-
Henry系数和等量吸附热是表征气体吸附中气-固作用的重要参数. 为了更好地理解气体在粗糙孔隙中的吸附特征, 首先构造并计算了矩形波纹粗糙狭缝及其外势分布. 进一步, 采用经典统计力学研究了狭缝中
$ \text{H}_{2} $ 分子在低压范围内的纵向Henry系数和等量吸附热. 研究结果表明, 粗糙狭缝的几何形貌和基板间距等因素可对狭缝中气体的纵向Henry系数和等量吸附热产生显著的影响与调制作用. 进一步, 在Henry范围内计算了$ \text{CO}_{2}/ \text{H}_{2} $ 二元混合物气体在矩形波纹粗糙狭缝中的吸附选择性, 并研究了狭缝几何形貌的调制作用. 此外, 还研究了不同形状的凸起对气体吸附性质的影响. 相关的结果可为理解多孔材料中气体的吸附、分离和提纯等过程提供可靠的理论依据, 并有望为设计与研发新型纳米功能材料提供有益的参考.Henry constant and isosteric heat of adsorption are important parameters for characterizing the gas-solid interaction in an adsorption process. In order to better understand the adsorption behavior of gas in rough pores, we construct rough slit pores by using two rectangular corrugated substrates, and then calculate the potential profile in it. By utilizing classical statistical mechanics, the longitudinal Henry constant and isosteric heat of$ \text{H}_{2} $ are further calculated in the Henry region. The results suggest that both geometric morphology and pore width can significantly influence and modulate the longitudinal Henry constant and isosteric heat of the gas in the pore. Furthermore, the selectivity of adsorption in the binary$ \text{CO}_{2}/ \text{H}_{2} $ mixture is also calculated and investigated in the Henry region. In addition, the effects of corrugated substrates with different geometries on adsorption properties are also calculated and studied. The result can provide reliable theoretical basis for understanding the adsorption, separation and purification of the gas in porous materials, and it is also expected to provide helpful idea for designing and exploring new nano functional materials.-
Keywords:
- rough pore /
- Henry’s law /
- isosteric heat /
- adsorption selectivity
[1] Roque-Malherbe R M A 2018 Adsorption and Diffusion in Nanoporous Materials (2nd Ed.) (New York: CRC Press) pp51–77
[2] Hill T L 1949 J. Chem. Phys. 17 520
 Google Scholar
Google Scholar
[3] Balbuena P B, Gubbins K E 1993 Langmuir 9 1801
 Google Scholar
Google Scholar
[4] Liu J, LeVan M D 2010 Carbon 48 3454
 Google Scholar
Google Scholar
[5] Do D D, Nicholson D, Do H D 2008 J. Colloid Interf. Sci. 324 15
 Google Scholar
Google Scholar
[6] Steele W 1973 Surf. Sci. 36 317
 Google Scholar
Google Scholar
[7] Maurer S, Mersmann A, Peukert W 2001 Chem. Eng. Sci. 56 3443
 Google Scholar
Google Scholar
[8] Jiang J W, Wagner N J, Sandler S I 2004 Phys. Chem. Chem. Phys. 6 4440
 Google Scholar
Google Scholar
[9] Do D D, Do H D, Wongkoblap A, Nicholson D 2008 Phys. Chem. Chem. Phys. 10 7293
 Google Scholar
Google Scholar
[10] Schindler B J, LeVan M D 2008 Carbon 46 644
 Google Scholar
Google Scholar
[11] Liu J, LeVan M D 2009 Carbon 47 3415
 Google Scholar
Google Scholar
[12] Do D D 1998 Adsorption Analysis: Equilibria and Kinetics (New Jersey: Imperial College Press) pp249–336
[13] Wongkoblap A, Do D D 2007 Carbon 45 1527
 Google Scholar
Google Scholar
[14] Loi Q K, Horikawa T, Tan S, Prasetyo L, Do D D, Nicholson D 2020 Microporous Mesoporous Mater. 293 109762
 Google Scholar
Google Scholar
[15] Cracknell R F, Nicholson D, Quirke N 1993 Mol. Phys. 80 885
 Google Scholar
Google Scholar
[16] Cracknell R F, Nicholson D, Tennison S R, Bromhead J 1996 Carbon 2 193
[17] Ben T, Li Y Q, Zhu L K, Zhang D L, Cao D P, Xiang Z H, Yao X D, Qiu S L 2012 Energy Environ. Sci. 5 8370
 Google Scholar
Google Scholar
[18] Principe I A, Fletcher A J 2020 Adsorption 26 723
 Google Scholar
Google Scholar
[19] Tan Z M, Gubbins K E 1992 J. Phys. Chem. 96 845
 Google Scholar
Google Scholar
[20] Maddox M W, Sowers S L, Gubbins K E 1996 Adsorption 2 23
 Google Scholar
Google Scholar
[21] Peng X, Cao D P, Zhao J S 2009 Sep. Purif. Technol. 68 50
 Google Scholar
Google Scholar
[22] Kumar K V, Rodriguez-Reinoso F 2012 RSC Adv. 2 9671
 Google Scholar
Google Scholar
[23] Wu T H, Firoozabadi A 2018 J. Phys. Chem. C 122 20727
 Google Scholar
Google Scholar
[24] 袁俊鹏, 刘秀英, 李晓东, 于景新 2021 70 156801
 Google Scholar
Google Scholar
Yuan J P, Liu X Y, Li X D, Yu J X 2021 Acta Phys. Sin. 70 156801
 Google Scholar
Google Scholar
[25] Nicholson D 2002 Mol. Phys. 100 2151
 Google Scholar
Google Scholar
[26] Trinh T T, Vlugt T J H, Hägg M B, Bedeaux D, Kjelstrup S 2013 Front. Chem. 1 38
[27] Zeng Y H, Xu H, Horikawa T, Do D D, Nicholson D 2018 J. Phys. Chem. C 122 24171
 Google Scholar
Google Scholar
[28] Prasetyo L, Do D D, Nicholson D 2018 Chem. Eng. J. 334 143
 Google Scholar
Google Scholar
[29] Steele W A 1974 The Interaction of Gases with Solid Surfaces (Oxford: Pergamon Press) pp317–320
[30] Maitland G C, Rigby M, Smith E B, Wakeham W A 1987 Intermolecular Forces: Their Origin and Determination (Oxford: Science Publications) pp493–504
[31] Poling B E, Prausnitz J M, O’Connell J P 2001 The Properties of Gases and Liquids (5th Ed.) (New York: McGraw-Hill) ppB.1–B.2
[32] Ravikovitch P I, Nishnyakov A, Russo R, Neimark A V 2000 Langmuir 16 2311
 Google Scholar
Google Scholar
[33] Ravikovitch P I, Nishnyakov A, Neimark A V 2001 Phys. Rev. E 64 011602
 Google Scholar
Google Scholar
[34] Frink L J D, van Swol F 1998 J. Chem. Phys. 108 5588
 Google Scholar
Google Scholar
[35] Ghatak C, Ayappa K G 2004 J. Chem. Phys. 120 9703
 Google Scholar
Google Scholar
-
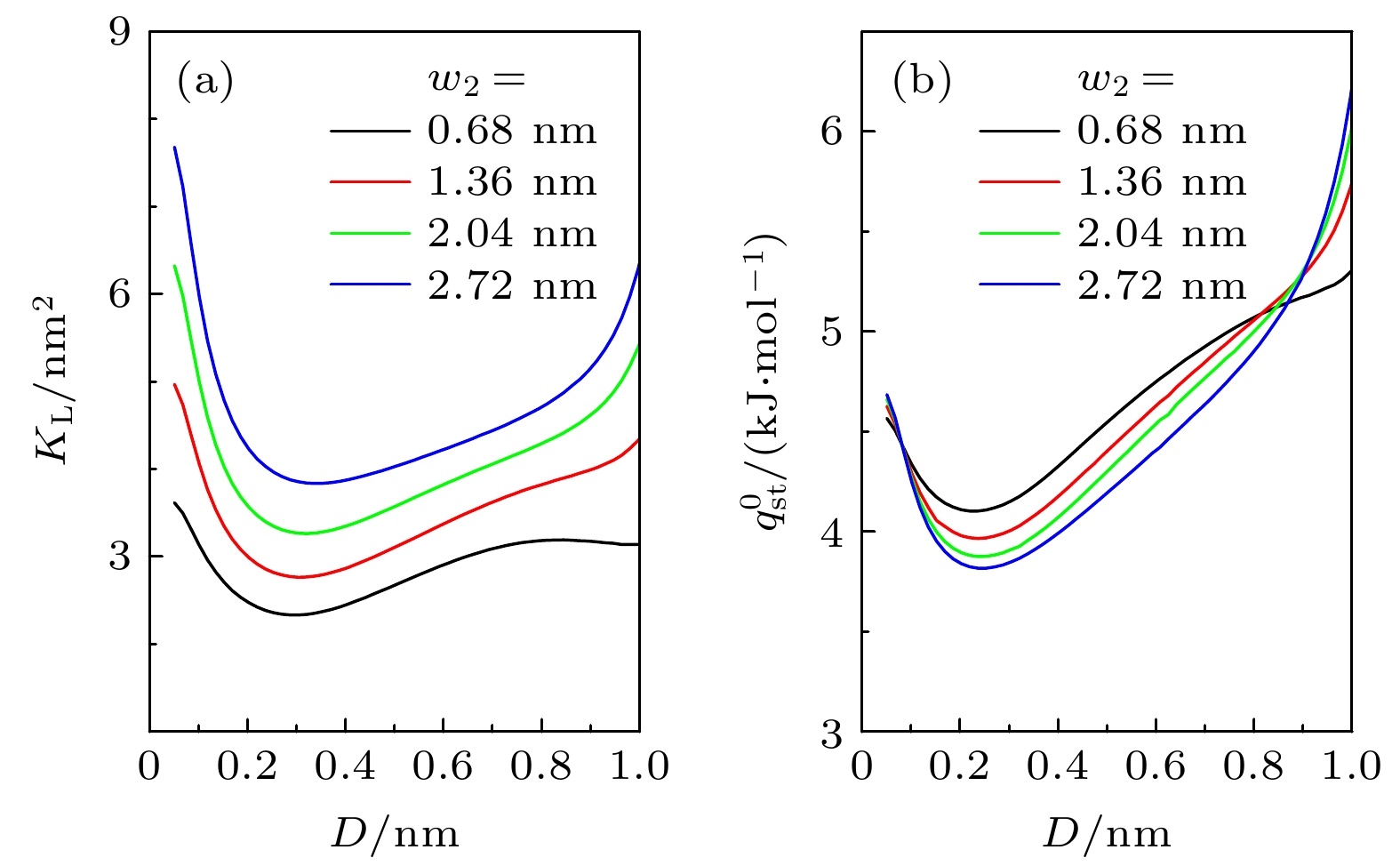
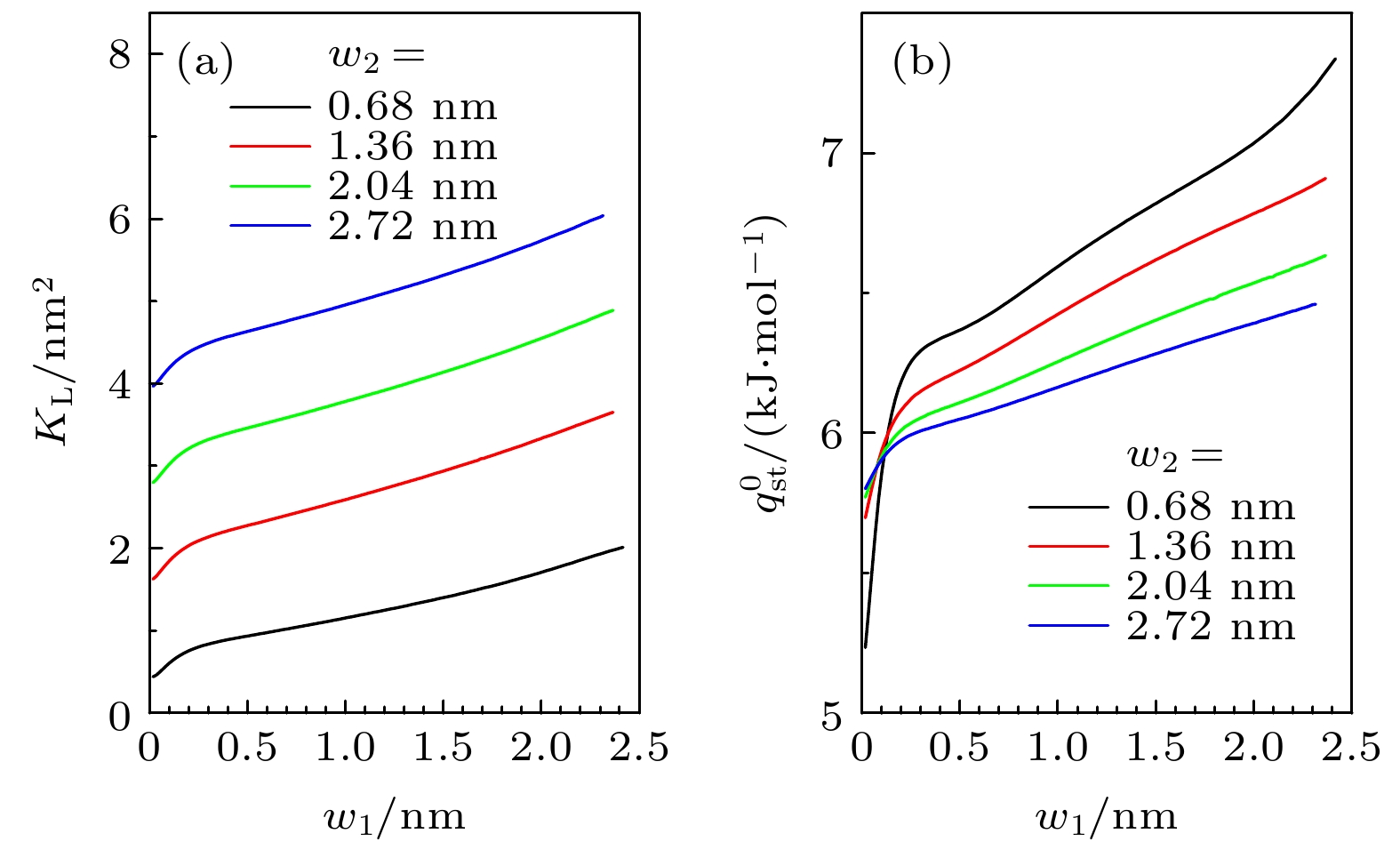
图 1 (a)粗糙狭缝示意图, 绿色虚线包围的区域定义为狭缝中的I-类和II-类区域, 蓝色虚线表示气体分子可达空间的边界; (b)单个粗糙基板附近的气体分子的外势分布为
$ V_{ \text{sub}}(y, z) $ . 计算中取$ w_{1} = 1.36 $ nm,$ w_{2} = 2.04 $ nm,${M} = 3 $ Fig. 1. (a) Sketch of the rough slit pore. The regions surrounded by the green dash lines are type-I and type-II regions in the rough pore, while the blue dashed line stands for the boundary of the accessible volume of the gas molecular. (b) Profile of external potential
$ V_{ \text{sub}}(y, z) $ near a rough substrate with$ w_{1} = 1.36 $ nm,$ w_{2} = 2.04 $ nm and${M} = 3$ 图 2 石墨表面上不同气体的(a)等量吸附热
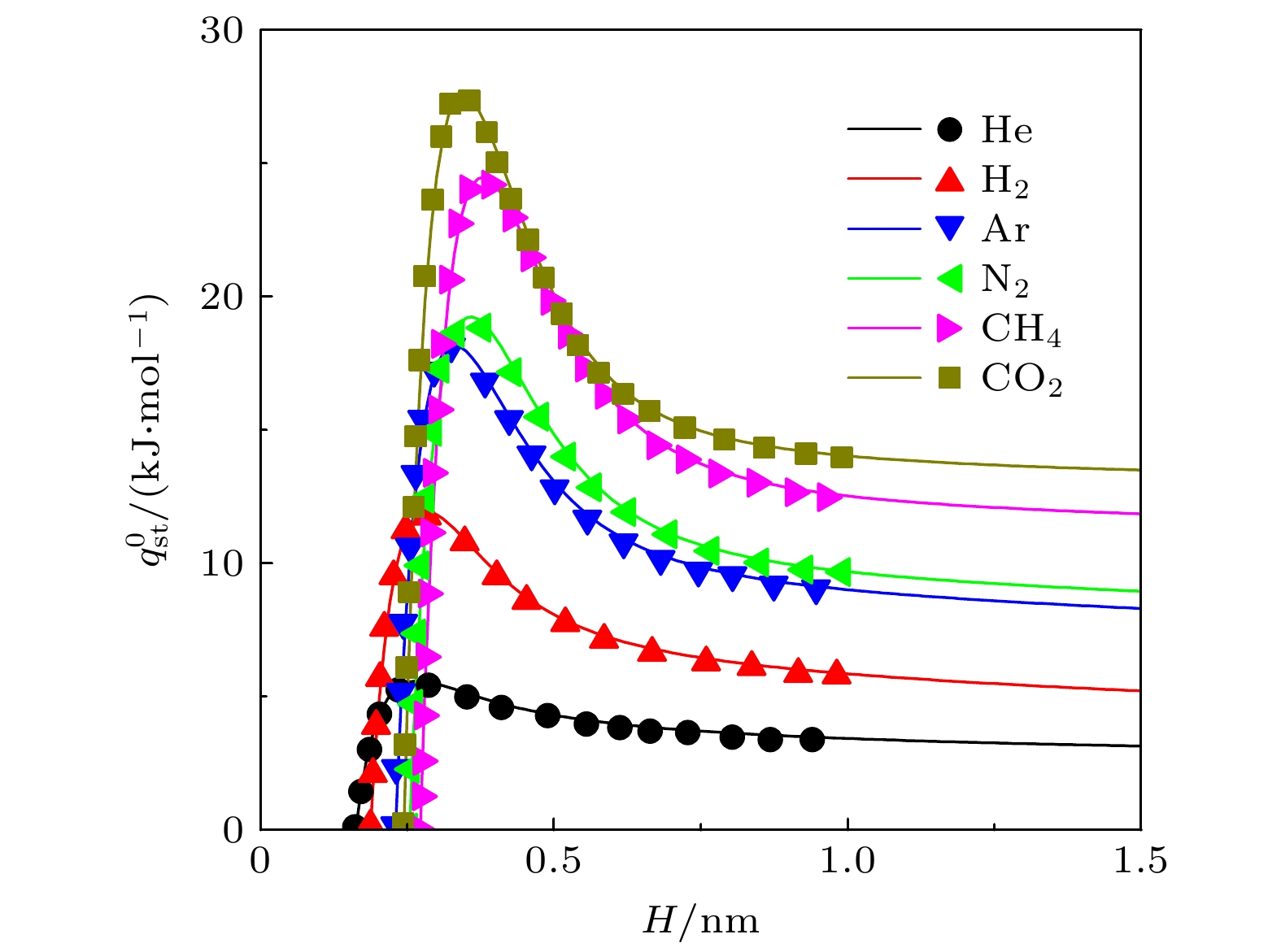
$ q_{ \text{st}}^{0} $ 和(b)内禀Henry系数$ K_{ \text{H}} $ . 线: 本文结果; 符号: MC模拟结果[28]. 其中S为基板面积Fig. 2. Isoteric heat of (a) adsorption
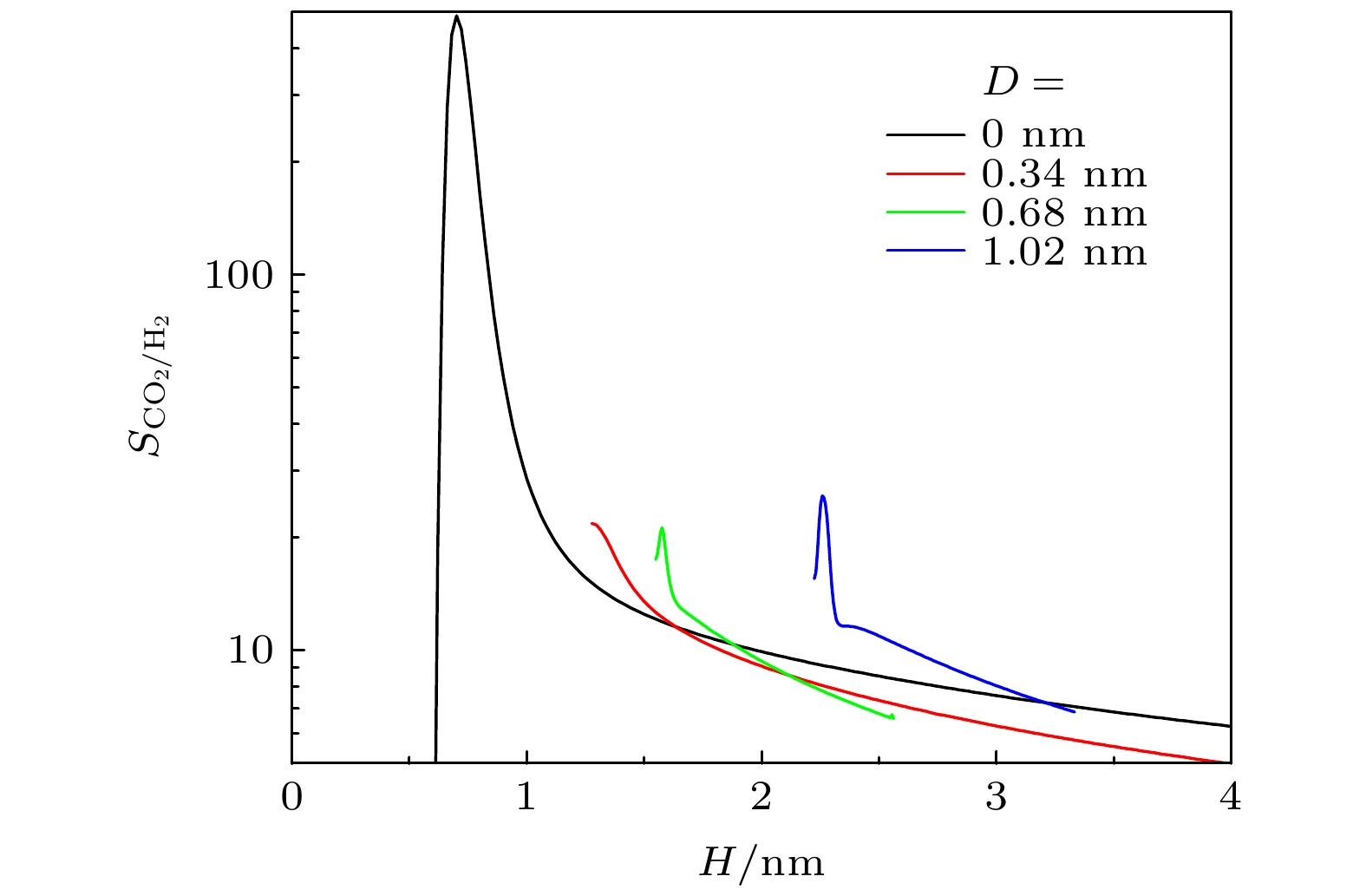
$ q_{ \text{st}}^{0} $ and (b) inherent Henry constant$ K_{ \text{H}} $ of different gases on graphite surface. Lines: results of this work; symbols: results from MC simulations[28]. S is the area of substrate图 8 不同条件下, 基板几何形貌对吸附选择性
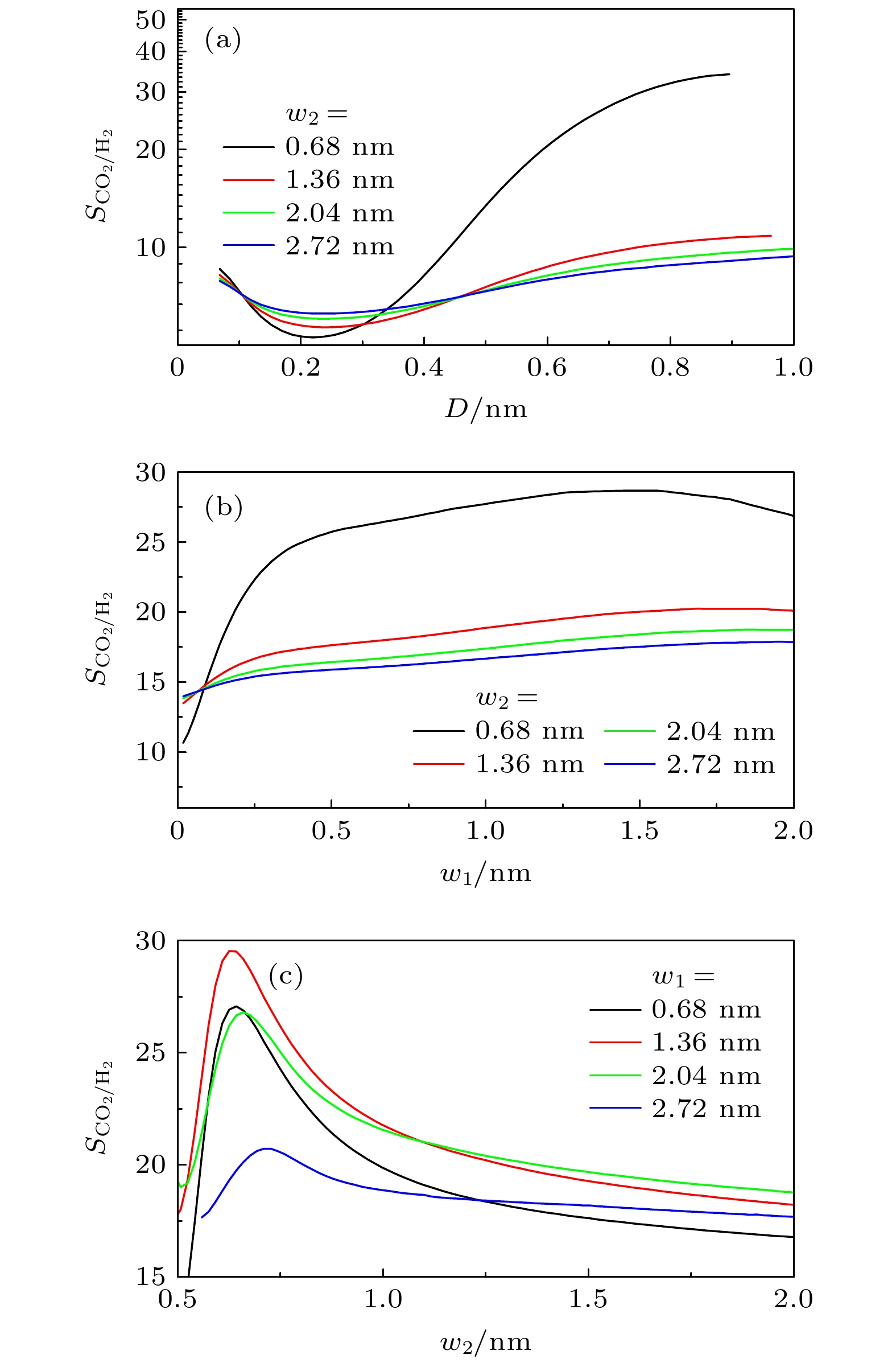
$ S_{ \text{CO}_{2}/ \text{H}_{2}} $ 的影响 (a)$ w_{1} = 1.36 $ nm,$ H = 3.0 $ nm时, D对$ S_{ \text{CO}_{2}/ \text{H}_{2}} $ 的影响; (b)$ D = 0.34 $ nm,$ H = 1.5 $ nm时,$ w_{1} $ 对$ S_{ \text{CO}_{2}/ \text{H}_{2}} $ 的影响; (c)$ D = 0.34 $ nm,$ H = 1.5 $ nm时,$ w_{2} $ 对$ S_{ \text{CO}_{2}/ \text{H}_{2}} $ 的影响Fig. 8. Effect of geometric morphology on the selectivity
$ S_{ \text{CO}_{2}/ \text{H}_{2}} $ in rough slit pore: (a) Effect of D on$ S_{ \text{CO}_{2}/ \text{H}_{2}} $ with$ w_{1} = 1.36 $ nm and$ H = 3.0 $ nm; (b) effect of$ w_{1} $ on$ S_{ \text{CO}_{2}/ \text{H}_{2}} $ with$ D = 0.34 $ nm and$ H = 1.5 $ nm; (c) effect of$ w_{2} $ on$ S_{ \text{CO}_{2}/ \text{H}_{2}} $ with$ D = 0.34 $ nm and$ H = 1.5 $ nm图 10 (a)—(c) 三角形和半圆形凸起对狭缝中
$ \text{H}_{2} $ 的$ K_{ \text{L}} $ 和$ q_{ \text{st}}^{0} $ 的影响. 图中, 黑线和红线分别为$ K_{ \text{L}} $ 和$ q_{ \text{st}}^{0} $ 的结果, 实线和虚线分别为三角形凸起和半圆形凸起情况下的结果. (d)—(f)三角形和半圆形凸起对对$ S_{ \text{CO}_{2}/ \text{H}_{2}} $ 的影响. 图中, 实线和虚线分别为三角形凸起和半圆形凸起情况下的结果. 需要说明的是, 在图(b)和图(e)中, 三角形凸起取$ w_{1} = 0.68 $ nm, 而在图(c)和图(f)中, 三角形凸起取$ w_{1} = 1.36 $ nmFig. 10. (a)–(c) Effect of geometry on
$ K_{ \text{L}} $ and$ q_{ \text{st}}^{0} $ of$ \text{H}_{2} $ in rough slit pore with triangular and semicircular corrugated substrates. In these figures, black lines and red lines correspond to results for$ K_{ \text{L}} $ and$ q_{ \text{st}}^{0} $ , respectively. In addition, solid and dashed lines stand for results of triangular and semicircular condition, respectively. (d)–(f) Effect of geometry on selectivity$ S_{ \text{CO}_{2}/ \text{H}_{2}} $ of$ \text{CO}_{2}/ \text{H}_{2} $ mixture in rough slit pore with triangular and semicircular corrugated substrates. In these figures, solid and dashed lines stand for results of triangular and semicircular condition, respectively. It should be noted that in panels (b) and (e),$ w_{1} = 0.68 $ nm is set for the triangular corrugated substrate, while in panels (c) and (f), it is set as$ w_{1} = 1.36 $ nm表 1 不同气体分子的LJ模型参数
Table 1. LJ model parameters of different gas molecular
分子种类 $\sigma_{\text{gg}}$/nm $\varepsilon_{\text{gg}}/k_\text{B}$/K $\sigma_{\text{gs}}$/nm $\varepsilon_{\text{gs}}/k_\text{B}$/K 文献 He 0.2560 10.21 0.2980 16.90 [29] Ne 0.2780 34.90 0.3080 31.26 [30] Ar 0.3405 119.80 0.3393 57.92 [30] Kr 0.3685 164.40 0.3533 67.85 [30] Xe 0.4100 221.00 0.3740 78.66 [30] $\text{H}_{2}$ 0.2830 59.70 0.3105 40.87 [31] $\text{N}_{2}$ 0.3575 94.45 0.3494 53.22 [32] $\text{CH}_{4}$ 0.3820 148.20 0.3600 64.40 [33] $\text{CO}_{2}$ 0.3454 235.90 0.3430 81.50 [32] -
[1] Roque-Malherbe R M A 2018 Adsorption and Diffusion in Nanoporous Materials (2nd Ed.) (New York: CRC Press) pp51–77
[2] Hill T L 1949 J. Chem. Phys. 17 520
 Google Scholar
Google Scholar
[3] Balbuena P B, Gubbins K E 1993 Langmuir 9 1801
 Google Scholar
Google Scholar
[4] Liu J, LeVan M D 2010 Carbon 48 3454
 Google Scholar
Google Scholar
[5] Do D D, Nicholson D, Do H D 2008 J. Colloid Interf. Sci. 324 15
 Google Scholar
Google Scholar
[6] Steele W 1973 Surf. Sci. 36 317
 Google Scholar
Google Scholar
[7] Maurer S, Mersmann A, Peukert W 2001 Chem. Eng. Sci. 56 3443
 Google Scholar
Google Scholar
[8] Jiang J W, Wagner N J, Sandler S I 2004 Phys. Chem. Chem. Phys. 6 4440
 Google Scholar
Google Scholar
[9] Do D D, Do H D, Wongkoblap A, Nicholson D 2008 Phys. Chem. Chem. Phys. 10 7293
 Google Scholar
Google Scholar
[10] Schindler B J, LeVan M D 2008 Carbon 46 644
 Google Scholar
Google Scholar
[11] Liu J, LeVan M D 2009 Carbon 47 3415
 Google Scholar
Google Scholar
[12] Do D D 1998 Adsorption Analysis: Equilibria and Kinetics (New Jersey: Imperial College Press) pp249–336
[13] Wongkoblap A, Do D D 2007 Carbon 45 1527
 Google Scholar
Google Scholar
[14] Loi Q K, Horikawa T, Tan S, Prasetyo L, Do D D, Nicholson D 2020 Microporous Mesoporous Mater. 293 109762
 Google Scholar
Google Scholar
[15] Cracknell R F, Nicholson D, Quirke N 1993 Mol. Phys. 80 885
 Google Scholar
Google Scholar
[16] Cracknell R F, Nicholson D, Tennison S R, Bromhead J 1996 Carbon 2 193
[17] Ben T, Li Y Q, Zhu L K, Zhang D L, Cao D P, Xiang Z H, Yao X D, Qiu S L 2012 Energy Environ. Sci. 5 8370
 Google Scholar
Google Scholar
[18] Principe I A, Fletcher A J 2020 Adsorption 26 723
 Google Scholar
Google Scholar
[19] Tan Z M, Gubbins K E 1992 J. Phys. Chem. 96 845
 Google Scholar
Google Scholar
[20] Maddox M W, Sowers S L, Gubbins K E 1996 Adsorption 2 23
 Google Scholar
Google Scholar
[21] Peng X, Cao D P, Zhao J S 2009 Sep. Purif. Technol. 68 50
 Google Scholar
Google Scholar
[22] Kumar K V, Rodriguez-Reinoso F 2012 RSC Adv. 2 9671
 Google Scholar
Google Scholar
[23] Wu T H, Firoozabadi A 2018 J. Phys. Chem. C 122 20727
 Google Scholar
Google Scholar
[24] 袁俊鹏, 刘秀英, 李晓东, 于景新 2021 70 156801
 Google Scholar
Google Scholar
Yuan J P, Liu X Y, Li X D, Yu J X 2021 Acta Phys. Sin. 70 156801
 Google Scholar
Google Scholar
[25] Nicholson D 2002 Mol. Phys. 100 2151
 Google Scholar
Google Scholar
[26] Trinh T T, Vlugt T J H, Hägg M B, Bedeaux D, Kjelstrup S 2013 Front. Chem. 1 38
[27] Zeng Y H, Xu H, Horikawa T, Do D D, Nicholson D 2018 J. Phys. Chem. C 122 24171
 Google Scholar
Google Scholar
[28] Prasetyo L, Do D D, Nicholson D 2018 Chem. Eng. J. 334 143
 Google Scholar
Google Scholar
[29] Steele W A 1974 The Interaction of Gases with Solid Surfaces (Oxford: Pergamon Press) pp317–320
[30] Maitland G C, Rigby M, Smith E B, Wakeham W A 1987 Intermolecular Forces: Their Origin and Determination (Oxford: Science Publications) pp493–504
[31] Poling B E, Prausnitz J M, O’Connell J P 2001 The Properties of Gases and Liquids (5th Ed.) (New York: McGraw-Hill) ppB.1–B.2
[32] Ravikovitch P I, Nishnyakov A, Russo R, Neimark A V 2000 Langmuir 16 2311
 Google Scholar
Google Scholar
[33] Ravikovitch P I, Nishnyakov A, Neimark A V 2001 Phys. Rev. E 64 011602
 Google Scholar
Google Scholar
[34] Frink L J D, van Swol F 1998 J. Chem. Phys. 108 5588
 Google Scholar
Google Scholar
[35] Ghatak C, Ayappa K G 2004 J. Chem. Phys. 120 9703
 Google Scholar
Google Scholar
计量
- 文章访问数: 7201
- PDF下载量: 129
- 被引次数: 0


























 下载:
下载: