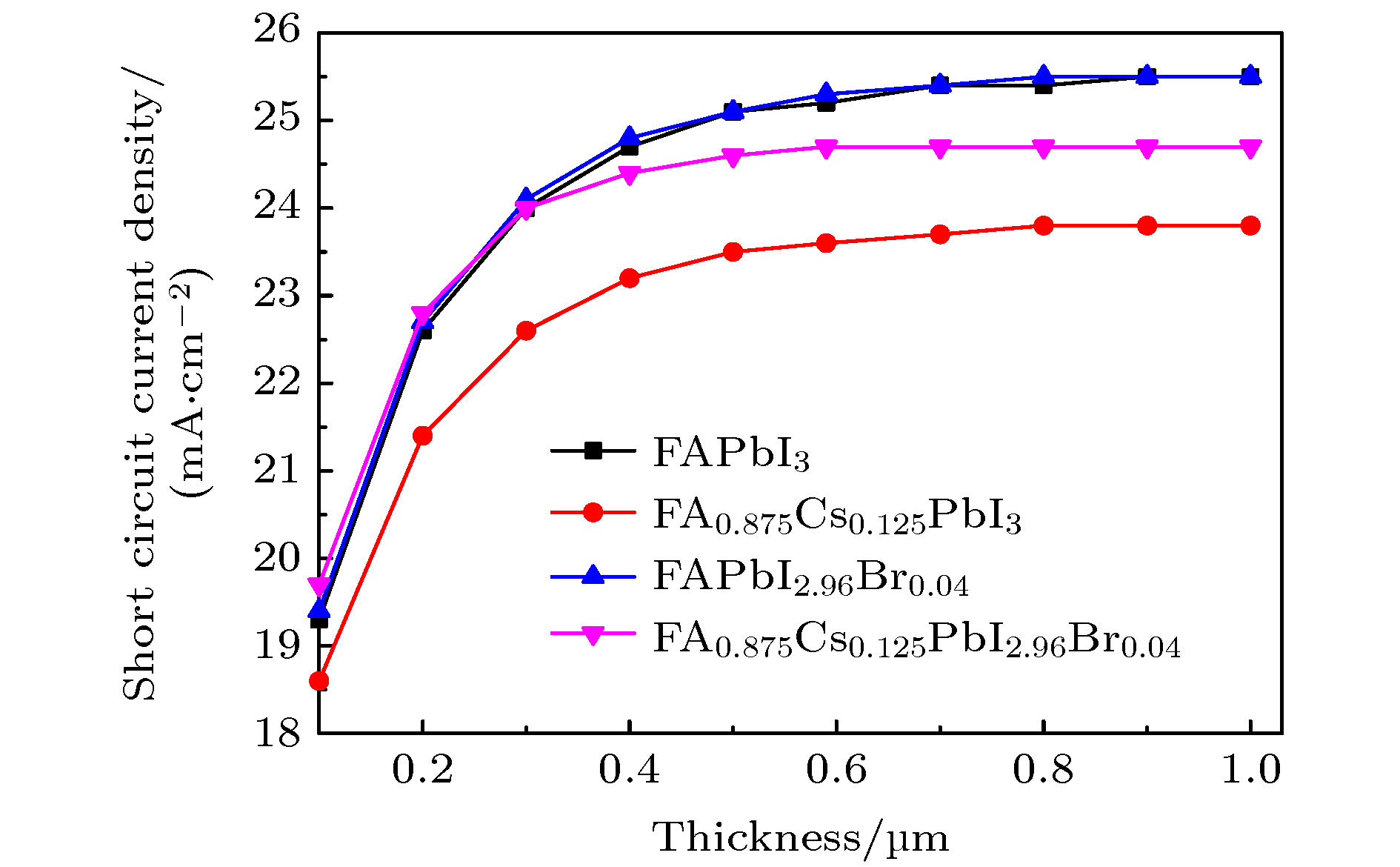
甲脒铅碘钙钛矿(FAPbI3)因其优异的光电性能而成为新兴太阳电池最具潜力的候选材料, 但是稳定性较差成为制约其发展的主要瓶颈. 通过离子掺杂可以有效地改善FAPbI3的稳定性, 如通过共掺杂Cs+和Br–形成FA1–xCsxPbI3–yBry钙钛矿材料, 其耐热及耐水稳定性得到显著改善. 本文利用第一性原理计算了FA1–xCsxPbI3–yBry (x = 0.125, y = 0—0.6)体系的几何结构、电子结构和光学性质. 通过分析发现Cs+和Br–的掺入使得体系能量降低, FA0.875Cs0.125PbI2.96 Br0.04最稳定. 利用等效光学导纳法模拟计算了平面结构钙钛矿太阳电池的吸收率、载流子收集效率、外量子效率、短路电流密度、开路电压和伏安特性. 对于FA1–xCsxPbI3–yBry钙钛矿太阳电池, 当x = 0.125, y = 0.04, 厚度为0.5—1.0 μm时, 电池的短路电流密度均为24.7 mA·cm–2, 开路电压为1.06 V. 结果表明Cs+和Br–的共掺杂在没有降低电池短路电流的同时提高了体系的稳定性, 可为实验上制备高效稳定的钙钛矿太阳电池提供理论参考.Formamdinium lead triiodide (FAPbI3) perovskite has developed as a promising candidate in solar cells for its excellent optoelectronic property. However, the poor environmental stability is still a critical hurdle for its further commercial application. Element doping is an effective method of improving the stability of FAPbI3 materials. It has been reported that the FA1–xCsxPbI3–yBry stability for heat and water resistance were greatly improved by Cs cations and Br anions co-doping. In this study, we perform first-principles calculations to systematically investigate the crystal structures, electronic structures, and optical properties of FA1–xCsxPbI3–yBry. We obtain several stable crystal structures of FA1–xCsxPbI3–yBry (x = 0.125, y = 0—0.6) in the cubic phase for different ratios of Cs cations to Br anions. By analyzing the structures of these mixed ion perovskites, it is revealed that the lattice parameters decrease linearly with the increase of concentration of Cs cations and Br anions, which is consistent with previous experimental result. In this work, the formation energy difference (∆E) is calculated and our results show that the mixing of Cs cations and Br anions could increase the thermodynamic stability compared with pure FAPbI3. The FA0.875Cs0.125PbI2.96Br0.04 is found to be the most stable in all composites investigated. Furthermore, the band gap, hole and electron effective mass increase with increasing proportion of Br anions, indicating an effective strategy for extending the absorption range of FAPbI3 perovskites into the ultraviolet of the solar spectrum, thereby affecting the carrier transport mechanism in this material. Density of states (DOS) analysis indicates that the DOS of valence band edge increases with increasing proportion of Br anions and enhancing transitions between the valence and conduction bands. Finally, the absorption rate, carrier collection efficiency, external quantum efficiency, short-circuit current density, open circuit voltage and volt-ampere characteristics for the planar structure perovskite solar cell are analyzed by the equivalent optical admittance method. For the FA1–xCsxPbI3–yBry (x = 0.125, y = 0.04, thickness = 0.5—1.0 μm) solar cell, the short-circuit current density and the open circuit voltage are estimated at about 24.7 mA·cm–2 and 1.06 V. It is demonstrated that the co-doping Cs cations and Br anions can improve the stability of the system without reducing short-circuit current density, which may provide some theoretical guidance in preparing the perovskite solar cells with high efficiency and excellent stability.
-
Keywords:
- first-principle calculations /
- electronic structure theory /
- optical properties /
- perovskite solar cell
[1] Burschka J, Pellet N, Moon S J, Humphry B R, Gao P, Nazeeruddin M K, Gratzel M 2013 Nature 499 316
 Google Scholar
Google Scholar
[2] Dong Q F, Fang Y J, Shao Y C, Mulligan P, Qiu J, Cao L, Huang J S 2015 Science 347 967
 Google Scholar
Google Scholar
[3] Wang C H, Zhang C J, Tong S C, Shen J Q, Wang C, Li Y Z, Xiao S, He J, Zhang J, Gao Y L, Yang J L 2017 J. Phys. Chem. C 121 6575
 Google Scholar
Google Scholar
[4] Zhang W H, Xiong J, Jiang L, Wang J Y, Mei T, Wang X B, Gu H S, Daoud W A, Li J H 2017 Appl. Mater. Interfaces 9 38467
 Google Scholar
Google Scholar
[5] Kojima A, Teshima K, Shirai Y, Miyasaka T 2009 J. Am. Chem. Soc. 131 6050
 Google Scholar
Google Scholar
[6] Park N G, Zhu K 2020 Nat. Rev. Mater. 5 333
 Google Scholar
Google Scholar
[7] Green M A, Dunlop E D, Levi D H, Hohl-Ebinger J, Yoshita M, Ho-Baillie A W Y 2019 Prog. Photovolt. Res. Appl. 27 565
 Google Scholar
Google Scholar
[8] Chen H, Ye F, Tang W T, He J J, Yin M S, Wang Y B, Xie F X, Bi E B, Yang X D, Gratzel M, Han L Y 2017 Nature 550 92
 Google Scholar
Google Scholar
[9] Jiang Q, Zhao Y, Zhang X W, Yang X L, Chen Y, Chu Z M, Ye Q F, Li X X, Yin Z G, You J B 2019 Nat. Photonics 13 460
 Google Scholar
Google Scholar
[10] Mellouhi F E, Bentria E T, Rashkeev S N, Kais S, Alharbi F H 2016 Sci. Rep. 6 30305
 Google Scholar
Google Scholar
[11] Lin R X, Xiao K, Qin Z Y, Han Q L, Zhang C F, Wei M Y, Saidaminov M I, Gao Y, Xu J, Xiao M, Li A D, Zhu J, Sargent E H, Tan H R 2019 Nat. Energy 4 864
 Google Scholar
Google Scholar
[12] Lee J W, Dai Z Z, Lee C, Lee H M, Han T H, Marco N D, Lin O, Choi C S, Dunn B S, Koh J, Carlo D D, Ko J H, Maynard H D, Yang Y 2018 J. Am. Chem. Soc. 140 6317
 Google Scholar
Google Scholar
[13] 毕富珍, 郑晓, 任志勇 2019 物理化学学报 35 69
 Google Scholar
Google Scholar
Bi F Z, Zheng X, Reng Z Y 2019 Acta Phys.-Chim. Sin. 35 69
 Google Scholar
Google Scholar
[14] Huang Y, Li L, Liu Z H, Jiao H Y, He Y Q, Wang X G, Zhu R, Wang D, Sun J L, Chen Q, Zhou H P 2017 J. Mater. Chem. A 5 8537
 Google Scholar
Google Scholar
[15] Li L, Liu N, Xu Z Q, Chen Q, Wang X D, Zhou H P 2017 ACS Nano 11 8804
 Google Scholar
Google Scholar
[16] Li N X, Luo Y Q, Chen Z H, Niu X X, Zhang X, Lu J Z, Kumar R S, Jiang J K, Liu H F, Guo X, Lai B, Brocks G, Chen Q, Tao S X, Fenning D P, Zhou H P 2020 Joule 4 1
 Google Scholar
Google Scholar
[17] Yi C Y, Luo J S, Meloni S, Boziki A, Astani N A, Gratzel C, Zakeeruddin S M, Rothlisberger U, Gratzel M 2016 Energy Environ. Sci. 9 656
 Google Scholar
Google Scholar
[18] Wang L G, Zhou H P, Hu J N, Huang B L, Sun M Z, Dong B W, Zheng J G H, Huang Y, Chen Y H, LI L, Xu Z Q, Li N G, Liu Z, Chen Q, Sun L D, Yan C H 2019 Science 363 265
 Google Scholar
Google Scholar
[19] Liu W, Liu N J, Ji S L, Hua H F, Ma Y H, Hu R Y, Zhang J, Chu L, Li X A, Huang W 2020 Nano-Micro Lett. 12 119
 Google Scholar
Google Scholar
[20] Correa-Baena J B, Luo Y Q, Brenner T M, Snaider J, Sun S J, Li X Y, Jensen M A, Hartono N P T, Nienhaus L, Wieghold S, Poindexter J R, Wang S, Meng Y S, Wang T, Lai B, Holt M V, Cai Z H, Bawendi M G, Huang L B, Buonassisi T, Fenning D P 2019 Science 363 627
 Google Scholar
Google Scholar
[21] Charles B, Weller M T, Rieger S, Hatcher L E, Henry P F, Feldmann J, Wolverson D, Wilson C C 2020 Chem. Mater. 32 2282
 Google Scholar
Google Scholar
[22] Nakane A, Tampo H, Tamakoshi M, Fujimoto S, Kim K M, Kim S, Shibata H, Niki S, Fujiwara H 2016 J. Appl. Phys. 120 064505
 Google Scholar
Google Scholar
[23] Dewan R, Vasilev I, Jovanov V, Knipp D 2011 J. Appl. Phys. 110 013101
 Google Scholar
Google Scholar
[24] 石将建, 卫会云, 朱立峰, 许信, 徐余颛, 吕松涛, 吴会觉, 罗艳红, 李冬梅, 孟庆波 2015 64 038402
 Google Scholar
Google Scholar
Shi J J, Wei H Y, Zhu L F, Xu X, Xu Y Z, Lü S T, Wu H J, Luo Y H, Li D M, Bo M Q 2015 Acta Phys. Sin. 64 038402
 Google Scholar
Google Scholar
[25] Kato Y, Fujimoto S, Kozawa M, Fujiwara H 2019 Phys. Rev. Appl. 12 024039
 Google Scholar
Google Scholar
[26] Weller M T, Weber O J, Frost J M, Walsh A 2015 J. Phys. Chem. Lett. 6 3209
 Google Scholar
Google Scholar
[27] Kato M, Fujiseki T, Miyadera T, Sugita T, Fujimoto S, Tamakoshi M, Chikamatsu M, Fujiwara H 2017 J. Appl. Phys. 121 115501
 Google Scholar
Google Scholar
[28] Eperon G E, Stranks S D, Menelaou S, Johnston M B, Herz L M, Snaith H J 2014 Energy Environ. Sci. 7 982
 Google Scholar
Google Scholar
[29] Stoumpos C C, Malliakas C D, Kanatzidis M G 2013 Inorg. Chem. 52 9019
 Google Scholar
Google Scholar
[30] 柴磊, 钟敏 2016 65 237902
 Google Scholar
Google Scholar
Chai L, Zhong M 2016 Acta Phys. Sin. 65 237902
 Google Scholar
Google Scholar
[31] 王福芝, 谭占鳌, 戴松元, 李永舫 2015 64 038401
 Google Scholar
Google Scholar
Wang F Z, Tan Z A, Dai S Y, Li Y F 2015 Acta Phys. Sin. 64 038401
 Google Scholar
Google Scholar
[32] Yang W S, Noh J H, Jeon N J, Kim Y C, Ryu S, Seo J, Seok S I 2015 Science 348 1234
 Google Scholar
Google Scholar
[33] Pham N D, Zhang C M, Tiong V T, Zhang S L, Will G, Bou A, Bisquert J, Shaw P E, Du A J, Wilson G J, Wang H X 2019 Adv. Funct. Mater. 29 1806479
 Google Scholar
Google Scholar
[34] Tavakoli M M, Yadav P, Tavakoli R, Kong J 2018 Adv. Energy Mater. 1800794
[35] Nazarenko O, Yakunin S, Morad V, Cherniukh I, Kovalenko V 2017 NPG Asia Mater. 9 e373
 Google Scholar
Google Scholar
[36] 刘娜, 危阳, 马新国, 祝林, 徐国旺, 楚亮, 黄楚云 2017 66 057103
 Google Scholar
Google Scholar
Liu N, Wei Y, Ma X G, Xu G W, Chu L, Huang C Y 2017 Acta Phys. Sin. 66 057103
 Google Scholar
Google Scholar
[37] 蒋泵, 陈思良, 崔晓磊, 胡紫婷, 李跃, 张笑铮, 吴康敬, 王文贞, 蒋最敏, 洪峰, 马忠权, 赵磊, 徐飞, 徐闰, 詹义强 2019 68 246801
 Google Scholar
Google Scholar
Jiang B, Chen S L, Cui X L, Hu Z T, Li Y, Zhang X Z, Wu K J, Wang W Z, Jiang Z M, Hong F, Ma Z Q, Zhao L, Xu F, Xu R, Zhan Y Q 2019 Acta Phys. Sin. 68 246801
 Google Scholar
Google Scholar
-
图 1 (a) α-FAPbI3的赝立方2 × 2 × 2超胞; (b) 正交晶格的第一布里渊区中各对称点和对称轴的记号示意图; (c) 利用图(b)中k空间路径得到α-FAPbI3的2 × 2 × 2超胞的能带图和态密度; (d) 钙钛矿太阳电池结构示意图
Fig. 1. (a) Pseudocubic crystal structures of α-FAPbI3 with 2 × 2 × 2 supercell; (b) the first Brillouin zone for the orthorhombic lattice of α-FAPbI3 and the k-path (red line) used to plot the band structure in the present paper; (c) band structure and DOS of α-FAPbI3 calculated using 2 × 2 × 2 supercell; (d) schematic diagram of perovskite solar cell structure.
图 4 FA1–xCsxPbI3–yBry的能带结构、总态密度和分态密度 (a), (d) FA0.875Cs0.125PbI3; (b), (e) FAPbI2.96Br0.04; (c), (f) FA0.875Cs0.125PbI2.96Br0.04
Fig. 4. Band structure, total and partial density of states of α perovskite phase: (a), (d) FA0.875Cs0.125PbI3; (b), (e) FAPbI2.96Br0.04; (c), (f) FA0.875Cs0.125PbI2.96Br0.04.
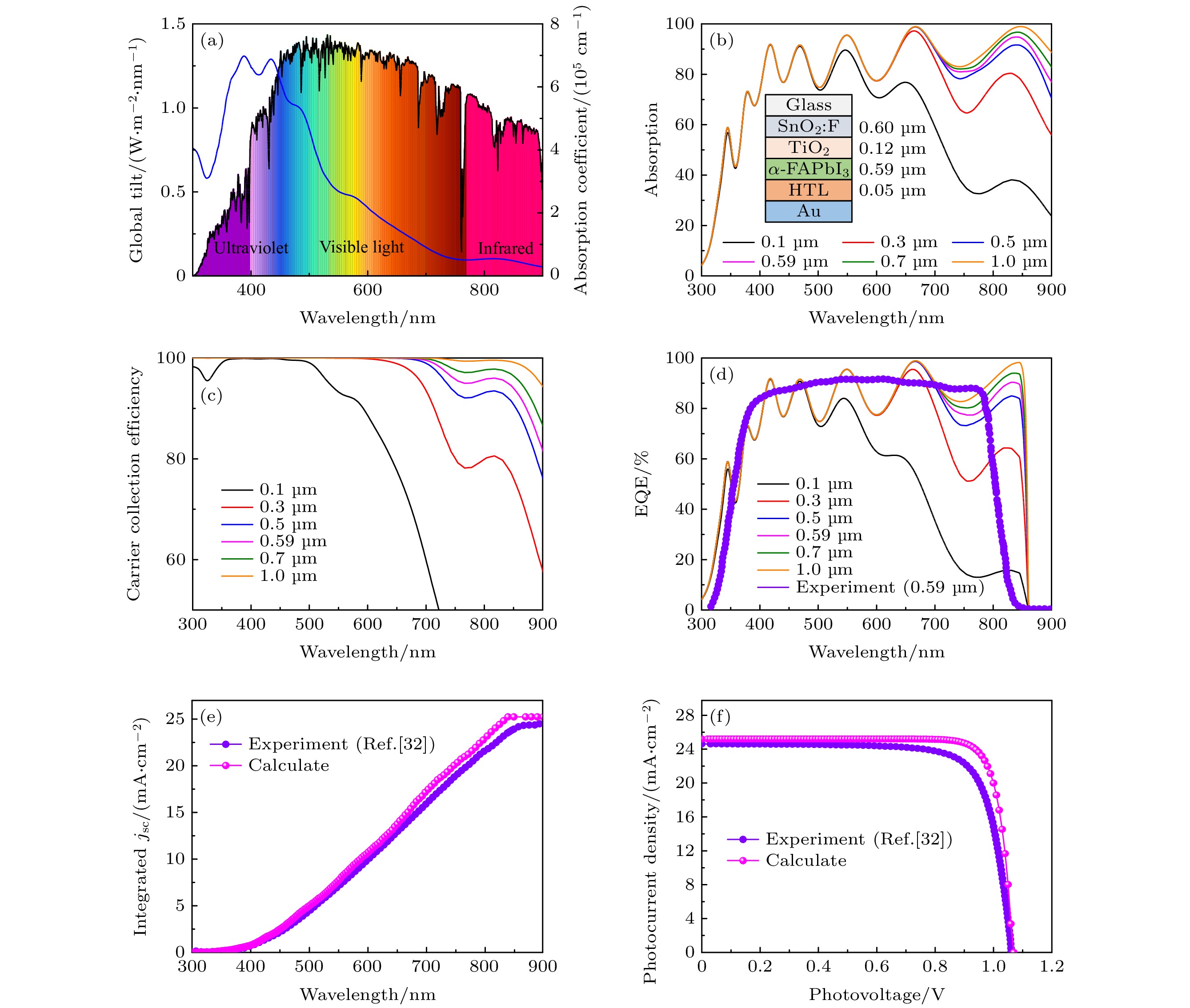
图 6 FAPbI3钙钛矿太阳电池的光电性能参数 (a) 吸收系数和AM1.5 G光谱; (b) 不同厚度的FAPbI3的吸收率; (c) 由FAPbI3的吸收系数α(ω)计算不同载流子收集长度(ΔZ)下载流子的收集效率H(λ); (d) 外量子效率; (e) 积分电流密度; (f) J-V特性
Fig. 6. Photovoltaic performance parameters of FAPbI3 perovskite solar cell: (a) Absorption coefficient and AM1.5 G illumination; (b) absorptance spectra of the FAPbI3 layer; (c) carrier collection efficiency H(λ) calculated from the α(ω) of the FAPbI3 using different values of carrier collection length (ΔZ); (d) external quantum efficiency spectrum; (e) integrated current density; (f) J-V curves
表 1 FA1–xCsxPbI3–yBry太阳电池的电学参数
Table 1. Photovoltaic parameters of the pure FA and the dopes Cs and Br perovskite solar cells.
Composition Jsc/(mA·cm–2) Voc/V FF/% η/% FAPbI3 (experiment) 24.7 1.06 77.5 20.3 FAPbI3 25.2 1.06 81.2 21.7 FA0.875Cs0.125PbI3 23.6 1.06 82.2 20.6 FAPbI2.96Br0.04 25.3 1.06 78.8 21.1 FA0.875Cs0.125PbI2.96Br0.04 24.7 1.06 82.4 21.6 -
[1] Burschka J, Pellet N, Moon S J, Humphry B R, Gao P, Nazeeruddin M K, Gratzel M 2013 Nature 499 316
 Google Scholar
Google Scholar
[2] Dong Q F, Fang Y J, Shao Y C, Mulligan P, Qiu J, Cao L, Huang J S 2015 Science 347 967
 Google Scholar
Google Scholar
[3] Wang C H, Zhang C J, Tong S C, Shen J Q, Wang C, Li Y Z, Xiao S, He J, Zhang J, Gao Y L, Yang J L 2017 J. Phys. Chem. C 121 6575
 Google Scholar
Google Scholar
[4] Zhang W H, Xiong J, Jiang L, Wang J Y, Mei T, Wang X B, Gu H S, Daoud W A, Li J H 2017 Appl. Mater. Interfaces 9 38467
 Google Scholar
Google Scholar
[5] Kojima A, Teshima K, Shirai Y, Miyasaka T 2009 J. Am. Chem. Soc. 131 6050
 Google Scholar
Google Scholar
[6] Park N G, Zhu K 2020 Nat. Rev. Mater. 5 333
 Google Scholar
Google Scholar
[7] Green M A, Dunlop E D, Levi D H, Hohl-Ebinger J, Yoshita M, Ho-Baillie A W Y 2019 Prog. Photovolt. Res. Appl. 27 565
 Google Scholar
Google Scholar
[8] Chen H, Ye F, Tang W T, He J J, Yin M S, Wang Y B, Xie F X, Bi E B, Yang X D, Gratzel M, Han L Y 2017 Nature 550 92
 Google Scholar
Google Scholar
[9] Jiang Q, Zhao Y, Zhang X W, Yang X L, Chen Y, Chu Z M, Ye Q F, Li X X, Yin Z G, You J B 2019 Nat. Photonics 13 460
 Google Scholar
Google Scholar
[10] Mellouhi F E, Bentria E T, Rashkeev S N, Kais S, Alharbi F H 2016 Sci. Rep. 6 30305
 Google Scholar
Google Scholar
[11] Lin R X, Xiao K, Qin Z Y, Han Q L, Zhang C F, Wei M Y, Saidaminov M I, Gao Y, Xu J, Xiao M, Li A D, Zhu J, Sargent E H, Tan H R 2019 Nat. Energy 4 864
 Google Scholar
Google Scholar
[12] Lee J W, Dai Z Z, Lee C, Lee H M, Han T H, Marco N D, Lin O, Choi C S, Dunn B S, Koh J, Carlo D D, Ko J H, Maynard H D, Yang Y 2018 J. Am. Chem. Soc. 140 6317
 Google Scholar
Google Scholar
[13] 毕富珍, 郑晓, 任志勇 2019 物理化学学报 35 69
 Google Scholar
Google Scholar
Bi F Z, Zheng X, Reng Z Y 2019 Acta Phys.-Chim. Sin. 35 69
 Google Scholar
Google Scholar
[14] Huang Y, Li L, Liu Z H, Jiao H Y, He Y Q, Wang X G, Zhu R, Wang D, Sun J L, Chen Q, Zhou H P 2017 J. Mater. Chem. A 5 8537
 Google Scholar
Google Scholar
[15] Li L, Liu N, Xu Z Q, Chen Q, Wang X D, Zhou H P 2017 ACS Nano 11 8804
 Google Scholar
Google Scholar
[16] Li N X, Luo Y Q, Chen Z H, Niu X X, Zhang X, Lu J Z, Kumar R S, Jiang J K, Liu H F, Guo X, Lai B, Brocks G, Chen Q, Tao S X, Fenning D P, Zhou H P 2020 Joule 4 1
 Google Scholar
Google Scholar
[17] Yi C Y, Luo J S, Meloni S, Boziki A, Astani N A, Gratzel C, Zakeeruddin S M, Rothlisberger U, Gratzel M 2016 Energy Environ. Sci. 9 656
 Google Scholar
Google Scholar
[18] Wang L G, Zhou H P, Hu J N, Huang B L, Sun M Z, Dong B W, Zheng J G H, Huang Y, Chen Y H, LI L, Xu Z Q, Li N G, Liu Z, Chen Q, Sun L D, Yan C H 2019 Science 363 265
 Google Scholar
Google Scholar
[19] Liu W, Liu N J, Ji S L, Hua H F, Ma Y H, Hu R Y, Zhang J, Chu L, Li X A, Huang W 2020 Nano-Micro Lett. 12 119
 Google Scholar
Google Scholar
[20] Correa-Baena J B, Luo Y Q, Brenner T M, Snaider J, Sun S J, Li X Y, Jensen M A, Hartono N P T, Nienhaus L, Wieghold S, Poindexter J R, Wang S, Meng Y S, Wang T, Lai B, Holt M V, Cai Z H, Bawendi M G, Huang L B, Buonassisi T, Fenning D P 2019 Science 363 627
 Google Scholar
Google Scholar
[21] Charles B, Weller M T, Rieger S, Hatcher L E, Henry P F, Feldmann J, Wolverson D, Wilson C C 2020 Chem. Mater. 32 2282
 Google Scholar
Google Scholar
[22] Nakane A, Tampo H, Tamakoshi M, Fujimoto S, Kim K M, Kim S, Shibata H, Niki S, Fujiwara H 2016 J. Appl. Phys. 120 064505
 Google Scholar
Google Scholar
[23] Dewan R, Vasilev I, Jovanov V, Knipp D 2011 J. Appl. Phys. 110 013101
 Google Scholar
Google Scholar
[24] 石将建, 卫会云, 朱立峰, 许信, 徐余颛, 吕松涛, 吴会觉, 罗艳红, 李冬梅, 孟庆波 2015 64 038402
 Google Scholar
Google Scholar
Shi J J, Wei H Y, Zhu L F, Xu X, Xu Y Z, Lü S T, Wu H J, Luo Y H, Li D M, Bo M Q 2015 Acta Phys. Sin. 64 038402
 Google Scholar
Google Scholar
[25] Kato Y, Fujimoto S, Kozawa M, Fujiwara H 2019 Phys. Rev. Appl. 12 024039
 Google Scholar
Google Scholar
[26] Weller M T, Weber O J, Frost J M, Walsh A 2015 J. Phys. Chem. Lett. 6 3209
 Google Scholar
Google Scholar
[27] Kato M, Fujiseki T, Miyadera T, Sugita T, Fujimoto S, Tamakoshi M, Chikamatsu M, Fujiwara H 2017 J. Appl. Phys. 121 115501
 Google Scholar
Google Scholar
[28] Eperon G E, Stranks S D, Menelaou S, Johnston M B, Herz L M, Snaith H J 2014 Energy Environ. Sci. 7 982
 Google Scholar
Google Scholar
[29] Stoumpos C C, Malliakas C D, Kanatzidis M G 2013 Inorg. Chem. 52 9019
 Google Scholar
Google Scholar
[30] 柴磊, 钟敏 2016 65 237902
 Google Scholar
Google Scholar
Chai L, Zhong M 2016 Acta Phys. Sin. 65 237902
 Google Scholar
Google Scholar
[31] 王福芝, 谭占鳌, 戴松元, 李永舫 2015 64 038401
 Google Scholar
Google Scholar
Wang F Z, Tan Z A, Dai S Y, Li Y F 2015 Acta Phys. Sin. 64 038401
 Google Scholar
Google Scholar
[32] Yang W S, Noh J H, Jeon N J, Kim Y C, Ryu S, Seo J, Seok S I 2015 Science 348 1234
 Google Scholar
Google Scholar
[33] Pham N D, Zhang C M, Tiong V T, Zhang S L, Will G, Bou A, Bisquert J, Shaw P E, Du A J, Wilson G J, Wang H X 2019 Adv. Funct. Mater. 29 1806479
 Google Scholar
Google Scholar
[34] Tavakoli M M, Yadav P, Tavakoli R, Kong J 2018 Adv. Energy Mater. 1800794
[35] Nazarenko O, Yakunin S, Morad V, Cherniukh I, Kovalenko V 2017 NPG Asia Mater. 9 e373
 Google Scholar
Google Scholar
[36] 刘娜, 危阳, 马新国, 祝林, 徐国旺, 楚亮, 黄楚云 2017 66 057103
 Google Scholar
Google Scholar
Liu N, Wei Y, Ma X G, Xu G W, Chu L, Huang C Y 2017 Acta Phys. Sin. 66 057103
 Google Scholar
Google Scholar
[37] 蒋泵, 陈思良, 崔晓磊, 胡紫婷, 李跃, 张笑铮, 吴康敬, 王文贞, 蒋最敏, 洪峰, 马忠权, 赵磊, 徐飞, 徐闰, 詹义强 2019 68 246801
 Google Scholar
Google Scholar
Jiang B, Chen S L, Cui X L, Hu Z T, Li Y, Zhang X Z, Wu K J, Wang W Z, Jiang Z M, Hong F, Ma Z Q, Zhao L, Xu F, Xu R, Zhan Y Q 2019 Acta Phys. Sin. 68 246801
 Google Scholar
Google Scholar
计量
- 文章访问数: 10832
- PDF下载量: 276
- 被引次数: 0














 下载:
下载: