-
通过测定及分析纳米颗粒和表面活性剂-纳米颗粒复配体系在自由吸附过程与动态收缩过程中表面张力的变化, 总结了纳米颗粒在气-液界面的吸附排布规律以及表面活性剂对其吸附规律的影响. 实验结果表明, 自由吸附过程中, 随矿化度增加、阳离子活性剂浓度增加, 平衡表面张力降低, 这与颗粒吸附密度增加及颗粒润湿性改变有关. 浓度低于临界胶束浓度(CMC)时, 阳离子活性剂体系与混合体系的表面张力差异证明了阳离子活性剂可以通过静电作用吸附于纳米颗粒表面, 进而部分溶解于水相; 而阴离子活性剂与纳米颗粒相互作用力较弱, 对表面张力影响较小. 纳米颗粒体系在液滴收缩过程中, 表面张力从自由吸附平衡态进一步降低大约9 mN/m, 说明自由吸附过程中纳米颗粒不能达到紧密排布; 同时表面张力呈现为缓慢降低、快速降低和达到平衡三部分, 表面压缩模量可达70 mN/m, 满足了液膜Gibbs稳定准则, 这将有助于提高泡沫或者乳液稳定性. 纳米颗粒-表面活性剂体系在液滴收缩过程中表面张力降低值随活性剂浓度增加而减小; 表面压缩模量由高到低依次为: 纳米颗粒 > 阳离子活性剂-纳米颗粒 > 阴离子-纳米颗粒 > 表面活性剂.Silica nanoparticles (NPs) are more and more useful in many engineering areas, but the dynamic behaviors of adsorption of NPs at surface are not clear, especially when there exist surfactants on the surface. The modified NPs with the nonionic dimethyl silane are partially hydrophobic, and in this paper, the surface behavior is investigated which is determined by interfacial tension and surface compression modulus. It is concluded that the dimethyl silane coverage, the brine salinity and the surfactant would affect the NPs’ adsorption. Higher salinity in brine or higher dimethyl silane coverage causes lower steady state surface tension, which is related to the hydrophobicity and adsorption amount of NPs at the surface. When the cationic surfactant concentration is lower than critical micelle concentration (CMC), the surface tension of mixture system is a little bit higher than cationic surfactant’s. Cationic surfactant can be adsorbed at NPs’ surface to change the hydrophobicity based on the electrostatic attraction, and then some surfactants are dissolved in liquid phase together with NPs, while the anionic surfactant will not do so. In the shrinking droplet process, the surface tension of the NPs with 1.5 μmol/m2 dimethyl silane decreases from ~59 mN/m at the steady state to ~50 mN/m, which proves that the NPs’ adsorption density can be higher even after infinite long time equilibrium due to the repulsive force between the NPs. Besides, the curve of interfacial tension (IFT) versus surface area shows three parts with different declining slopes. In the first part, the relatively low adsorption of NPs at the surface means weak interaction between NPs. Then in the second part, due to the irreversible adsorption, the spacing between NPs decreases with adsorption amount increasing and surface area lowering, so the increasing of NPs’ interaction leads to high surface compression modulus. After that, the IFT curve keeps flat since the NPs assembly reaches to the closest peck. With the increase of NPs’ hydrophobicity, the compression modulus increases up to ~70 mN/m, which satisfies the Gibbs criterion to resist coarsening of the foam or emulsion. However, for the mixing system, increasing surfactant concentration leads to a lower surface tension at steady state, then the surface tension difference or compression modulus decreases too. Finally, we find that the compression modulus order from high to low is as follows: NPs, cationic surfactant-NPs, anionic surfactant-NPs, surfactants. This investigation is meaningful for accounting for the enhancement of foam or emulsion stability with NPs affected by salinity and surfactant.
-
Keywords:
- nanoparticles /
- adsorption at interface /
- surface tension /
- surface dilatational modulus and compression modulus
[1] Sozer N, Kokini J L 2009 Trends Biotechnol. 27 82
 Google Scholar
Google Scholar
[2] 侯静菲, 杨延莲, 王琛 2017 物理化学学报 33 63
 Google Scholar
Google Scholar
Hou J F, Yang Y L, Wang C 2017 Acta Phys.-Chim. Sin. 33 63
 Google Scholar
Google Scholar
[3] Soleimani M, Khani A, Najafzadeh K 2012 J. Mol. Catal. B: Enzym. 74 1
 Google Scholar
Google Scholar
[4] Arjang S, Manteghian M, Mohammadi A 2013 Chem. Eng. Res. Des. 91 1050
 Google Scholar
Google Scholar
[5] Zhang C, Li Z, Sun Q, Wang P, Wang S, Liu W 2015 Soft Matter 12 946
[6] Kim I, Worthen A J, Johnston K P, DiCarlo D A, Huh C 2016 J. Nanopart. Res. 18 82
 Google Scholar
Google Scholar
[7] 叶学民, 李明兰, 张湘珊, 李春曦 2018 67 214703
Ye X M, Li M L, Zhang X S, Li C X 2018 Acta Phys. Sin. 67 214703
[8] Binks B P 2002 Curr. Opin. Colloid. Interface Sci. 7 21
 Google Scholar
Google Scholar
[9] Bizmark N, Ioannidis M A, Henneke D E 2014 Langmuir 30 710
 Google Scholar
Google Scholar
[10] Roger K, Cabane B 2012 Angew. Chem. Int. Ed. 51 5625
 Google Scholar
Google Scholar
[11] Kutuzov S, He J, Tangirala R, Emrick T, Russell T, Böker A 2007 Phys. Chem. Chem. Phys. 9 6351
 Google Scholar
Google Scholar
[12] Zang D, Zhang Y, Hou Q 2012 Colloids Surf., A 395 262
 Google Scholar
Google Scholar
[13] Zhang Y, Wang S, Zhou J, Zhao R, Benz G, Tcheimou S, Meredith J C, Behrens S H 2017 Langmuir 33 4511
 Google Scholar
Google Scholar
[14] Du K, Glogowski E, Emrick T, Russell T P, Dinsmore A D 2010 Langmuir 26 12518
 Google Scholar
Google Scholar
[15] Hua X, Frechette J, Bevan M A 2018 Soft matter 14 3818
 Google Scholar
Google Scholar
[16] Chai Y, Lukito A, Jiang Y, Ashby P D, Russell T P 2017 Nano Lett. 17 6453
 Google Scholar
Google Scholar
[17] Maestro A, Rio E, Drenckhan W, Langevin D, Salonen A 2014 Soft Matter 10 6975
 Google Scholar
Google Scholar
[18] Zang D Y, Rio E, Langevin D, Wei B, Binks B P 2010 Eur. Phys. J. E 31 125
 Google Scholar
Google Scholar
[19] Safouane M, Langevin D, Binks B 2007 Langmuir 23 11546
 Google Scholar
Google Scholar
[20] Monteux C, Fuller G G, Bergeron V 2004 J. Phys. Chem. B 108 16473
 Google Scholar
Google Scholar
[21] Santini E, Krägel J, Ravera F, Liggieri L, Miller R 2011 Colloids Surf. A 382 186
 Google Scholar
Google Scholar
[22] Wang J, Nguyen A V, Farrokhpay S 2016 Adv. Colloid Interface Sci. 228 55
 Google Scholar
Google Scholar
[23] Worthen A J, Tran V, Cornell K A, Truskett T M, Johnston K P 2016 Soft Matter 12 2025
 Google Scholar
Google Scholar
[24] Cui Z G, Yang L L, Cui Y Z, Binks B 2009 Langmuir 26 4717
[25] Zhang H, Yu K, Cayre O J, Harbottle D 2016 Langmuir 32 13472
 Google Scholar
Google Scholar
[26] 臧渡洋, 张永建, Langevin Dominique 2011 60 076801
Zang D Y, Zhang Y J, Langevin D 2011 Acta Phys. Sin. 60 076801
[27] Zang D, Rio E, Delon G, Langevin D, Wei B, Binks B 2011 Mol. Phys. 109 1057
 Google Scholar
Google Scholar
-
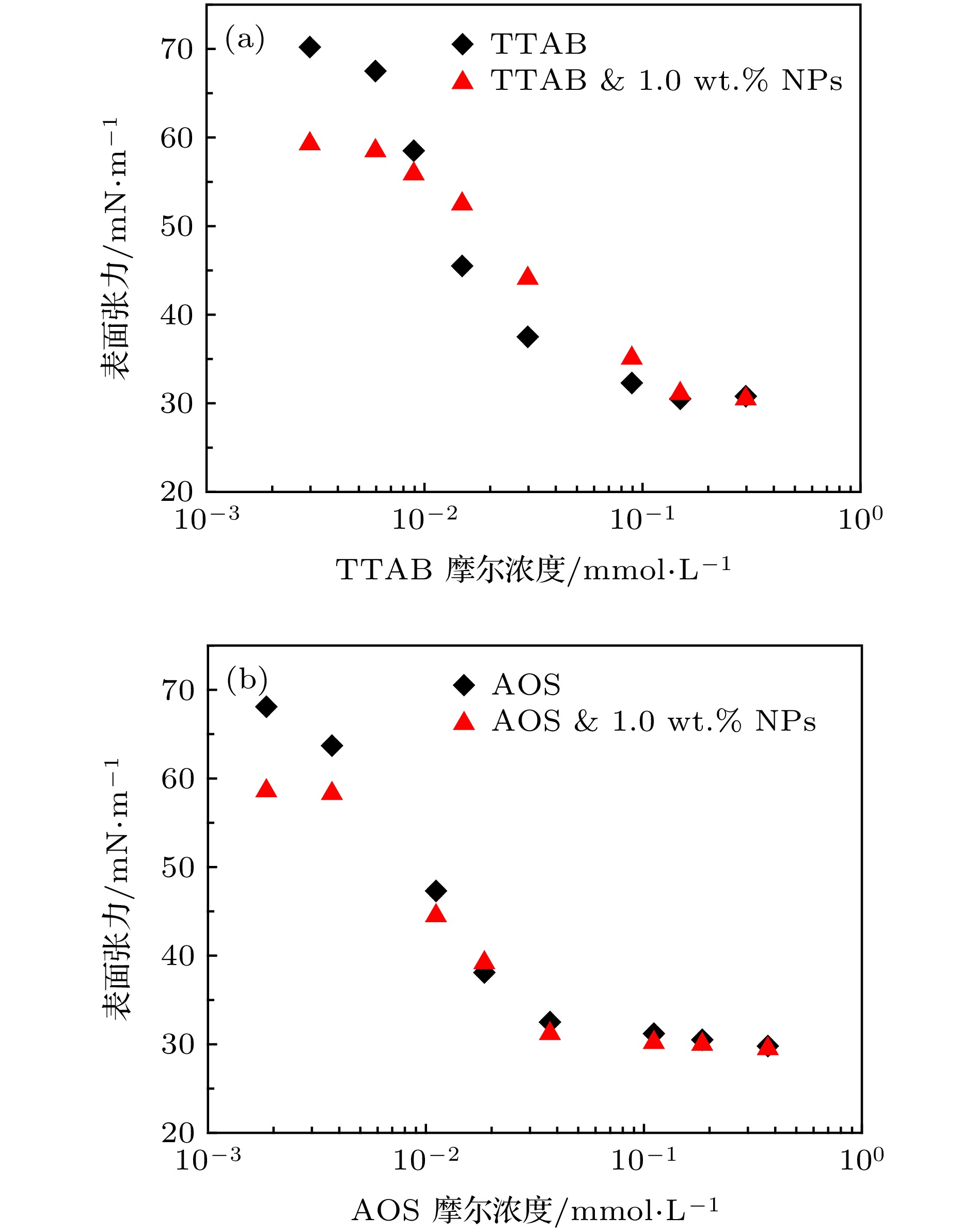
图 3 阴、阳离子活性剂与改性纳米颗粒复配体系表面张力 (a) 1.5 μmol/m2 DM纳米颗粒溶液中加入不同浓度TTAB; (b) 1.5 μmol/m2 DM纳米颗粒中加入不同浓度AOS
Fig. 3. The IFT of mixing solution of different surfactant with 1.0 wt.% NPs: (a) 1.5 μmol/m2 DM NPs solutions with different concentrations of TTAB; (b) 1.5 μmol/m2 DM NPs solutions with different concentrations of AOS
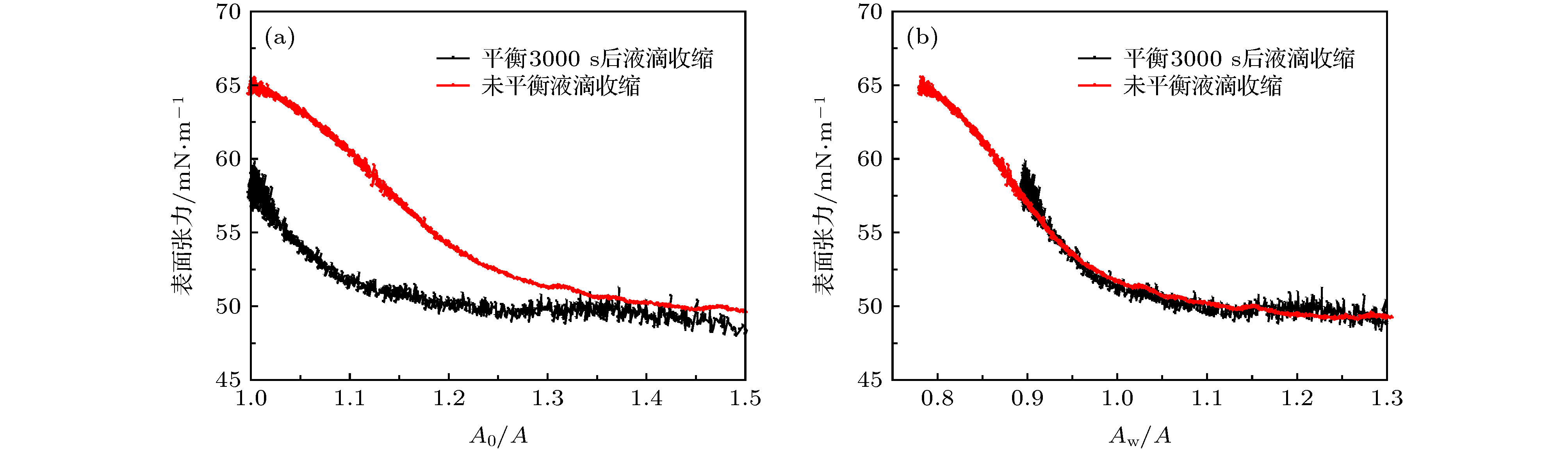
图 5 不同体系液滴收缩过程中表面张力的变化, 溶液质量密度为1 wt.% (a), (b) 不同疏水性纳米颗粒溶液, 盐含量2 M NaCl+0.25 M CaCl2; (c), (d) 不同盐浓度溶液, 1.5 μmol/m2 DM纳米颗粒
Fig. 5. IFT at different Aw/A ratios during droplet shrinking for (a) and (b) NPs with difference DM coverage at the fixed concentration of 1 wt.% in 2 M NaCl+0.25 M CaCl2 brine; for (c) and (d) 1 wt.% 1.5 μmol/m2 DM NPs solutions with various salinities
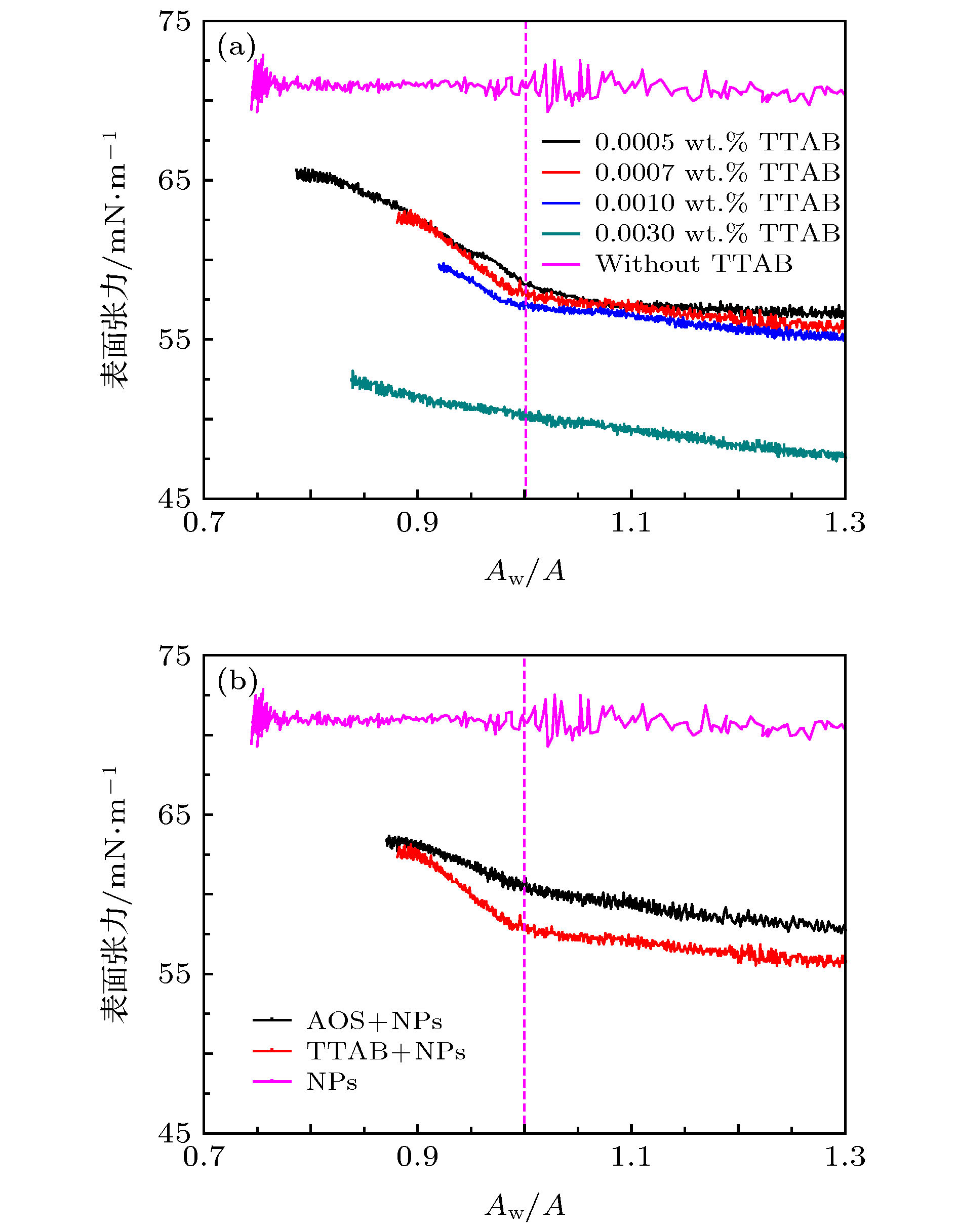
图 6 质量浓度为1.0 wt.%的不同纳米颗粒-活性剂体系液滴收缩过程中表面张力的变化 (a) 1.5 μmol/m2 DM纳米颗粒中加入不同浓度TTAB; (b) 1.5 μmol/m2 DM纳米颗粒中加入TTAB或AOS, 表面张力初始值相近
Fig. 6. IFT at different Aw/A ratios during droplet shrinking in DI water with 1.0 wt.% NPs: (a) 1.5 μmol/m2 DM NPs solutions with different TTAB concentrations; (b) 1.5 μmol/m2 DM NPs solutions with TTAB and AOS with same beginning IFT
表 1 液滴持续收缩过程中不同改性纳米颗粒溶液体系的平均表面压缩模量
Table 1. The compression modulus of various hydrophobic NPs solutions in 2 M NaCl + 0.25 M CaCl2 brine with continuous shrinking droplet.
0.8 μmol/m2 DM 1.2 μmol/m2 DM 1.5 μmol/m2 DM 前段 中段 前段 中段 前段 中段 表面压缩模量/mN·m–1 ~26 ~58 ~39 ~70 ~36 ~67 表 2 液滴持续收缩过程中改性纳米颗粒溶液在不同盐含量条件下的表面压缩模量
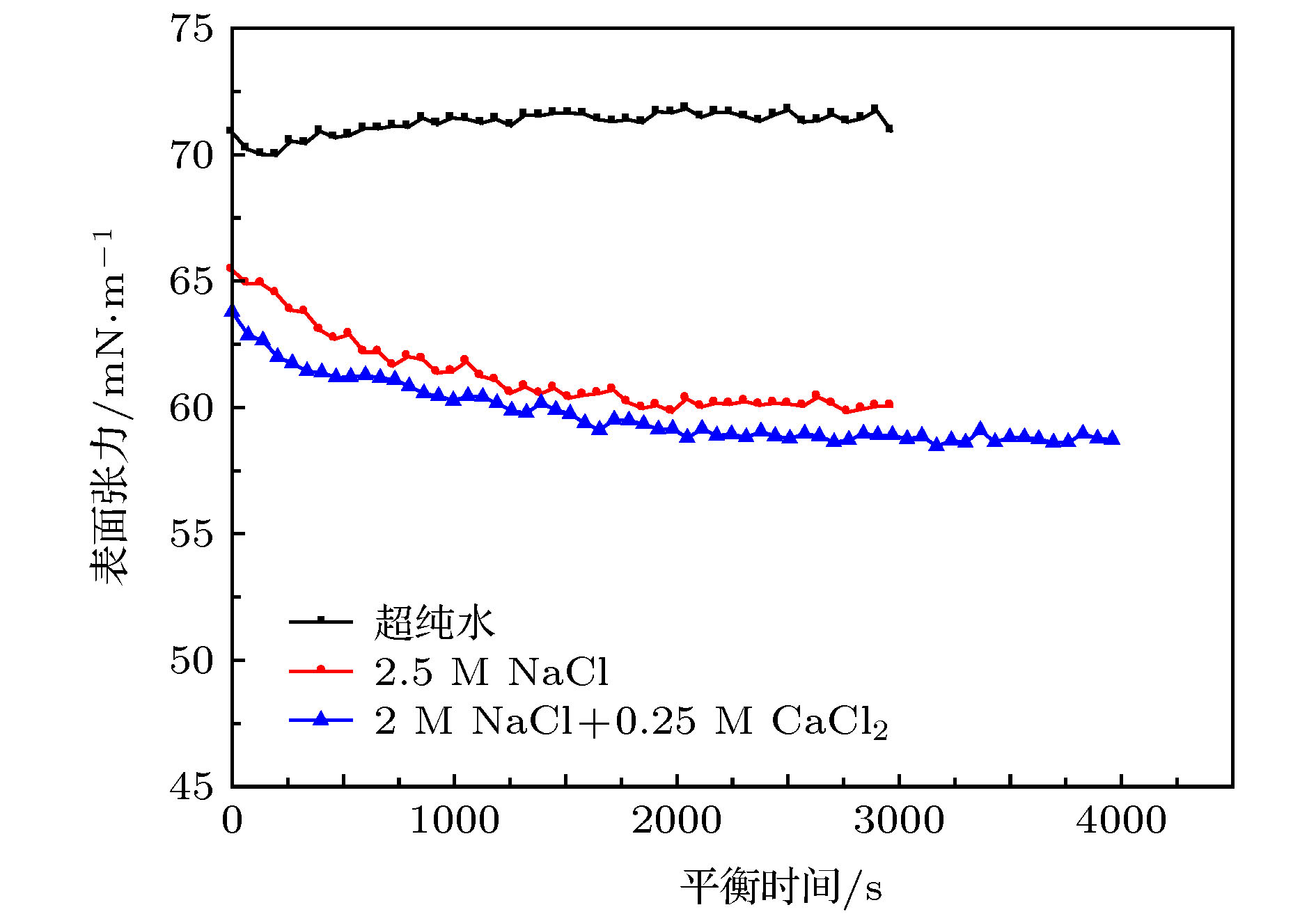
Table 2. The compression modulus of 1.5 μmol/m2 DM NPs solutions in various salinity brine with continuous shrinking droplet
超纯水 2.5 M NaCl 2 M NaCl + 0.25 M CaCl2 前段 中段 前段 中段 前段 中段 表面压缩模量/mN·m–1 ~0 ~0 ~22 ~68 ~36 ~67 -
[1] Sozer N, Kokini J L 2009 Trends Biotechnol. 27 82
 Google Scholar
Google Scholar
[2] 侯静菲, 杨延莲, 王琛 2017 物理化学学报 33 63
 Google Scholar
Google Scholar
Hou J F, Yang Y L, Wang C 2017 Acta Phys.-Chim. Sin. 33 63
 Google Scholar
Google Scholar
[3] Soleimani M, Khani A, Najafzadeh K 2012 J. Mol. Catal. B: Enzym. 74 1
 Google Scholar
Google Scholar
[4] Arjang S, Manteghian M, Mohammadi A 2013 Chem. Eng. Res. Des. 91 1050
 Google Scholar
Google Scholar
[5] Zhang C, Li Z, Sun Q, Wang P, Wang S, Liu W 2015 Soft Matter 12 946
[6] Kim I, Worthen A J, Johnston K P, DiCarlo D A, Huh C 2016 J. Nanopart. Res. 18 82
 Google Scholar
Google Scholar
[7] 叶学民, 李明兰, 张湘珊, 李春曦 2018 67 214703
Ye X M, Li M L, Zhang X S, Li C X 2018 Acta Phys. Sin. 67 214703
[8] Binks B P 2002 Curr. Opin. Colloid. Interface Sci. 7 21
 Google Scholar
Google Scholar
[9] Bizmark N, Ioannidis M A, Henneke D E 2014 Langmuir 30 710
 Google Scholar
Google Scholar
[10] Roger K, Cabane B 2012 Angew. Chem. Int. Ed. 51 5625
 Google Scholar
Google Scholar
[11] Kutuzov S, He J, Tangirala R, Emrick T, Russell T, Böker A 2007 Phys. Chem. Chem. Phys. 9 6351
 Google Scholar
Google Scholar
[12] Zang D, Zhang Y, Hou Q 2012 Colloids Surf., A 395 262
 Google Scholar
Google Scholar
[13] Zhang Y, Wang S, Zhou J, Zhao R, Benz G, Tcheimou S, Meredith J C, Behrens S H 2017 Langmuir 33 4511
 Google Scholar
Google Scholar
[14] Du K, Glogowski E, Emrick T, Russell T P, Dinsmore A D 2010 Langmuir 26 12518
 Google Scholar
Google Scholar
[15] Hua X, Frechette J, Bevan M A 2018 Soft matter 14 3818
 Google Scholar
Google Scholar
[16] Chai Y, Lukito A, Jiang Y, Ashby P D, Russell T P 2017 Nano Lett. 17 6453
 Google Scholar
Google Scholar
[17] Maestro A, Rio E, Drenckhan W, Langevin D, Salonen A 2014 Soft Matter 10 6975
 Google Scholar
Google Scholar
[18] Zang D Y, Rio E, Langevin D, Wei B, Binks B P 2010 Eur. Phys. J. E 31 125
 Google Scholar
Google Scholar
[19] Safouane M, Langevin D, Binks B 2007 Langmuir 23 11546
 Google Scholar
Google Scholar
[20] Monteux C, Fuller G G, Bergeron V 2004 J. Phys. Chem. B 108 16473
 Google Scholar
Google Scholar
[21] Santini E, Krägel J, Ravera F, Liggieri L, Miller R 2011 Colloids Surf. A 382 186
 Google Scholar
Google Scholar
[22] Wang J, Nguyen A V, Farrokhpay S 2016 Adv. Colloid Interface Sci. 228 55
 Google Scholar
Google Scholar
[23] Worthen A J, Tran V, Cornell K A, Truskett T M, Johnston K P 2016 Soft Matter 12 2025
 Google Scholar
Google Scholar
[24] Cui Z G, Yang L L, Cui Y Z, Binks B 2009 Langmuir 26 4717
[25] Zhang H, Yu K, Cayre O J, Harbottle D 2016 Langmuir 32 13472
 Google Scholar
Google Scholar
[26] 臧渡洋, 张永建, Langevin Dominique 2011 60 076801
Zang D Y, Zhang Y J, Langevin D 2011 Acta Phys. Sin. 60 076801
[27] Zang D, Rio E, Delon G, Langevin D, Wei B, Binks B 2011 Mol. Phys. 109 1057
 Google Scholar
Google Scholar
计量
- 文章访问数: 17922
- PDF下载量: 232
- 被引次数: 0













 下载:
下载: