-
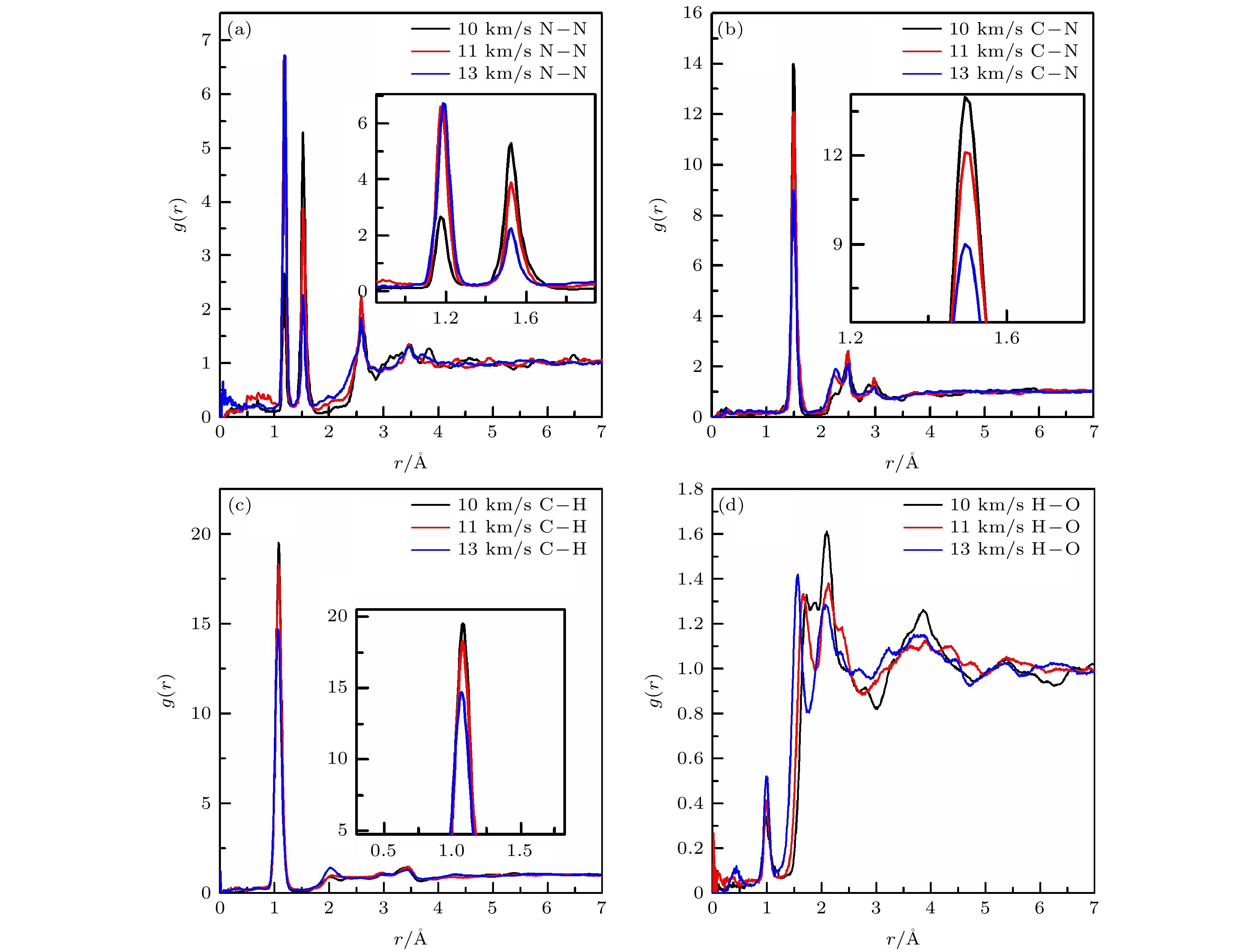
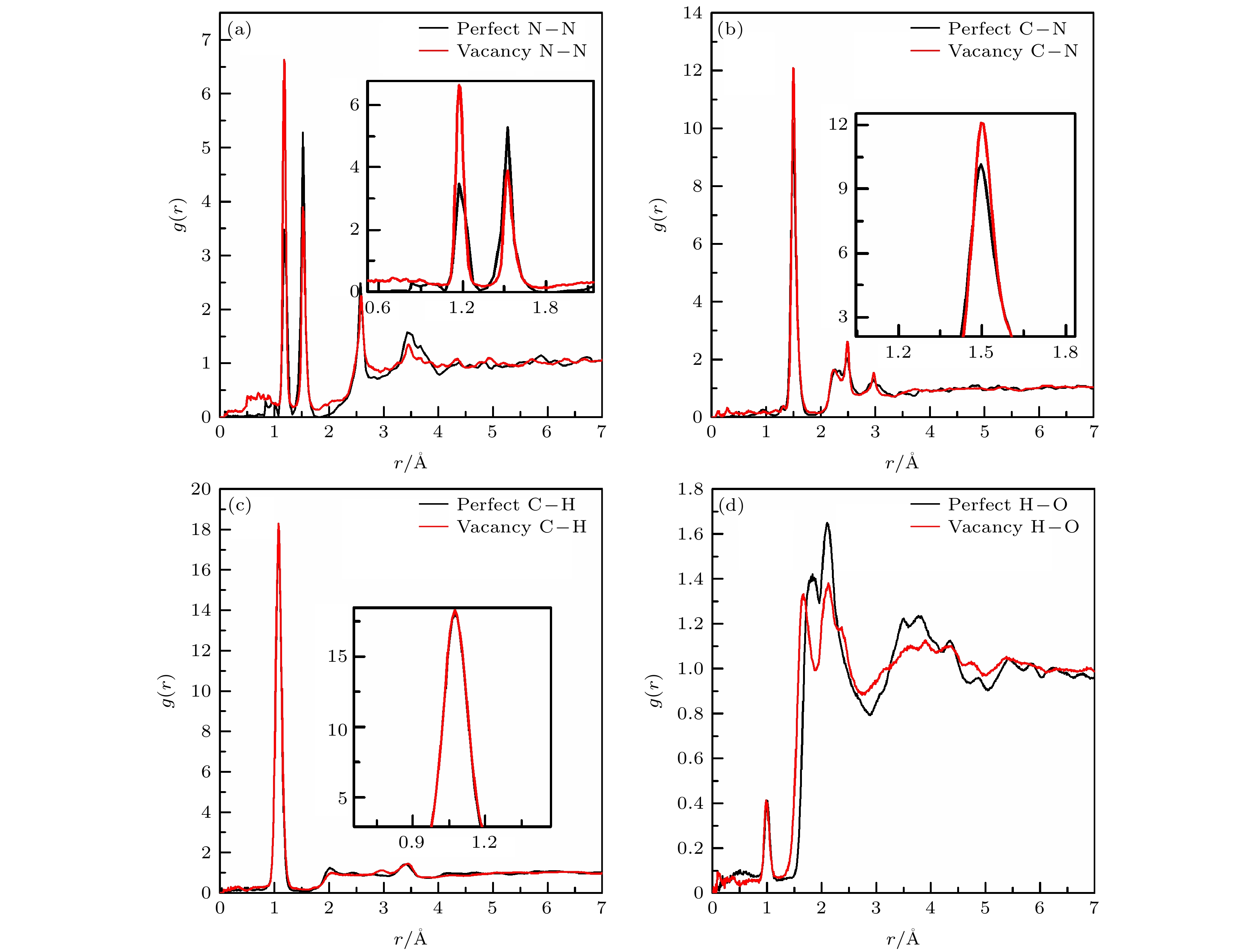
当前, 对暴露于极端环境中的含能材料的相对安全性的关注日益增加. 理解含能材料在冲击加载下的初始分解机理是探索新型高能顿感材料的基础. 本文利用多尺度冲击技术(multi-scale shock technique, MSST)结合反应力场(ReaxFF)分子动力学的方法研究冲击加载下环三亚甲基三硝胺(RDX)的完美晶体和分子空位晶体的初始动态响应及反应机理, 计算了可能参与反应的原子间径向分布函数, 分析了不同冲击速度及分子空位缺陷对冲击加载过程的影响. 结果表明: 在冲击加载下, 完美RDX晶体和空位RDX晶体的初始分解方式均为首先发生N—NO2键断裂, 随后是C—N键的断裂. 此外, 还可能会出现C—H键断裂, 并有氢转移到硝基中的氧原子上形成HONO. 随着冲击速度的增加, 两种RDX晶体的化学键断裂数目增多, 反应更强烈. 分子空位缺陷的存在增强了N—NO2的反应活性, 使其更易发生断裂, 进而加速空位RDX晶体的初始反应.At present, the relative safety of energetic materials exposed to extreme environments is concerned widely. Understanding the initial decomposition mechanism of energetic materials under impact loading is the basis for exploring new energetic materials with high energy and low sensitivity. In this paper, we study the initial dynamic response and reaction mechanism of perfect cyclotrimethylenetrinitramine (RDX) crystal and RDX crystal with a molecular vacancy defect under shock loading by using the multiscale shock technique (MSST) combined with reactive force field (ReaxFF) molecular dynamics method. The RDX perfect supercell and supercell containing a molecular vacancy are constructed to simulate the shock process by using the generalized gradient approximation method in density functional theory and Perdew-Burke-Ernzerhof functional. Before loading the shock wave, one NVE ensemble and Berendsen thermostat are used to control the RDX equilibrium process. A multi-scale impact compression is loaded along the crystal A direction. The initial temperature is 300 K and the initial pressure is set to be an atmospheric pressure. The radial distribution functions between main atoms are calculated, and the influences of shock velocity and molecular vacancy defect on shock loading process are analyzed. The evolution of N—NO2 bond and C—N bond with time in RDX perfect crystals and vacancy crystals under shock velocity of 11 km/s are given. As a result, the possible initial decomposition path of perfect RDX crystal and vacancy RDX crystal are the first fracture of N—NO2 bond, followed by the cleavage of C—N bond at small shock velocity. The initial reaction of the RDX crystal with a molecule vacancy is earlier than that of the perfect crystal, which indicates that the vacancy crystal is more sensitive to shock and more prone to decomposition. Furthermore, the fracture of C—H bond is possible after the initial cleavage of N—NO2 bond and C—N bond, and then the H atom is transferred to oxygen atom in nitro group, forming HONO. As the shock velocity increases, the number of broken chemical bonds in the two kinds of RDX crystals increases, and the reaction becomes strong. The presence of molecular vacancy defect enhances the activity of N—NO2 bond and makes it easier to break, thus accelerating the initial reaction of the vacancy crystal. The shock velocity and the particle velocity of the RDX crystal are consistent with previous experimental results and theoretical data, which shows the validity of our calculation results.
-
Keywords:
- energetic materials /
- multiscale shock technique /
- molecular dynamics /
- vacancy defect /
- radial distribution function
[1] Zhu W, Xiao J J, Zhu W H, Xiao H M 2009 J. Hazard. Mater. 164 1082
 Google Scholar
Google Scholar
[2] Xiao J J, Li S Y, Chen J, Ji G F, Zhu W, Zhao F, Wu Q, Xiao H M 2013 J. Mol. Model. 19 803
 Google Scholar
Google Scholar
[3] Deng C, Liu J, Xue X G, Long X P, Zhang C Y 2018 J. Phys. Chem. C 122 27875
 Google Scholar
Google Scholar
[4] Qin H, Zhong M, Zhu S H, Liu F S, Tang B, Gan Y D, Liu Q J 2020 Chem. Phys. Lett. 749 137470
 Google Scholar
Google Scholar
[5] Brown J A, LaBarbera D A, Zikry M A 2014 Model. Simul. Mater. SC. 22 055013
 Google Scholar
Google Scholar
[6] Wang N, Peng J H, Pang A, Hu J J, He T 2016 J. Mol. Model. 22 229
 Google Scholar
Google Scholar
[7] Kuklja M M, Kunz A B 1999 J. Phys. Chem. B 103 8427
 Google Scholar
Google Scholar
[8] 彭亚晶, 蒋艳雪 2015 64 243102
 Google Scholar
Google Scholar
Peng Y J, Jiang Y X 2015 Acta Phys. Sin. 64 243102
 Google Scholar
Google Scholar
[9] Chakraborty D, Muller R P, Dasgupta S, Goddard III W A 2001 J. Comput-Aided Mater. 8 203
 Google Scholar
Google Scholar
[10] Ge N N, Wei Y K, Ji G F, Chen X R, Zhao F, Wei D Q 2012 J. Phys. Chem. B 116 13696
 Google Scholar
Google Scholar
[11] Ge N N, Bai S, Chang J, Ji G F 2018 RSC Advances 8 17312
 Google Scholar
Google Scholar
[12] 郑朝阳, 赵纪军 2015 高压 29 81
 Google Scholar
Google Scholar
Zheng Z Y, Zhao J J 2015 Chin. J. High Press. Phys. 29 81
 Google Scholar
Google Scholar
[13] 刘海, 李启楷, 何远航 2015 64 018201
 Google Scholar
Google Scholar
Liu H, Li Q K, He Y H 2015 Acta Phys. Sin. 64 018201
 Google Scholar
Google Scholar
[14] Miao M S, Dreger Z A, Patterson J E, Gupta Y M 2008 J. Phys. Chem. A 112 7383
 Google Scholar
Google Scholar
[15] 陈芳, 程新路 2016 原子与分子 33 315
 Google Scholar
Google Scholar
Chen F, Cheng X L 2016 J. Atom. Mol. Phys. 33 315
 Google Scholar
Google Scholar
[16] Strachan A, van Duin A C T, Goddard W A 2004 Proceedings of the Conference of the American Physical Society Topical Group on Shock Compression of Condensed Matter, Portland, Oregon, USA, July 20−25, 706 p895
[17] Moore J D, Barnes B C, Izvekov S, Lísal M, Sellers M S, Taylor D E, Brennan J K 2016 J. Chem. Phys. 144 104501
 Google Scholar
Google Scholar
[18] Zhou T T, Huang F L 2011 J. Phys. Chem. B 115 278
 Google Scholar
Google Scholar
[19] Huang X N, Qiao Z Q, Dai X G, Zhang K L, Li M, Pei G, Wen Y S 2019 J. Appl. Phys. 125 195101
 Google Scholar
Google Scholar
[20] Van Duin A C T, Dasgupta S, Lorant F, Goddard W A 2001 J. Phys. Chem. A 105 9396
 Google Scholar
Google Scholar
[21] Xue X G, Wen Y S, Long X P, Li J S, Zhang C Y 2015 J. Phys. Chem. C 119 13735
 Google Scholar
Google Scholar
[22] Strachan A, Kober E M, Van Duin A C T, Oxgaard J, Goddard W A 2005 J. Chem. Phys. 122 54502
 Google Scholar
Google Scholar
[23] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[24] Choi C S, Prince E 1972 Acta Crystallogr. B 28 2857
 Google Scholar
Google Scholar
[25] Bai Z Q, Dai B, Chang J, Wang J, Ge N N 2018 J. Mol. Graph. Model. 85 316
 Google Scholar
Google Scholar
[26] 李志鹏, 龙新平, 李洪珍, 韩勇, 李明 2014 兵工学报 35 83
 Google Scholar
Google Scholar
Li Z P, Long X P, Li H Z, Han Y, Li M 2014 Acta Armament. 35 83
 Google Scholar
Google Scholar
[27] Gibbs T R 1980 LASL Explosive Property Data (Berkeley, Los Angeles, London: University of California Press)
[28] Zhou P 2016 M. S. Thesis (Chongqing: Chongqing University of Posts and Telecommunications) (in Chinese)
[29] Xu J C, Zhao J J, Sun L Z 2008 Mol. Simulat. 34 961
 Google Scholar
Google Scholar
[30] Chakraborty D, Muller R P, Dasgupta S, Goddard W A 2000 J.Phys. Chem. A 104 2261
 Google Scholar
Google Scholar
[31] Chen F, Zhang H, Duan M L, Wang J L, Chen L Z 2013 J. Atom. Mol. Phys. 30 1025
 Google Scholar
Google Scholar
[32] Zhong K, Liu J, Wang L Y, Zhang C Y 2019 J. Phys. Chem. C 123 1483
-
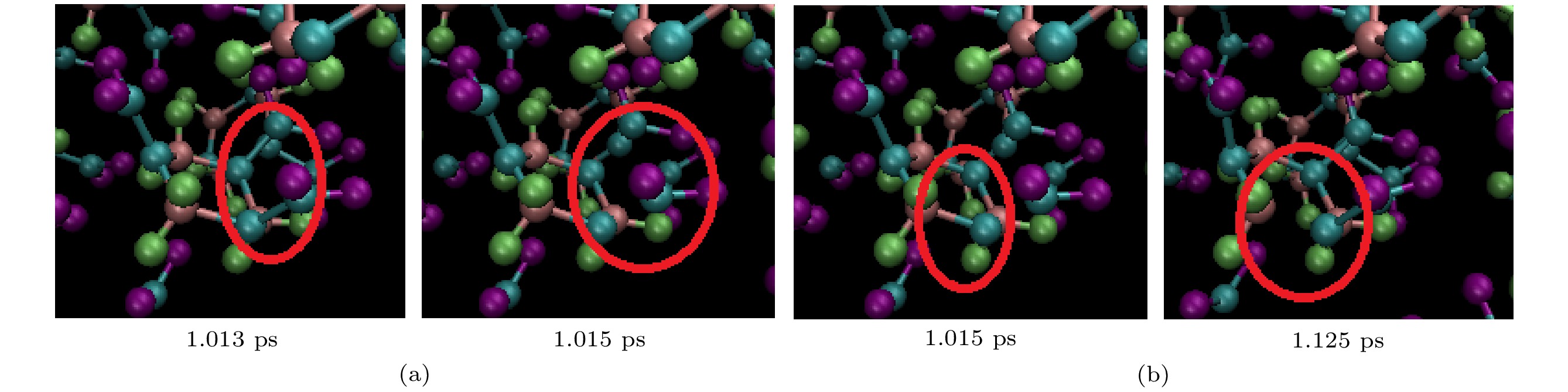
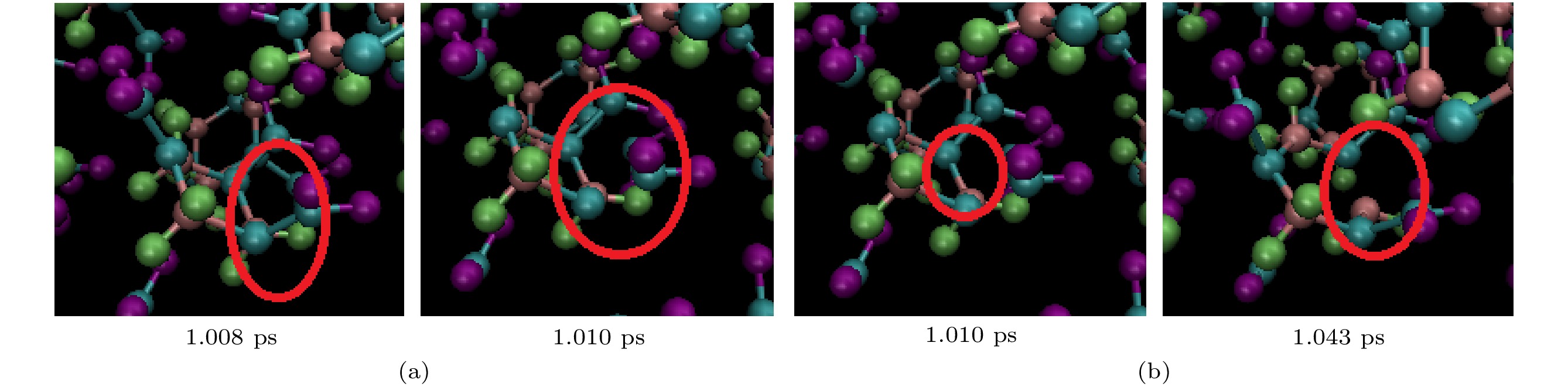
图 2 冲击速度为11 km/s加载下, 完美RDX超胞(a)和空位RDX超胞(b)在不同时刻的体系结构, 图中红色代表氧原子、黑色代表碳原子、蓝色代表氮原子、绿色代表氢原子
Fig. 2. System structure of perfect RDX supercell (a) and RDX supercell with a molecular vacancy (b) at different time under shock velocity of 11 km/s. The red for oxygen, the black for carbon, the blue for nitrogen and the green for hydrogen.
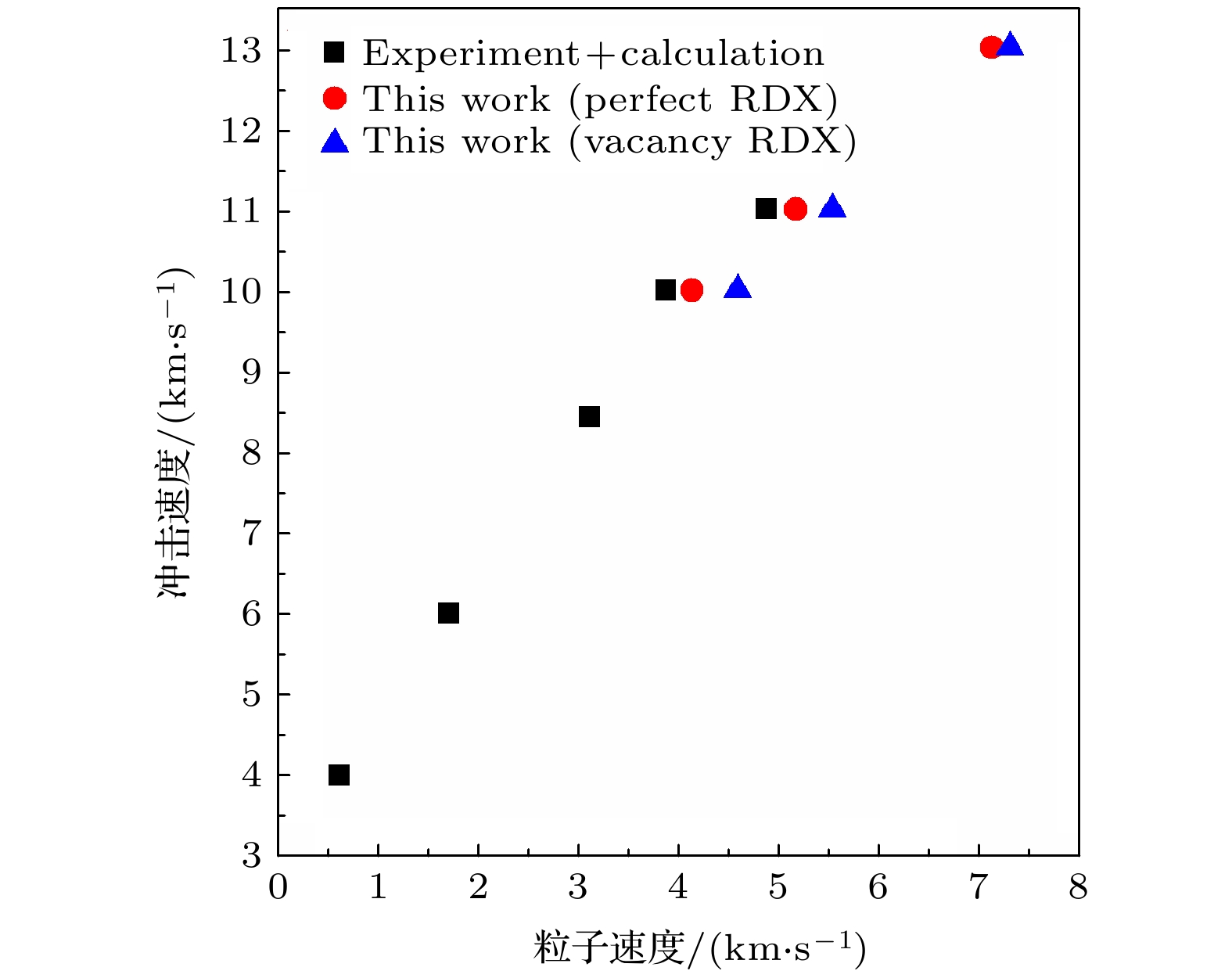
图 4 RDX冲击速度与粒子速度对应关系, 其中, 黑色方形数据为起爆前(冲击速度低于8.6 km/s)实验数据和起爆后的理论计算数据[27,28], 红色圆形和蓝色三角形数据为本计算的完美晶体和空位晶体的相应数据
Fig. 4. Shock velocity vs. particle velocity for RDX. Here, the black square data are the experimental data before detonation (below 8.6 km/s in shock velocity) and the theoretical calculation data after detonation[27,28], and the red circle and blue triangle data are respectively that of the perfect crystal and the vacancy crystal in this calculation.
图 5 在冲击速度为11 km/s作用下, RDX完美晶胞中N—NO2键随时间演化情况(a)和C—N键随时间演化情况(b), 其中蓝绿色代表氮、紫色代表氧、绿色代表氢、橘红色代表碳
Fig. 5. Evolution of N—NO2 bonds with time (a) and that of C—N bonds with time (b) in RDX perfect cell under shock velocity of 11 km/s. The blue-green represents nitrogen atom, the purple represents oxygen atom, the green represents hydrogen atom and the tangerine represents carbon atom.
图 6 在冲击速度为11 km/s作用下, 含分子空位的RDX晶胞中N—NO2键随时间的演化情况(a)和C—N键随时间的演化情况(b), 其中蓝绿色代表氮、紫色代表氧、绿色代表氢、橘红色代表碳
Fig. 6. Evolution of N—NO2 bonds with time (a) and that of C—N bonds with time (b) in RDX cell with a molecular vacancy under shock velocity of 11 km/s. The blue-green represents nitrogen atom, the purple represents oxygen atom, the green represents hydrogen atom and the tangerine represents carbon atom.
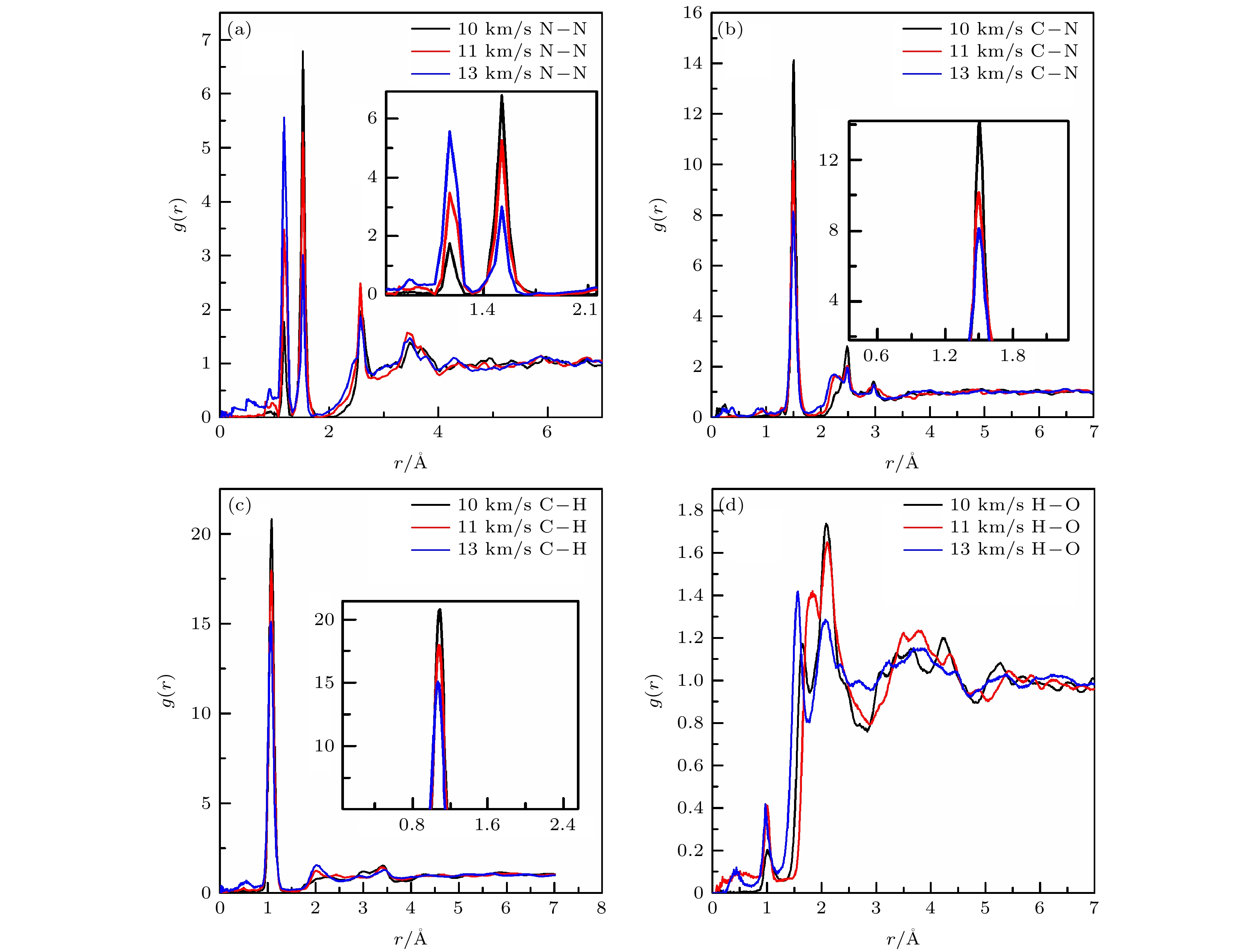
表 1 完美晶体中原子间的径向分布函数相关峰所包围的面积
Table 1. Area enclosed by a certain peak of inter-atoms radial distribution function in the perfect crystal
Shock velocity/(km·s–1) N—N C—N C—H H—O r = 1.18 Å r = 1.55 Å r = 1.45 Å r = 1.04 Å r = 1.00 Å r = 2.45 Å 10 0.1399 0.6488 1.3807 2.6913 0.0156 0.3824 11 0.3406 0.5048 1.2285 2.4445 0.0364 0.2695 13 0.5710 0.2831 1.1063 2.1975 0.0403 0.2616 表 2 空位晶体中原子间的径向分布函数峰所包围的面积
Table 2. Area enclosed by a certain peak of inter-atoms radial distribution function in the vacancy crystal.
Shock velocity/(km·s–1) N—N C—N C—H H—O r = 1.18 Å r = 1.55 Å r = 1.45 Å r = 1.04 Å r = 1.00 Å r = 2.15 Å 10 0.1631 0.7238 1.3794 2.5649 0.0342 0.2328 11 0.4722 0.4398 1.3427 2.5349 0.0372 0.2268 13 0.5619 0.3095 1.0985 2.1741 0.0477 0.2316 表 3 11 km/s冲击速度加载下完美晶体和空位晶体中原子间的径向分布函数峰所包围的面积
Table 3. Area enclosed by a certain peak of inter-atoms radial distribution function in the perfect and vacancy crystals at the shock velocity of 11 km/s.
RDX N—N C—N C—H H—O r = 1.18 Å r = 1.55 Å r = 1.45 Å r = 1.04 Å r = 1.00 Å r = 1.52 Å r = 2.15 Å Perfect 0.3406 0.5048 1.2285 2.4445 0.0364 0.2226 0.2695 vacancy 0.4722 0.4398 1.3427 2.5349 0.0372 0.2027 0.2268 -
[1] Zhu W, Xiao J J, Zhu W H, Xiao H M 2009 J. Hazard. Mater. 164 1082
 Google Scholar
Google Scholar
[2] Xiao J J, Li S Y, Chen J, Ji G F, Zhu W, Zhao F, Wu Q, Xiao H M 2013 J. Mol. Model. 19 803
 Google Scholar
Google Scholar
[3] Deng C, Liu J, Xue X G, Long X P, Zhang C Y 2018 J. Phys. Chem. C 122 27875
 Google Scholar
Google Scholar
[4] Qin H, Zhong M, Zhu S H, Liu F S, Tang B, Gan Y D, Liu Q J 2020 Chem. Phys. Lett. 749 137470
 Google Scholar
Google Scholar
[5] Brown J A, LaBarbera D A, Zikry M A 2014 Model. Simul. Mater. SC. 22 055013
 Google Scholar
Google Scholar
[6] Wang N, Peng J H, Pang A, Hu J J, He T 2016 J. Mol. Model. 22 229
 Google Scholar
Google Scholar
[7] Kuklja M M, Kunz A B 1999 J. Phys. Chem. B 103 8427
 Google Scholar
Google Scholar
[8] 彭亚晶, 蒋艳雪 2015 64 243102
 Google Scholar
Google Scholar
Peng Y J, Jiang Y X 2015 Acta Phys. Sin. 64 243102
 Google Scholar
Google Scholar
[9] Chakraborty D, Muller R P, Dasgupta S, Goddard III W A 2001 J. Comput-Aided Mater. 8 203
 Google Scholar
Google Scholar
[10] Ge N N, Wei Y K, Ji G F, Chen X R, Zhao F, Wei D Q 2012 J. Phys. Chem. B 116 13696
 Google Scholar
Google Scholar
[11] Ge N N, Bai S, Chang J, Ji G F 2018 RSC Advances 8 17312
 Google Scholar
Google Scholar
[12] 郑朝阳, 赵纪军 2015 高压 29 81
 Google Scholar
Google Scholar
Zheng Z Y, Zhao J J 2015 Chin. J. High Press. Phys. 29 81
 Google Scholar
Google Scholar
[13] 刘海, 李启楷, 何远航 2015 64 018201
 Google Scholar
Google Scholar
Liu H, Li Q K, He Y H 2015 Acta Phys. Sin. 64 018201
 Google Scholar
Google Scholar
[14] Miao M S, Dreger Z A, Patterson J E, Gupta Y M 2008 J. Phys. Chem. A 112 7383
 Google Scholar
Google Scholar
[15] 陈芳, 程新路 2016 原子与分子 33 315
 Google Scholar
Google Scholar
Chen F, Cheng X L 2016 J. Atom. Mol. Phys. 33 315
 Google Scholar
Google Scholar
[16] Strachan A, van Duin A C T, Goddard W A 2004 Proceedings of the Conference of the American Physical Society Topical Group on Shock Compression of Condensed Matter, Portland, Oregon, USA, July 20−25, 706 p895
[17] Moore J D, Barnes B C, Izvekov S, Lísal M, Sellers M S, Taylor D E, Brennan J K 2016 J. Chem. Phys. 144 104501
 Google Scholar
Google Scholar
[18] Zhou T T, Huang F L 2011 J. Phys. Chem. B 115 278
 Google Scholar
Google Scholar
[19] Huang X N, Qiao Z Q, Dai X G, Zhang K L, Li M, Pei G, Wen Y S 2019 J. Appl. Phys. 125 195101
 Google Scholar
Google Scholar
[20] Van Duin A C T, Dasgupta S, Lorant F, Goddard W A 2001 J. Phys. Chem. A 105 9396
 Google Scholar
Google Scholar
[21] Xue X G, Wen Y S, Long X P, Li J S, Zhang C Y 2015 J. Phys. Chem. C 119 13735
 Google Scholar
Google Scholar
[22] Strachan A, Kober E M, Van Duin A C T, Oxgaard J, Goddard W A 2005 J. Chem. Phys. 122 54502
 Google Scholar
Google Scholar
[23] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[24] Choi C S, Prince E 1972 Acta Crystallogr. B 28 2857
 Google Scholar
Google Scholar
[25] Bai Z Q, Dai B, Chang J, Wang J, Ge N N 2018 J. Mol. Graph. Model. 85 316
 Google Scholar
Google Scholar
[26] 李志鹏, 龙新平, 李洪珍, 韩勇, 李明 2014 兵工学报 35 83
 Google Scholar
Google Scholar
Li Z P, Long X P, Li H Z, Han Y, Li M 2014 Acta Armament. 35 83
 Google Scholar
Google Scholar
[27] Gibbs T R 1980 LASL Explosive Property Data (Berkeley, Los Angeles, London: University of California Press)
[28] Zhou P 2016 M. S. Thesis (Chongqing: Chongqing University of Posts and Telecommunications) (in Chinese)
[29] Xu J C, Zhao J J, Sun L Z 2008 Mol. Simulat. 34 961
 Google Scholar
Google Scholar
[30] Chakraborty D, Muller R P, Dasgupta S, Goddard W A 2000 J.Phys. Chem. A 104 2261
 Google Scholar
Google Scholar
[31] Chen F, Zhang H, Duan M L, Wang J L, Chen L Z 2013 J. Atom. Mol. Phys. 30 1025
 Google Scholar
Google Scholar
[32] Zhong K, Liu J, Wang L Y, Zhang C Y 2019 J. Phys. Chem. C 123 1483
计量
- 文章访问数: 8320
- PDF下载量: 104
- 被引次数: 0














 下载:
下载: