-
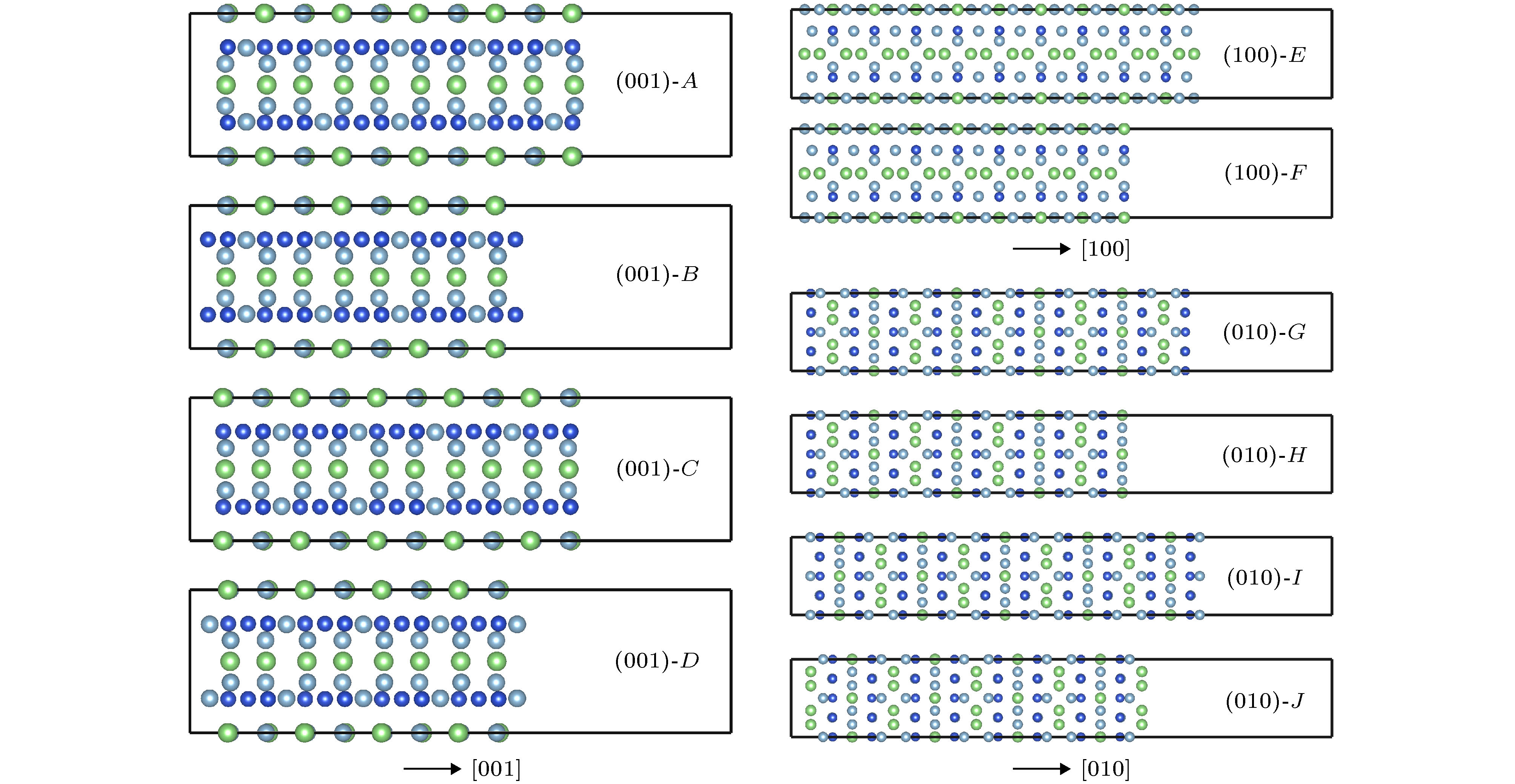
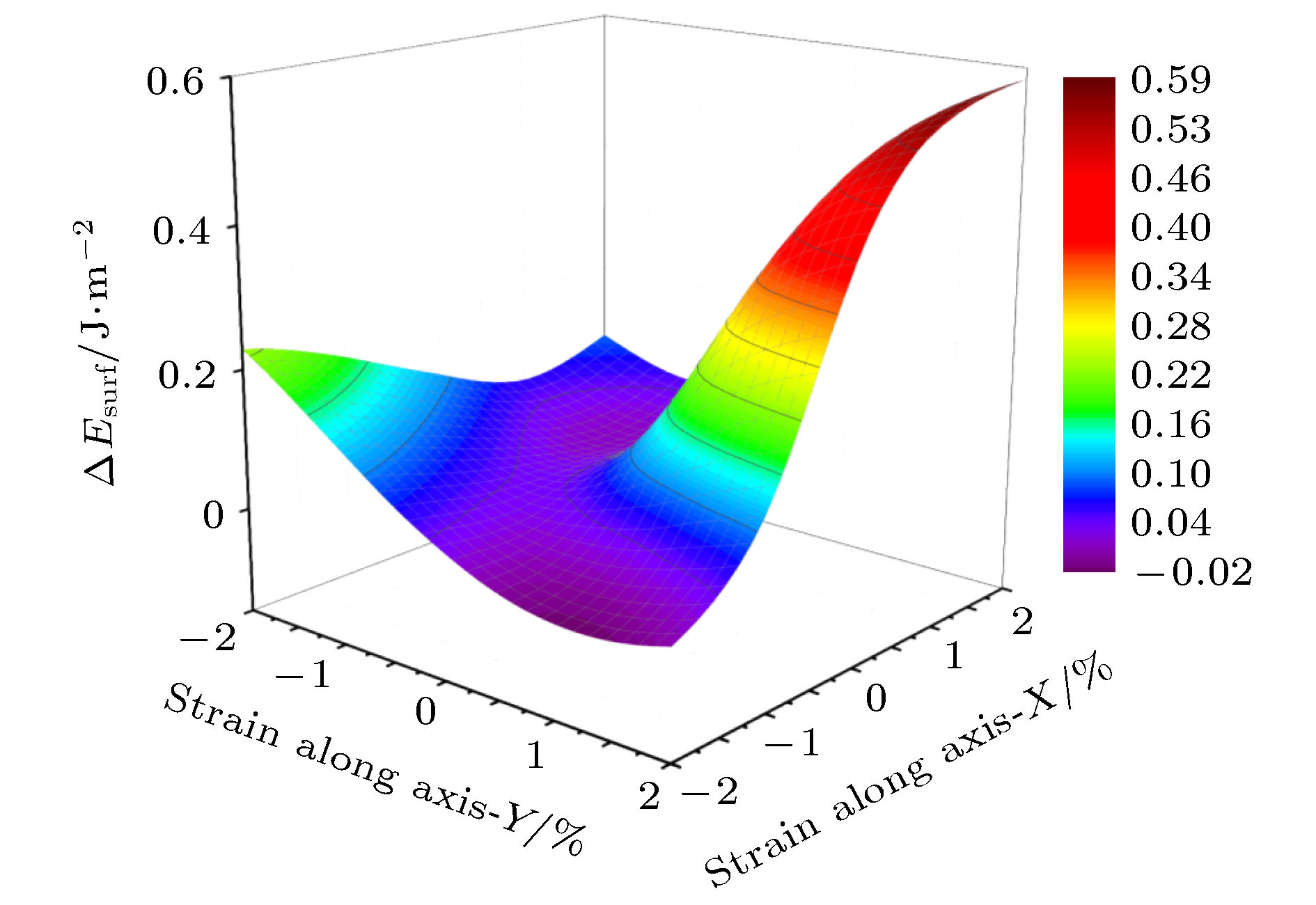
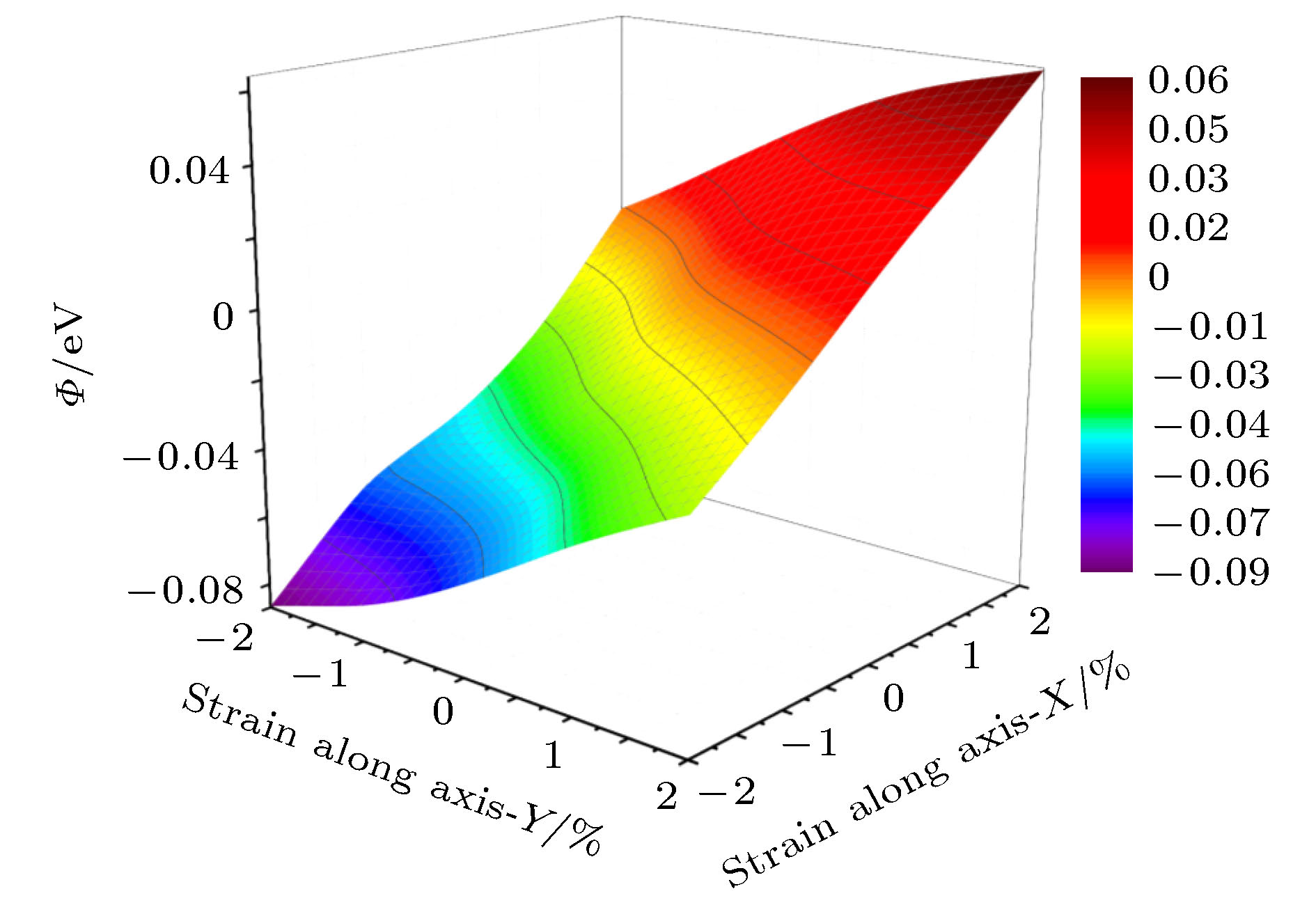
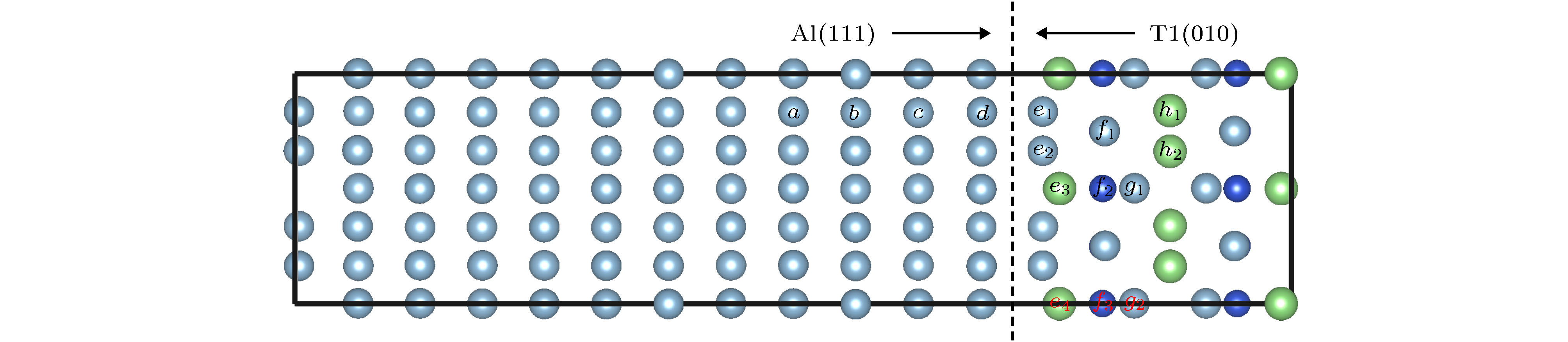
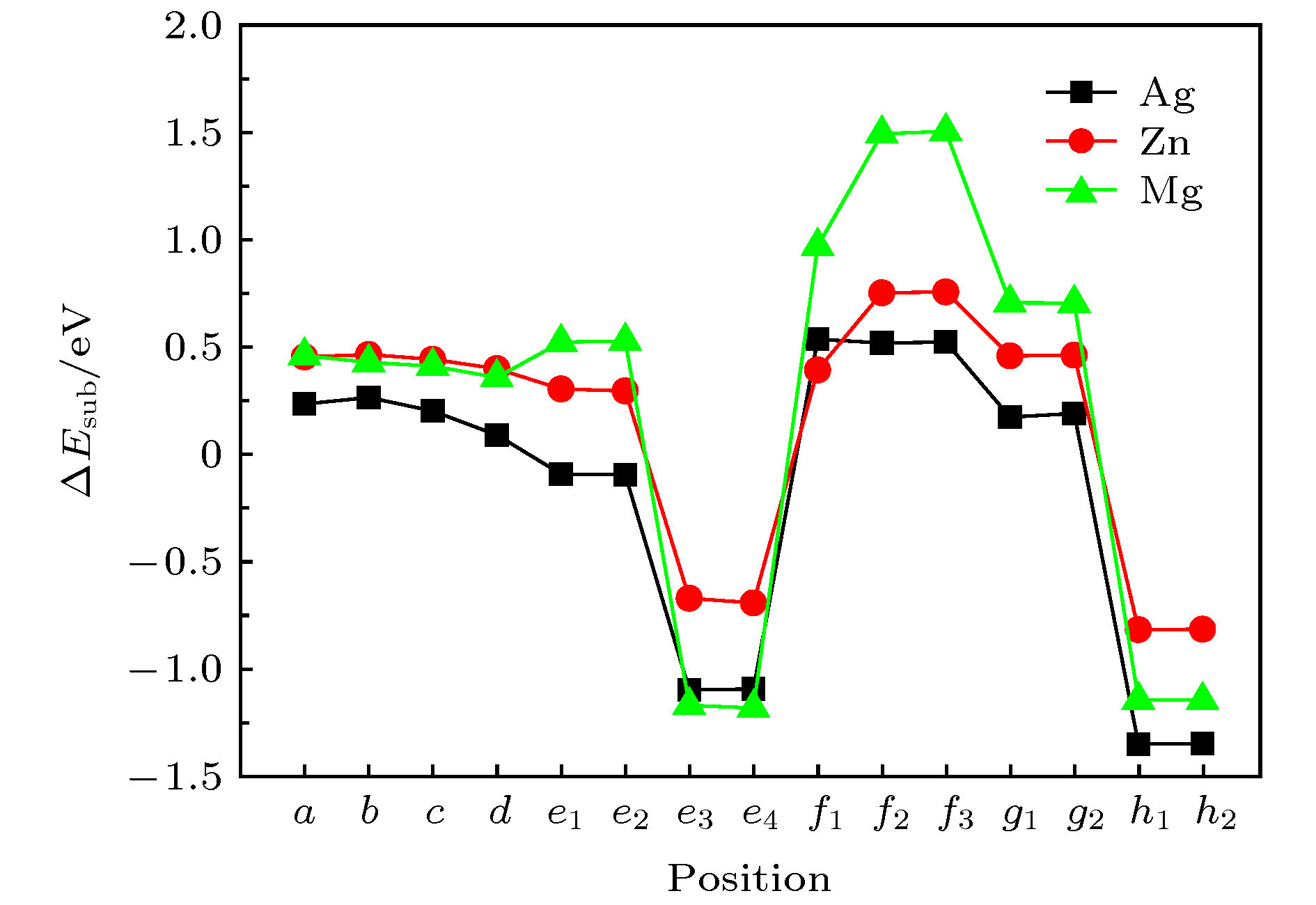
利用密度泛函理论的第一性原理, 讨论Al-Cu-Li合金中主要析出相T1相(Al6Cu4Li3)的表面性质, 计算不同终结面的表面能和表面电子功函数, 并探讨应力作用和常见合金元素对Al/T1界面的影响. 结果表明: T1相的表面能与表面的原子排列有关, 不同的表面通过应变释放重构, 进而获得不同的表面能. 表面电子功函数则与表面原子种类有关, 由于Li的电负性最小, 含Li原子的表面通常有较低的电子功函数, 进而降低材料的耐蚀性. 此外, 在应力作用下, T1相一些表面的电子功函数变化与纯金属是相反的. 压应力下T1相电子功函数降低, 材料更加容易被腐蚀; 张应力下T1相功函数增加, 材料更加耐腐蚀. 同时, 通过计算Al/T1界面中Ag, Zn和Mg 3种合金元素的替位能, 可以发现, 这3种元素都有利于降低界面能, 且Ag的作用最明显.
-
关键词:
- Al-Cu-Li合金 /
- T1相 /
- 电子功函数
First principle calculations in the framework of density functional theory are performed to calculate the T1 phase (Al6Cu4Li3), which is the main precipitation in Al-Cu-Li alloy. In this paper, the surface energy values and surface electron work functions of different termination surfaces in T1 phase are calculated. Meanwhile, the effects of stress and common alloying elements on the T1 phase are also discussed. There are 10 different termination surfaces for T1 phase. The surface energy varies between 0.59 and 1.28 J·m–2. It is found that the surface energy is dependent on the atomic configuration of the surface. The relaxation of the surficial atoms leads to low surface energy. For work function, it is controlled by the surficial atomic species. When a surface contains Li atoms, low work function is expected, which can be attributed to the low electronegativity of Li atom. The (010) T1 surface with Li termination has a minimum work function, 3.40 eV. In addition, as is different from pure metal, work function of some T1 surfaces shows unique behavior under stress state. The (010) T1 surface with Al and Cu termination has an increasing work function under the action of tensile strain. In fact, tensile strain induces the first and second surface layer to merge, which can improve the surface electronic density and raise work function. As a result, the corrosion resistance can be enhanced. Finally, the effect of alloying elements on the precipitation of T1 phase is studied. Al(111)/T1(010) interface is built and the substitution energy of Mg, Zn and Ag are calculated. Comparing with Mg and Zn atom, the energy of Ag atom to substitute the interfacial one is low, meaning that Ag can relax the strain in the interface. Ag atom has the closest atomic radius to Al atom, and the same chemical valence as Li atom. Therefore, Ag atom is more likely to promote the precipitation of T1 phase, which is also in agreement with the experimental result.-
Keywords:
- Al-Cu-Li alloy /
- T1 phase /
- electronic work function
[1] 程超, 王逊, 孙嘉兴, 曹超铭, 马云莉, 刘艳侠 2018 67 197101
 Google Scholar
Google Scholar
Cheng C, Wang X, Sun J X, Cao C M, Ma Y L, Liu Y X 2018 Acta Phys. Sin. 67 197101
 Google Scholar
Google Scholar
[2] Jr. E A S, Staley J T 1996 Prog. Aerosp. Sci 32 131
 Google Scholar
Google Scholar
[3] Kim K, Zhou B C, Wolverton C 2018 Acta Mater. 145 337
 Google Scholar
Google Scholar
[4] Rioja R J 1998 Mater Sci Eng, A 257 100
 Google Scholar
Google Scholar
[5] Kim Y S, Park I J, An B S, Park J G, Yang C W, Lee Y H, Kim J G 2020 Mater. Chem. Phys. 241 122275
 Google Scholar
Google Scholar
[6] 段永华, 孙勇, 何建洪, 彭明军, 郭中正 2012 61 046101
 Google Scholar
Google Scholar
Duan Y H, Sun Y, He J H, Peng M J, Guo Z Z 2012 Acta Phys. Sin. 61 046101
 Google Scholar
Google Scholar
[7] Tao Y, Xiong T, Chao S, Kong L, Cui X, Li T, Song G L 2010 Corros. Sci. 52 3191
 Google Scholar
Google Scholar
[8] Wadeson D A, Zhou X, Thompson G E, Skeldon P, Oosterkamp L D, Scamans G 2006 Corros. Sci. 48 887
 Google Scholar
Google Scholar
[9] Vera R, Delgado D, Rosales B M 2006 Corros. Sci. 48 2882
 Google Scholar
Google Scholar
[10] 刘贵立 2010 59 2708
Liu G L 2010 Acta Phys. Sin. 59 2708
[11] Wang X Y, Jang J T, Li G A, Wang X M, Sun J, Zhen L 2020 J. Alloy Compd. 815 152469
[12] Li J F, Zheng Z Q, Li S C, Chen W J, Ren W D, Zhao X S 2007 Corros. Sci. 49 2436
 Google Scholar
Google Scholar
[13] Eifert A J, Thomas J P, Jr R G R 1999 Scr. Mater. 40 929
 Google Scholar
Google Scholar
[14] Zhang X, Zhou X, Hashimoto T, Liu B, Luo C, Sun Z, Tang Z, Lu F, Ma Y 2018 Corros. Sci. 132 1
 Google Scholar
Google Scholar
[15] Buchheit R G, Moran J P, Stoner G E 1994 Corrosion 50 120
 Google Scholar
Google Scholar
[16] 王健, 王绍青 2014 物理化学学报 30 551
 Google Scholar
Google Scholar
Wang J, Wang S Q 2014 Acta Phys-Chim. Sin. 30 551
 Google Scholar
Google Scholar
[17] Wu J J, Tang X, Long F, Tang B 2018 Chin. Phys. B 27 057701
 Google Scholar
Google Scholar
[18] Pang X, Yang W, Yang J, Pang M, Zhan Y 2018 Intermetallics 93 329
 Google Scholar
Google Scholar
[19] Brik M G, Ma C-G, Krasnenko V 2013 Surf. Sci. 608 146
 Google Scholar
Google Scholar
[20] Heifets E, Eglitis R I, Kotomin E A, Maier J, Borstel G 2001 Phys. Rev. B 64 235417
 Google Scholar
Google Scholar
[21] Sharma A, Berger R, Lewis D A, Andersson G G 2015 Appl. Surf. Sci. 327 22
 Google Scholar
Google Scholar
[22] Liu J, Zhang X, Chen M, Li L, Zhu B, Tang J, Liu S 2011 Appl. Surf. Sci. 257 4004
 Google Scholar
Google Scholar
[23] Ma H, Chen X-Q, Li R, Wang S, Dong J, Ke W 2017 Acta Mater. 130 137
 Google Scholar
Google Scholar
[24] Cao F, Zheng J, Jiang Y, Chen B, Wang Y, Hu T 2019 Acta Mater. 164 207
 Google Scholar
Google Scholar
[25] Ye Z Y, Liu D X, Yuan M, Zhang X M, Yang Z, Lei M X 2015 Acta Metall. Sin. 28 608
 Google Scholar
Google Scholar
[26] Brewick P T, DeGiorgi V G, Geltmacher A B, Qidwai S M 2019 Corros. Sci. 158 108111
 Google Scholar
Google Scholar
[27] Nicolas A, Mello A W, Sangid M D 2019 Corros. Sci. 154 208
 Google Scholar
Google Scholar
[28] Scott P M, Combrade P 2019 J. Nucl. Mater. 524 340
 Google Scholar
Google Scholar
[29] Li W, Cai M, Wang Y, Yu S 2006 Scr. Mater. 54 921
 Google Scholar
Google Scholar
[30] Kiejna A, Pogosov V V 2000 Phys. Rev. B 62 10445
 Google Scholar
Google Scholar
[31] Kim K, Zhou B-C, Wolverton C 2019 Scr. Mater. 159 99
 Google Scholar
Google Scholar
[32] Murayama M, Hono K 2001 Scr. Mater. 44 701
 Google Scholar
Google Scholar
[33] Huang B P, Zheng Z Q 1998 Acta Mater. 46 4381
 Google Scholar
Google Scholar
[34] Gumbmann E, de Geuser F, Sigli C, Deschamps A 2017 Acta Mater. 133 172
 Google Scholar
Google Scholar
-
表 1 T1相10个终结面的表面能和电子功函数
Table 1. The surface energies and electron work functions of ten surfaces.
面 终结面 表面能/J·m–2 功函数/eV A Al-Cu-Li 1.24 3.64 B Cu 1.10 3.91 C Al-Cu-Li 1.20 3.70 D Al 1.28 4.27 E Al-Cu-Li 1.02 3.89 F Al-Cu-Li 1.07 4.35 G Al-Cu 0.84 4.29 H Al-Li 0.86 4.53 I Al-Cu 0.59 4.12 J Li 0.83 3.40 表 2 应变条件下表面能和功函数的波动量
Table 2. Fluctuation of surface energy and work function with strain.
面 表面能变化量/J·m–2 功函数变化量/eV A 0.41 0.14 B 0.59 0.09 C 0.25 0.09 D 0.16 0.12 E 0.21 0.14 F 0.31 0.07 G 0.34 0.08 H 0.37 0.05 I 0.37 0.15 J 0.18 0.04 -
[1] 程超, 王逊, 孙嘉兴, 曹超铭, 马云莉, 刘艳侠 2018 67 197101
 Google Scholar
Google Scholar
Cheng C, Wang X, Sun J X, Cao C M, Ma Y L, Liu Y X 2018 Acta Phys. Sin. 67 197101
 Google Scholar
Google Scholar
[2] Jr. E A S, Staley J T 1996 Prog. Aerosp. Sci 32 131
 Google Scholar
Google Scholar
[3] Kim K, Zhou B C, Wolverton C 2018 Acta Mater. 145 337
 Google Scholar
Google Scholar
[4] Rioja R J 1998 Mater Sci Eng, A 257 100
 Google Scholar
Google Scholar
[5] Kim Y S, Park I J, An B S, Park J G, Yang C W, Lee Y H, Kim J G 2020 Mater. Chem. Phys. 241 122275
 Google Scholar
Google Scholar
[6] 段永华, 孙勇, 何建洪, 彭明军, 郭中正 2012 61 046101
 Google Scholar
Google Scholar
Duan Y H, Sun Y, He J H, Peng M J, Guo Z Z 2012 Acta Phys. Sin. 61 046101
 Google Scholar
Google Scholar
[7] Tao Y, Xiong T, Chao S, Kong L, Cui X, Li T, Song G L 2010 Corros. Sci. 52 3191
 Google Scholar
Google Scholar
[8] Wadeson D A, Zhou X, Thompson G E, Skeldon P, Oosterkamp L D, Scamans G 2006 Corros. Sci. 48 887
 Google Scholar
Google Scholar
[9] Vera R, Delgado D, Rosales B M 2006 Corros. Sci. 48 2882
 Google Scholar
Google Scholar
[10] 刘贵立 2010 59 2708
Liu G L 2010 Acta Phys. Sin. 59 2708
[11] Wang X Y, Jang J T, Li G A, Wang X M, Sun J, Zhen L 2020 J. Alloy Compd. 815 152469
[12] Li J F, Zheng Z Q, Li S C, Chen W J, Ren W D, Zhao X S 2007 Corros. Sci. 49 2436
 Google Scholar
Google Scholar
[13] Eifert A J, Thomas J P, Jr R G R 1999 Scr. Mater. 40 929
 Google Scholar
Google Scholar
[14] Zhang X, Zhou X, Hashimoto T, Liu B, Luo C, Sun Z, Tang Z, Lu F, Ma Y 2018 Corros. Sci. 132 1
 Google Scholar
Google Scholar
[15] Buchheit R G, Moran J P, Stoner G E 1994 Corrosion 50 120
 Google Scholar
Google Scholar
[16] 王健, 王绍青 2014 物理化学学报 30 551
 Google Scholar
Google Scholar
Wang J, Wang S Q 2014 Acta Phys-Chim. Sin. 30 551
 Google Scholar
Google Scholar
[17] Wu J J, Tang X, Long F, Tang B 2018 Chin. Phys. B 27 057701
 Google Scholar
Google Scholar
[18] Pang X, Yang W, Yang J, Pang M, Zhan Y 2018 Intermetallics 93 329
 Google Scholar
Google Scholar
[19] Brik M G, Ma C-G, Krasnenko V 2013 Surf. Sci. 608 146
 Google Scholar
Google Scholar
[20] Heifets E, Eglitis R I, Kotomin E A, Maier J, Borstel G 2001 Phys. Rev. B 64 235417
 Google Scholar
Google Scholar
[21] Sharma A, Berger R, Lewis D A, Andersson G G 2015 Appl. Surf. Sci. 327 22
 Google Scholar
Google Scholar
[22] Liu J, Zhang X, Chen M, Li L, Zhu B, Tang J, Liu S 2011 Appl. Surf. Sci. 257 4004
 Google Scholar
Google Scholar
[23] Ma H, Chen X-Q, Li R, Wang S, Dong J, Ke W 2017 Acta Mater. 130 137
 Google Scholar
Google Scholar
[24] Cao F, Zheng J, Jiang Y, Chen B, Wang Y, Hu T 2019 Acta Mater. 164 207
 Google Scholar
Google Scholar
[25] Ye Z Y, Liu D X, Yuan M, Zhang X M, Yang Z, Lei M X 2015 Acta Metall. Sin. 28 608
 Google Scholar
Google Scholar
[26] Brewick P T, DeGiorgi V G, Geltmacher A B, Qidwai S M 2019 Corros. Sci. 158 108111
 Google Scholar
Google Scholar
[27] Nicolas A, Mello A W, Sangid M D 2019 Corros. Sci. 154 208
 Google Scholar
Google Scholar
[28] Scott P M, Combrade P 2019 J. Nucl. Mater. 524 340
 Google Scholar
Google Scholar
[29] Li W, Cai M, Wang Y, Yu S 2006 Scr. Mater. 54 921
 Google Scholar
Google Scholar
[30] Kiejna A, Pogosov V V 2000 Phys. Rev. B 62 10445
 Google Scholar
Google Scholar
[31] Kim K, Zhou B-C, Wolverton C 2019 Scr. Mater. 159 99
 Google Scholar
Google Scholar
[32] Murayama M, Hono K 2001 Scr. Mater. 44 701
 Google Scholar
Google Scholar
[33] Huang B P, Zheng Z Q 1998 Acta Mater. 46 4381
 Google Scholar
Google Scholar
[34] Gumbmann E, de Geuser F, Sigli C, Deschamps A 2017 Acta Mater. 133 172
 Google Scholar
Google Scholar
计量
- 文章访问数: 11124
- PDF下载量: 205
- 被引次数: 0














 下载:
下载: