-
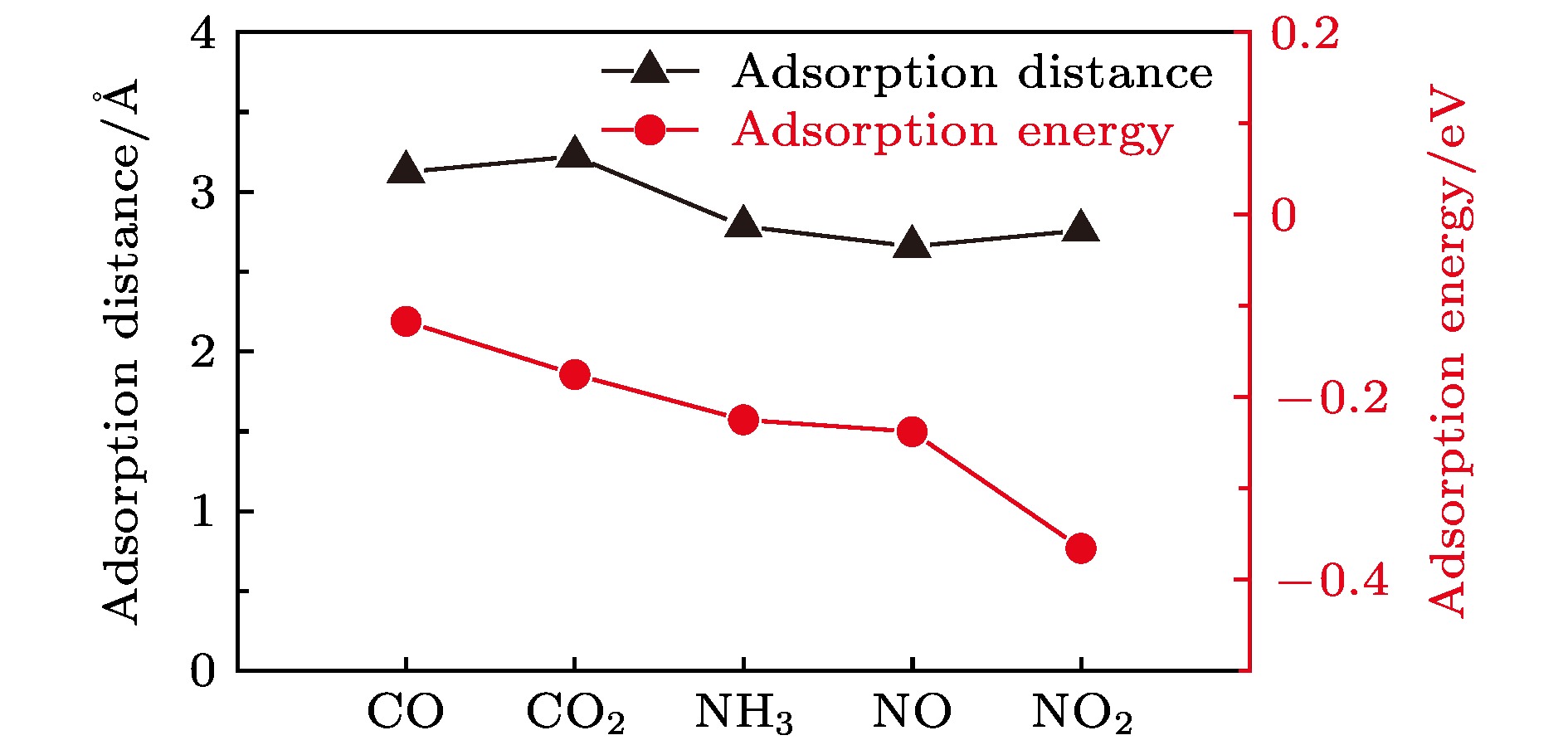
二维过渡金属硫族化合物由于具有大的比表面积、高的载流子迁移率以及快速响应等特性, 在高性能气体传感器应用方面具有显著优势. 本文通过密度泛函理论计算, 研究了CO, CO2, NH3, NO, NO2气体分子在单层WTe2表面的吸附构型、吸附能、电荷转移、电学及磁学特性. 结果表明, N基气体分子的吸附能小于C基气体分子的吸附能, 说明WTe2对N基气体分子的吸附更敏感. 电荷分析结果表明, NH3气体分子吸附在WTe2表面时表现为给电子体, 而其他四种气体分子都表现为得电子体. 能带结构方面, 与CO, CO2, NH3气体分子相比, 磁性气体分子NO和NO2的吸附在费米能级附近引入了杂质能带, 杂质能带主要来源于O原子和N原子的p轨道. 此外, NO和NO2气体分子分别诱导了0.99 μB和0.80 μB的磁矩. 本文的研究结果为实验上制备基于WTe2的超灵敏气体传感器提供理论指导.Since the discovery of graphene, graphene-based gas sensors have been widely studied, but the inherent zero band gap of graphene limits the response sensitivity of gas sensors. Transition metal dichalcogenides (TMDs) are ideal materials for designing nanoscaled highly-sensitive gas sensors due to their moderate band gaps, large surface-to-volume ratios and high carrier mobilities. Tungsten ditelluride (WTe2), as an important member of TMDs family, has outstanding advantages such as high specific surface area, excellent selectivity, and fast response. The WTe2 has quite a high carrier mobility and thus can provide a great response speed for gas sensor compared with graphene, which motivates us to further explore WTe2 as a promising sensing material. Recent studies have reported that monolayered and multilayered WTe2 films have been successfully synthesized, and the precise control of the number of atomic layers of monolayered WTe2 has been achieved. In this work, by density functional theory calculation, we examine the most stable adsorption configuration, adsorption energy, charge transfer, electrical and magnetic properties for each of the gas molecules (CO, CO2, NH3, NO and NO2) adsorbed on WTe2 monolayer. The results show that all the adsorptions of these gas molecules are physical adsorptions, and the adsorption energy of nitrogen-based gas is smaller than that of carbon-based gas, indicating that WTe2 is more sensitive to the adsorption of N-based gas molecules. The adsorption of NH3 behaves as a charge donor with electron obtained from WTe2 monolayer. The adsorption of CO, CO2, NO, and NO2 are charge acceptors, which accept charges from the WTe2 monolayer. Moreover, compared with the adsorption of CO, CO2 and NH3 gas molecules, the adsorption of NO and NO2 gas molecules introduce impurity states near the Fermi level, which are mainly contributed by the N p orbital and O p orbital. In addition, the adsorption of NO and NO2 induce magnetic moments of 0.99 μB and 0.80 μB, respectively. The results obtained in this work not only conduce to further understanding the charge transfer mechanism of gas molecules adsorbed on WTe2 monolayer, but also indicate the promising prospects of developing WTe2-based ultra-sensitivity gas sensing nanodevices.
[1] Kong J, Franklin NR, Zhou C 2000 Science 287 622
 Google Scholar
Google Scholar
[2] Abbas A N, Liu B, Chen L, Ma Y, Cong S, Aroonyadet N, Köpf M, Nilges T, Zhou C 2015 ACS Nano 9 5618
 Google Scholar
Google Scholar
[3] Yang A, Wang D, Wang X, Zhang D, Koratkar N, Rong M 2018 Nano Today 20 13
 Google Scholar
Google Scholar
[4] Liu X, Ma T, Pinna N, Zhang J 2017 Adv. Funct. Mater. 27 1702168
 Google Scholar
Google Scholar
[5] Ko K Y, Park K, Lee S, Kim Y, Woo W J, Kim D, Song J G, Park J, Kim H 2018 Acs Appl. Mater. Interfaces 10 23910
 Google Scholar
Google Scholar
[6] Ko K Y, Song J G, Kim Y, Choi T, Shin S, Lee C W, Lee K, Koo J, Lee H, Kim J, Lee T, Park J, Kim H 2016 ACS Nano 10 9287
 Google Scholar
Google Scholar
[7] Ma Y, Kou L, Li X, Dai Y, Heine T 2016 Phys. Rev. B 93 035442
 Google Scholar
Google Scholar
[8] Hu Z, Wu Z, Han C, He J, Ni Z, Chen W 2018 Chem. Soc. Rev. 47 3100
 Google Scholar
Google Scholar
[9] Zhao Y, Qiao J, Yu Z, Yu P, Xu K, Lau S P, Zhou W, Wang X, Ji W, Chai Y 2017 Adv. Mater. 29 1604230
 Google Scholar
Google Scholar
[10] Bandurin D A, Tyurnina A V, Geliang L Y, Mishchenko A, Zólyomi V, Morozov S V, et al. 2017 Nat. Nanotechnol. 12 223
 Google Scholar
Google Scholar
[11] Zhang S, Guo S, Chen Z, Wang Y, Gao H, Gomez-Herrero J, Ares P, Zamora F, Zhu Z, Zeng H 2018 Chem. Soc. Rev. 47 982
 Google Scholar
Google Scholar
[12] Zhang S, Xie M, Li F, Yan Z, Li Y, Kan E, Liu W, Chen Z, Zeng H 2016 Angew. Chem. Int. Ed. 55 1666
 Google Scholar
Google Scholar
[13] Perkins F K, Friedman A L, Cobas E, Campbell P M, Jernigan G G, Jonker B T 2013 Nano Lett. 13 668
 Google Scholar
Google Scholar
[14] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[15] Yuan W, Shi G 2013 J. Mater. Chem. A 1 10078
 Google Scholar
Google Scholar
[16] Mousavi H 2011 Commun. Theor. Phys. 56 373
 Google Scholar
Google Scholar
[17] Tang X, Du A, Kou L 2018 Wiley Interdiscip. Rev.: Comput. Mol. Sci. 8 e1361
 Google Scholar
Google Scholar
[18] Kuc A, Heine T, Kis A 2015 MRS Bull. 40 577
 Google Scholar
Google Scholar
[19] Zhou L, Kou L, Sun Y, Felser C, Hu F, Shan G, Smith S C, Yan B, Frauenheim T 2015 Nano Lett. 15 7867
 Google Scholar
Google Scholar
[20] Kou L, Du A, Chen C, Frauenheim T 2014 Nanoscale 6 5156
 Google Scholar
Google Scholar
[21] Huang Y, Guo J, Kang Y, Ai Y, Li C M 2015 Nanoscale 7 19358
 Google Scholar
Google Scholar
[22] Xie T, Xie G, Su Y, Hongfei D, Ye Z, Jiang Y 2016 Nanotechnol. 27 065502
 Google Scholar
Google Scholar
[23] Rao C N, Gopalakrishnan K, Maitra U 2015 ACS Appl. Mater. Interfaces 7 7809
 Google Scholar
Google Scholar
[24] Liu P F, Zhou L, Frauenheimc T, Wu L M 2016 Nanoscale 8 4915
 Google Scholar
Google Scholar
[25] Late D J, Huang Y K, Liu B, Acharya J, Shirodkar S N, Luo J, Yan A, Charles D, Waghmare U V, Dravid V P 2013 ACS Nano 7 4879
 Google Scholar
Google Scholar
[26] Lee K, Gatensby R, McEvoy N, Hallam T, Duesberg G S 2013 Adv. Mater. 25 6699
 Google Scholar
Google Scholar
[27] Yao Y, Tolentino L, Yang Z, Song X, Zhang W, Chen Y, Wong C P 2013 Adv. Funct. Mater. 23 3577
 Google Scholar
Google Scholar
[28] Giri A, Yang H, Jang W, Kwak J, Thiyagarajan K, Pal M, Lee D, Singh R, Kim C, Cho K 2018 Chem. Mater. 30 2463
 Google Scholar
Google Scholar
[29] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[30] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[31] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[32] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[33] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[34] Grimme S 2006 J. Comput. Chem. 27 1787
 Google Scholar
Google Scholar
[35] Bučko T, Hafner J, Lebègue S, Ángyán J G 2010 J. Phys. Chem. A 114 11814
 Google Scholar
Google Scholar
[36] Yue Q, Shao Z, Chang S, Li J 2013 Nanoscale Res. Lett. 8 425
 Google Scholar
Google Scholar
[37] Henkelman G, Arnaldsson A, Jónsson H 2006 Comput. Mater. Sci. 36 354
 Google Scholar
Google Scholar
[38] Ding Y, Wang Y, Ni J, Shi L, Shi S, Tang W 2011 Physica B 406 2254
 Google Scholar
Google Scholar
[39] Kang J, Tongay S, Zhou J, Li J, Wu J 2013 Appl. Phys. Lett. 102 012111
 Google Scholar
Google Scholar
[40] Hu X, Kou L, Sun L 2016 Sci. Rep. 6 31122
 Google Scholar
Google Scholar
[41] Hu X, Wang Y, Shen X, Krasheninnikov A V, Sun L, Chen Z 2018 2D Mater. 5 031012
 Google Scholar
Google Scholar
[42] Leenaerts O, Partoens B, Peeters F M 2008 Phys. Rev. B 77 125416
 Google Scholar
Google Scholar
-
图 1 (a) 四个不同的吸附位点示意图; (b) CO, (c) CO2, (d) NH3, (e) NO和(f) NO2吸附在单层WTe2表面最稳定构型的俯视图和侧视图; 吸附距离d的定义如 (b) 所示
Fig. 1. (a) Schematic diagram of four different adsorption sites; the top view and side view of the most favorable configurations of monolayer WTe2 with (b) CO, (c) CO2, (d) NH3, (e) NO, and (f) NO2 adsorption; the definition of adsorption distance d is shown in (b).
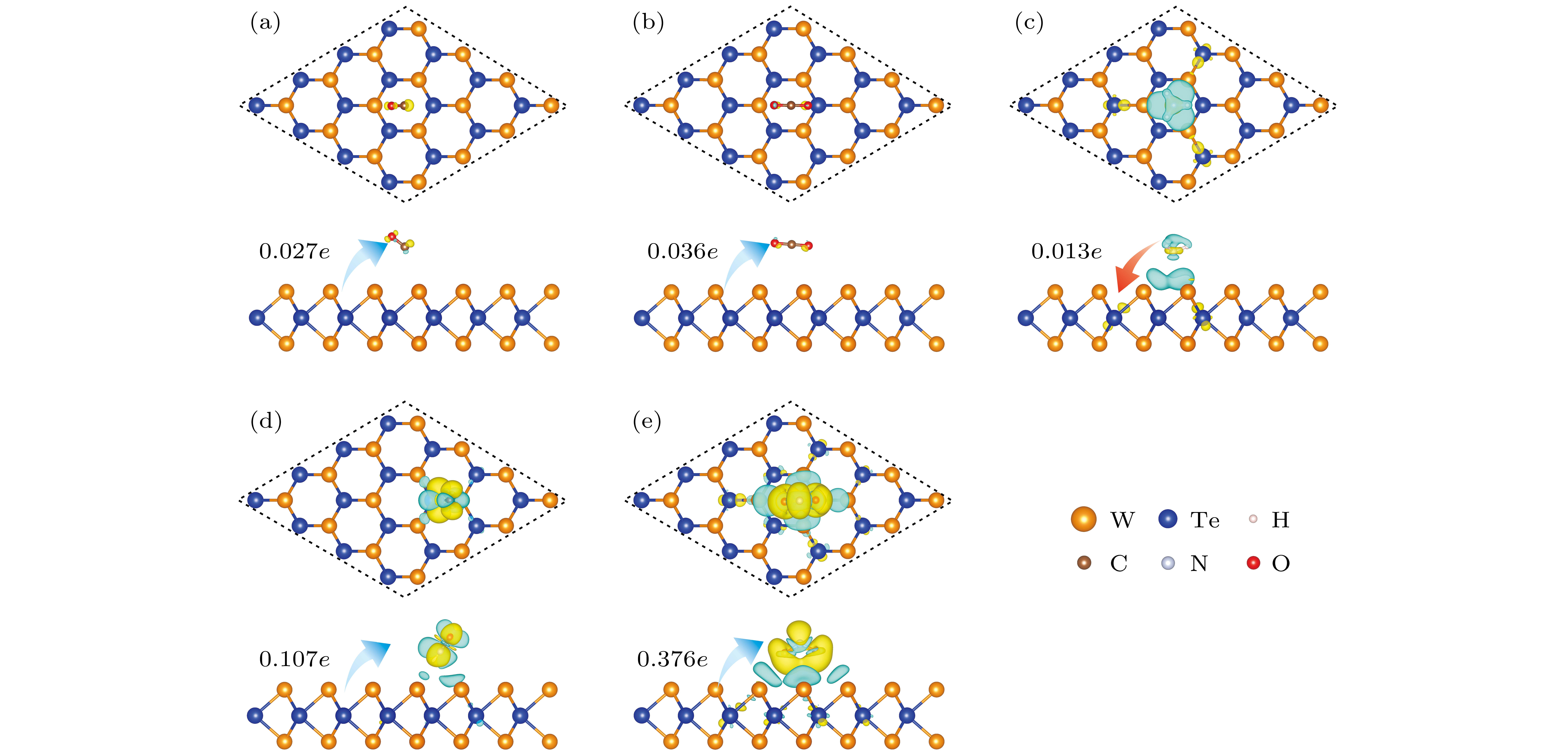
图 3 (a) CO, (b) CO2, (c) NH3, (d) NO和 (e) NO2气体分子与单层WTe2之间的差分电荷密度. 等值面取6.0 × 10–4 e/Å3, 电子积累(损耗)分别用黄色(蓝色)表示, 同时标注了电荷转移的方向(用箭头表示)和电荷转移量
Fig. 3. The charge difference between WTe2 monolayer and gas molecules for (a) CO, (b) CO2, (c) NH3, (d) NO and (e) NO2. The isosurface is taken as 6.0 × 10–4 e/Å3. The electron accumulation (depletion) is indicated by yellow (blue) color. The direction (indicated by an arrow) and value of the charge transfer are shown.
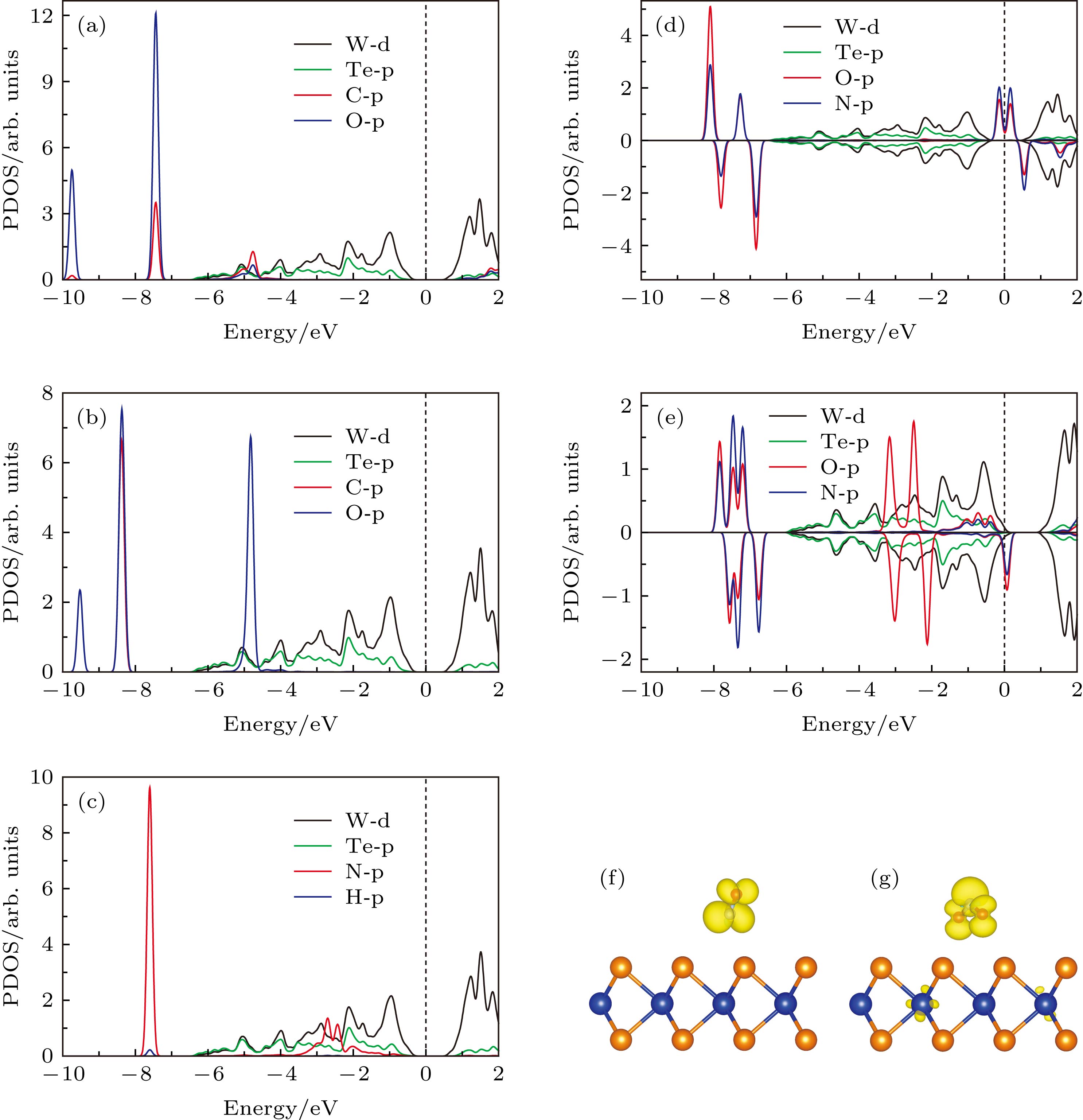
图 4 (a) 本征WTe2的能带结构图; (b) CO, (c) CO2和 (d) NH3吸附在单层WTe2表面的能带结构图; (e), (f) NO和 (g), (h) NO2吸附在单层WTe2表面的能带结构图. 其中蓝线和红线分别表示自旋向上和自旋向下的能带结构, 橄榄色的点线表示吸附气体分子的投影能带结构
Fig. 4. (a) The band structure of pristine WTe2; band structure of (b) CO, (c) CO2 and (d) NH3 adsorbed on WTe2 monolayer; band structure of (e), (f) NO and (g), (h) NO2 adsorbed on WTe2 monolayer, the blue and red lines represent the band structure of spin-up and spin-down, respectively. The olive dots represent the projected band structure of the adsorbed gas molecules.
-
[1] Kong J, Franklin NR, Zhou C 2000 Science 287 622
 Google Scholar
Google Scholar
[2] Abbas A N, Liu B, Chen L, Ma Y, Cong S, Aroonyadet N, Köpf M, Nilges T, Zhou C 2015 ACS Nano 9 5618
 Google Scholar
Google Scholar
[3] Yang A, Wang D, Wang X, Zhang D, Koratkar N, Rong M 2018 Nano Today 20 13
 Google Scholar
Google Scholar
[4] Liu X, Ma T, Pinna N, Zhang J 2017 Adv. Funct. Mater. 27 1702168
 Google Scholar
Google Scholar
[5] Ko K Y, Park K, Lee S, Kim Y, Woo W J, Kim D, Song J G, Park J, Kim H 2018 Acs Appl. Mater. Interfaces 10 23910
 Google Scholar
Google Scholar
[6] Ko K Y, Song J G, Kim Y, Choi T, Shin S, Lee C W, Lee K, Koo J, Lee H, Kim J, Lee T, Park J, Kim H 2016 ACS Nano 10 9287
 Google Scholar
Google Scholar
[7] Ma Y, Kou L, Li X, Dai Y, Heine T 2016 Phys. Rev. B 93 035442
 Google Scholar
Google Scholar
[8] Hu Z, Wu Z, Han C, He J, Ni Z, Chen W 2018 Chem. Soc. Rev. 47 3100
 Google Scholar
Google Scholar
[9] Zhao Y, Qiao J, Yu Z, Yu P, Xu K, Lau S P, Zhou W, Wang X, Ji W, Chai Y 2017 Adv. Mater. 29 1604230
 Google Scholar
Google Scholar
[10] Bandurin D A, Tyurnina A V, Geliang L Y, Mishchenko A, Zólyomi V, Morozov S V, et al. 2017 Nat. Nanotechnol. 12 223
 Google Scholar
Google Scholar
[11] Zhang S, Guo S, Chen Z, Wang Y, Gao H, Gomez-Herrero J, Ares P, Zamora F, Zhu Z, Zeng H 2018 Chem. Soc. Rev. 47 982
 Google Scholar
Google Scholar
[12] Zhang S, Xie M, Li F, Yan Z, Li Y, Kan E, Liu W, Chen Z, Zeng H 2016 Angew. Chem. Int. Ed. 55 1666
 Google Scholar
Google Scholar
[13] Perkins F K, Friedman A L, Cobas E, Campbell P M, Jernigan G G, Jonker B T 2013 Nano Lett. 13 668
 Google Scholar
Google Scholar
[14] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[15] Yuan W, Shi G 2013 J. Mater. Chem. A 1 10078
 Google Scholar
Google Scholar
[16] Mousavi H 2011 Commun. Theor. Phys. 56 373
 Google Scholar
Google Scholar
[17] Tang X, Du A, Kou L 2018 Wiley Interdiscip. Rev.: Comput. Mol. Sci. 8 e1361
 Google Scholar
Google Scholar
[18] Kuc A, Heine T, Kis A 2015 MRS Bull. 40 577
 Google Scholar
Google Scholar
[19] Zhou L, Kou L, Sun Y, Felser C, Hu F, Shan G, Smith S C, Yan B, Frauenheim T 2015 Nano Lett. 15 7867
 Google Scholar
Google Scholar
[20] Kou L, Du A, Chen C, Frauenheim T 2014 Nanoscale 6 5156
 Google Scholar
Google Scholar
[21] Huang Y, Guo J, Kang Y, Ai Y, Li C M 2015 Nanoscale 7 19358
 Google Scholar
Google Scholar
[22] Xie T, Xie G, Su Y, Hongfei D, Ye Z, Jiang Y 2016 Nanotechnol. 27 065502
 Google Scholar
Google Scholar
[23] Rao C N, Gopalakrishnan K, Maitra U 2015 ACS Appl. Mater. Interfaces 7 7809
 Google Scholar
Google Scholar
[24] Liu P F, Zhou L, Frauenheimc T, Wu L M 2016 Nanoscale 8 4915
 Google Scholar
Google Scholar
[25] Late D J, Huang Y K, Liu B, Acharya J, Shirodkar S N, Luo J, Yan A, Charles D, Waghmare U V, Dravid V P 2013 ACS Nano 7 4879
 Google Scholar
Google Scholar
[26] Lee K, Gatensby R, McEvoy N, Hallam T, Duesberg G S 2013 Adv. Mater. 25 6699
 Google Scholar
Google Scholar
[27] Yao Y, Tolentino L, Yang Z, Song X, Zhang W, Chen Y, Wong C P 2013 Adv. Funct. Mater. 23 3577
 Google Scholar
Google Scholar
[28] Giri A, Yang H, Jang W, Kwak J, Thiyagarajan K, Pal M, Lee D, Singh R, Kim C, Cho K 2018 Chem. Mater. 30 2463
 Google Scholar
Google Scholar
[29] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[30] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[31] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[32] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[33] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[34] Grimme S 2006 J. Comput. Chem. 27 1787
 Google Scholar
Google Scholar
[35] Bučko T, Hafner J, Lebègue S, Ángyán J G 2010 J. Phys. Chem. A 114 11814
 Google Scholar
Google Scholar
[36] Yue Q, Shao Z, Chang S, Li J 2013 Nanoscale Res. Lett. 8 425
 Google Scholar
Google Scholar
[37] Henkelman G, Arnaldsson A, Jónsson H 2006 Comput. Mater. Sci. 36 354
 Google Scholar
Google Scholar
[38] Ding Y, Wang Y, Ni J, Shi L, Shi S, Tang W 2011 Physica B 406 2254
 Google Scholar
Google Scholar
[39] Kang J, Tongay S, Zhou J, Li J, Wu J 2013 Appl. Phys. Lett. 102 012111
 Google Scholar
Google Scholar
[40] Hu X, Kou L, Sun L 2016 Sci. Rep. 6 31122
 Google Scholar
Google Scholar
[41] Hu X, Wang Y, Shen X, Krasheninnikov A V, Sun L, Chen Z 2018 2D Mater. 5 031012
 Google Scholar
Google Scholar
[42] Leenaerts O, Partoens B, Peeters F M 2008 Phys. Rev. B 77 125416
 Google Scholar
Google Scholar
计量
- 文章访问数: 16885
- PDF下载量: 347
- 被引次数: 0














 下载:
下载: